Abstract
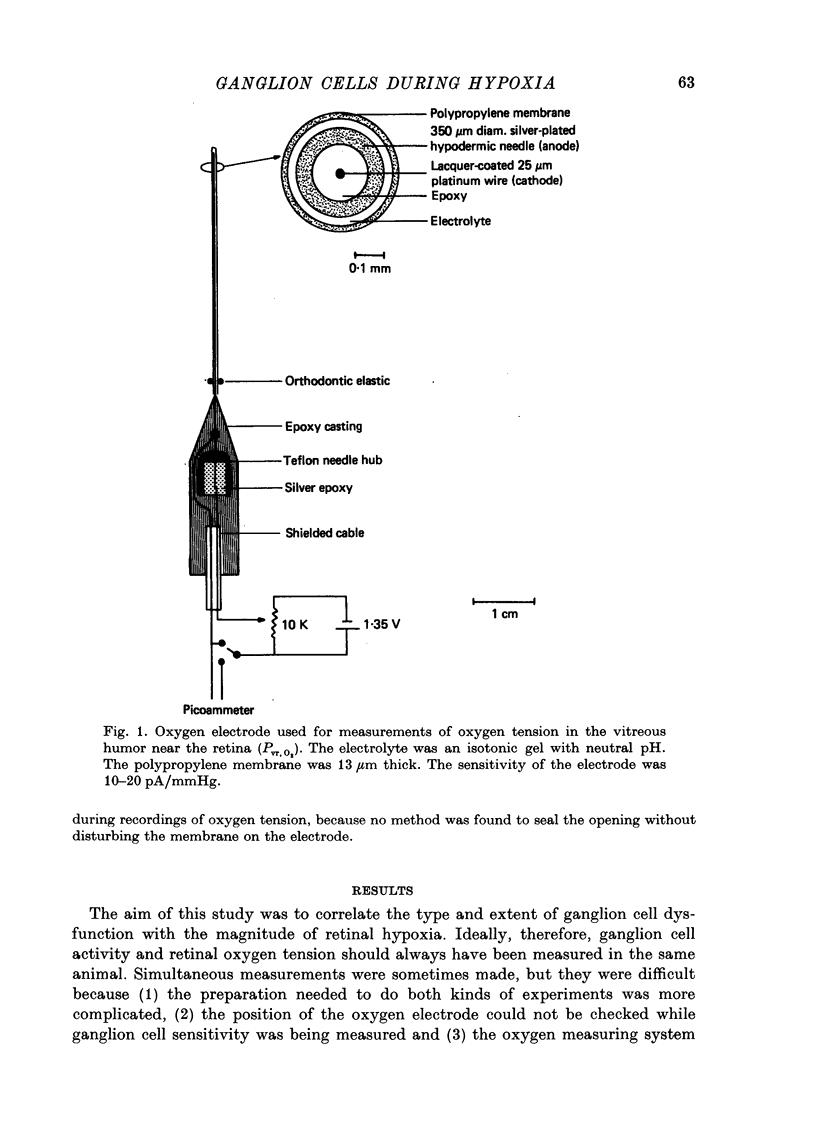
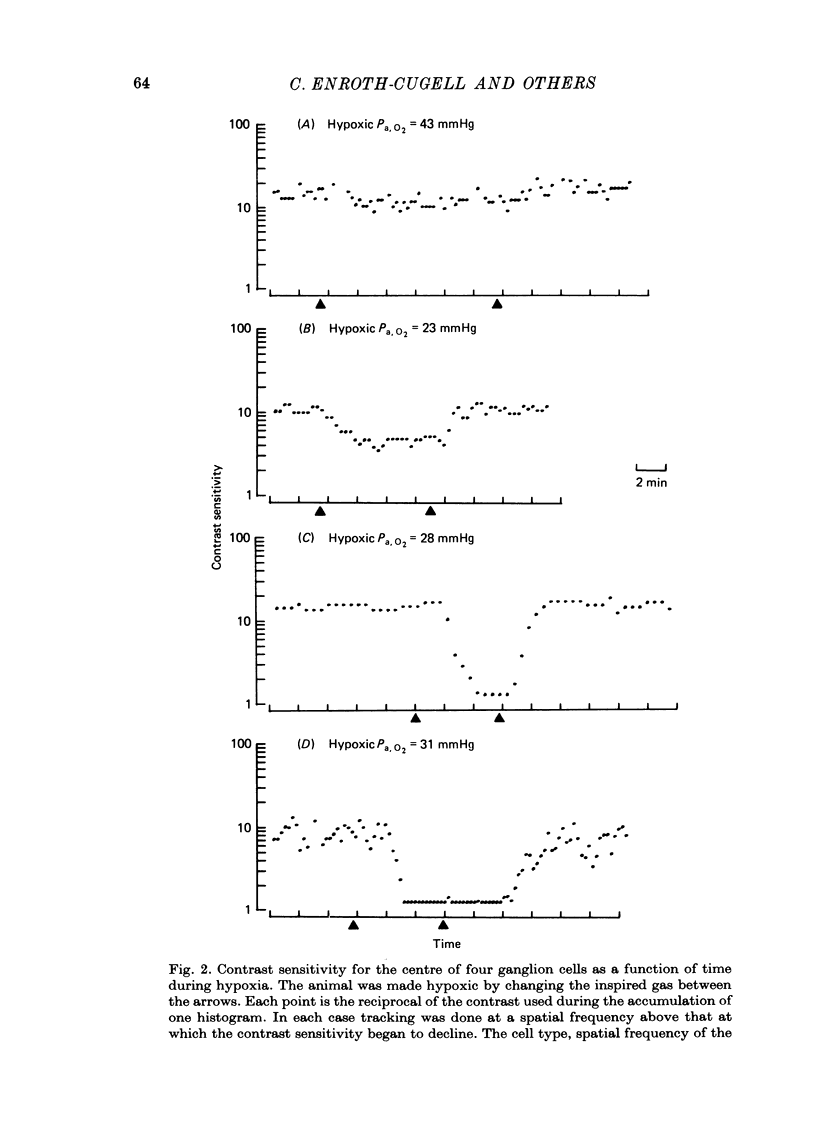
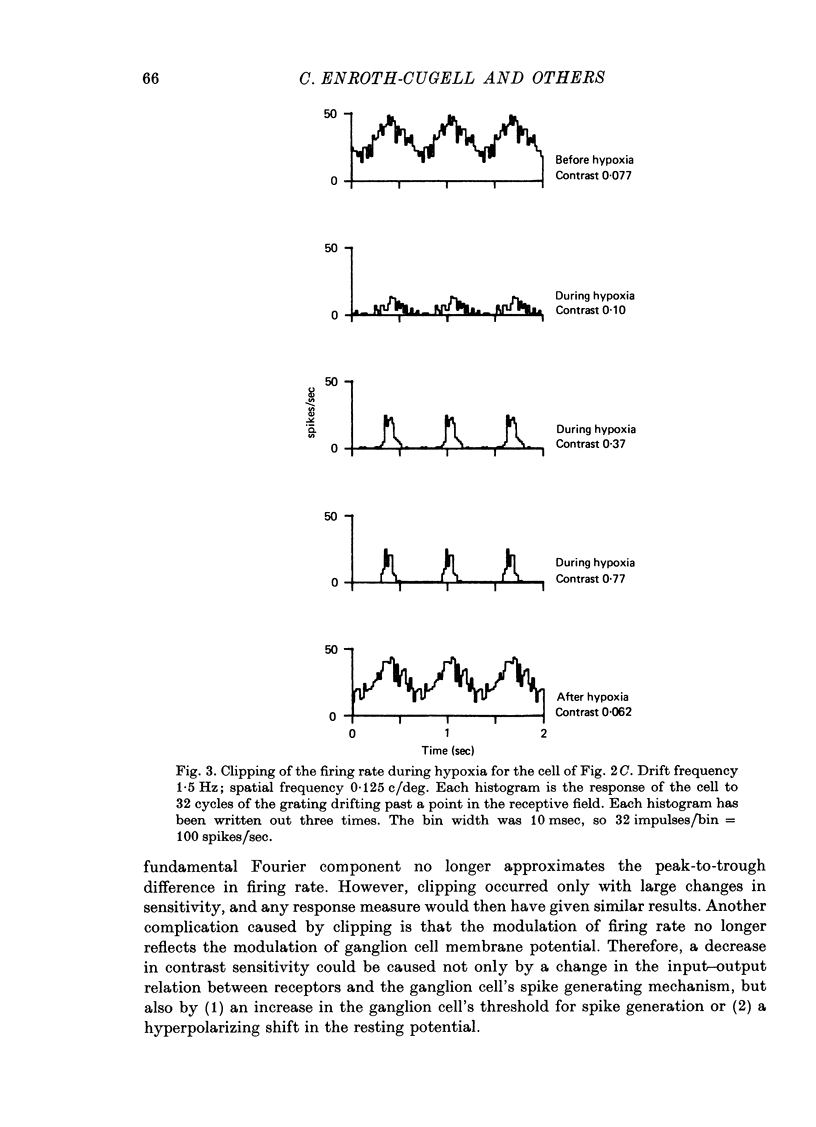
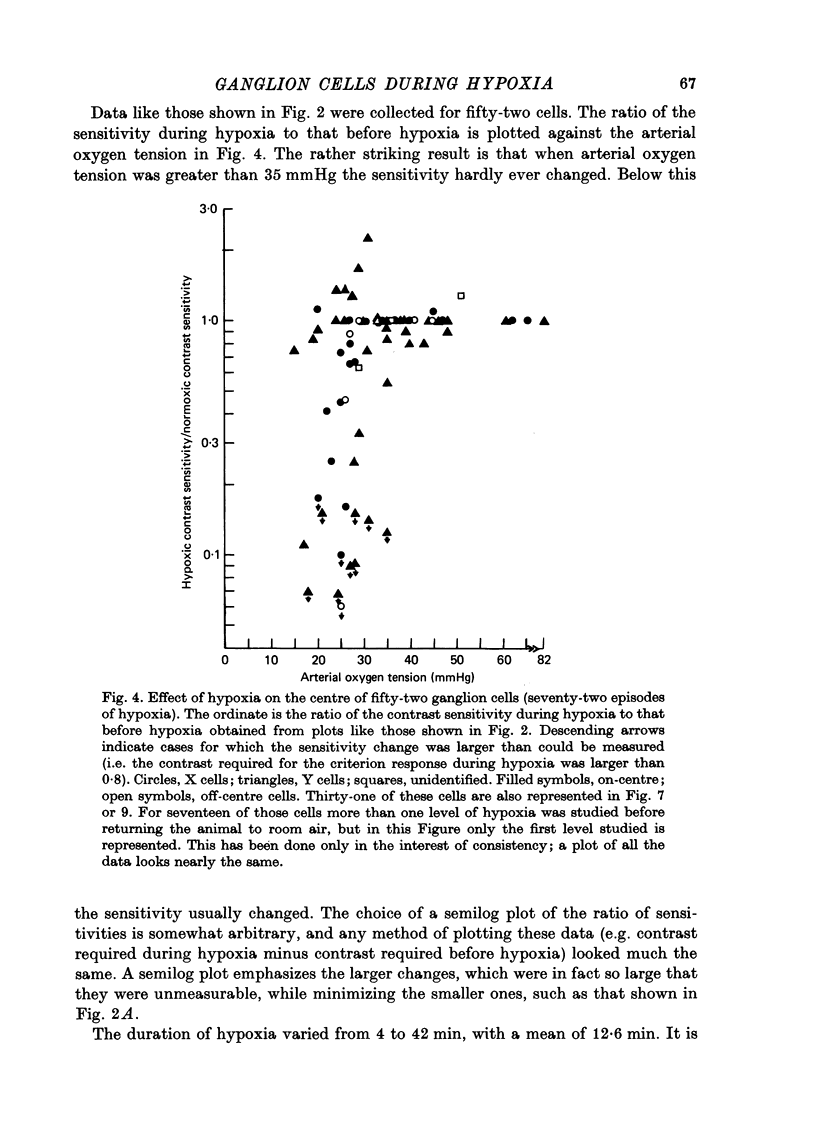
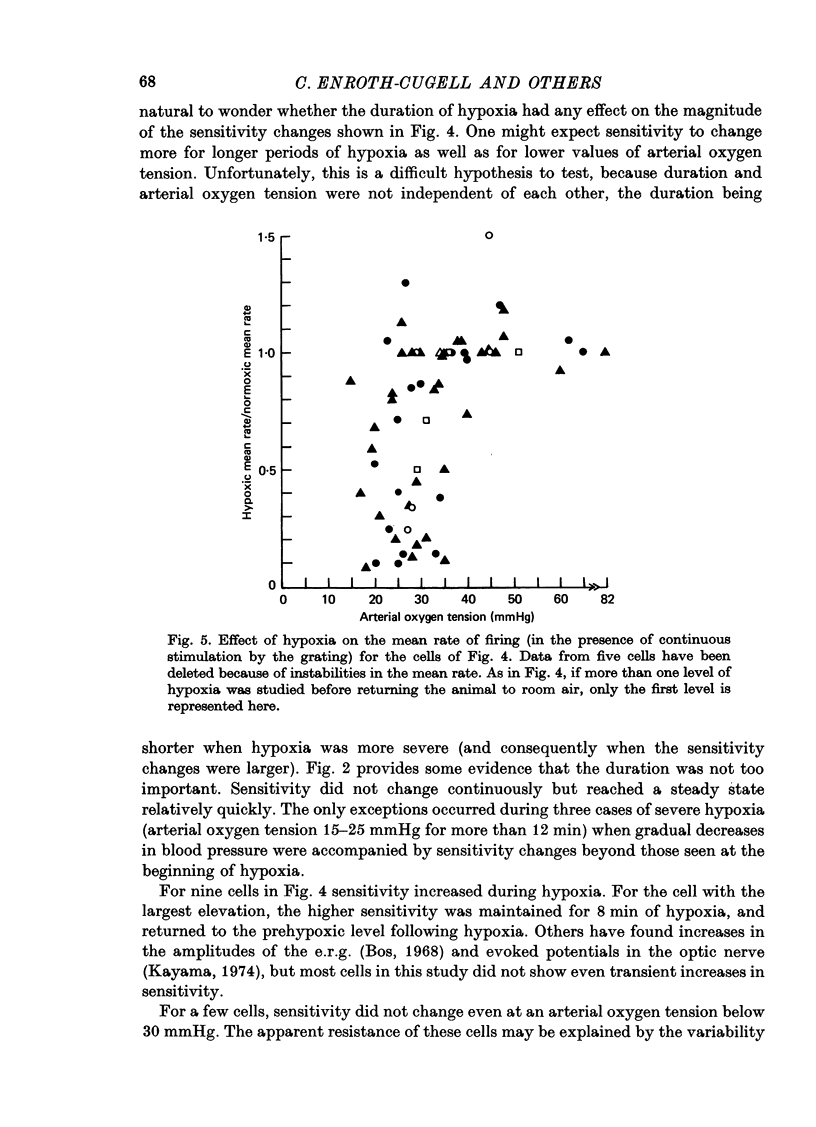
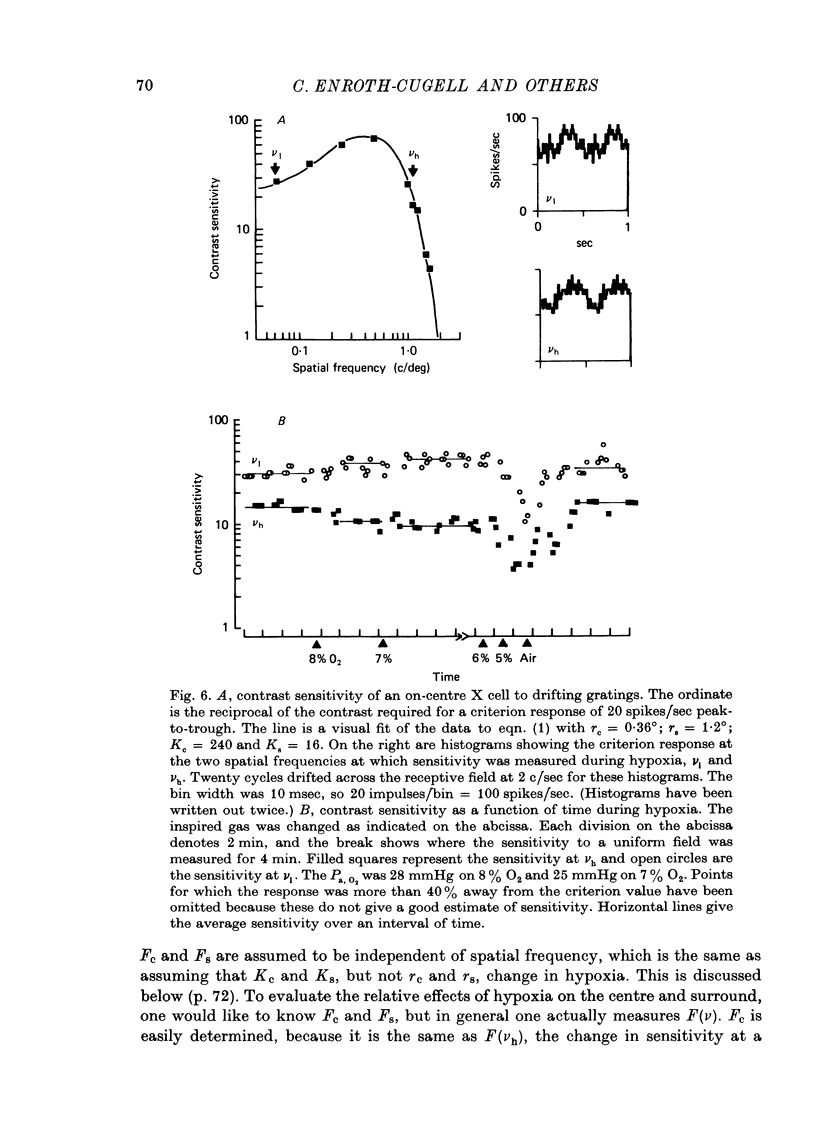
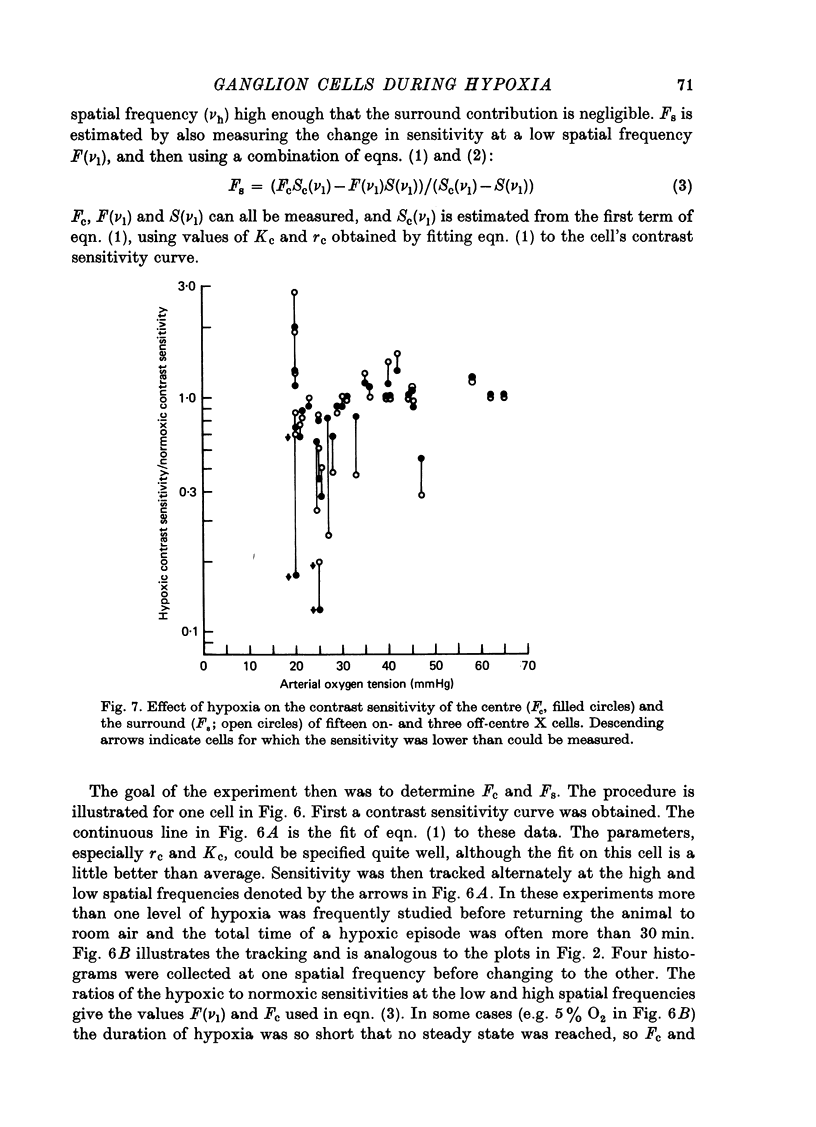
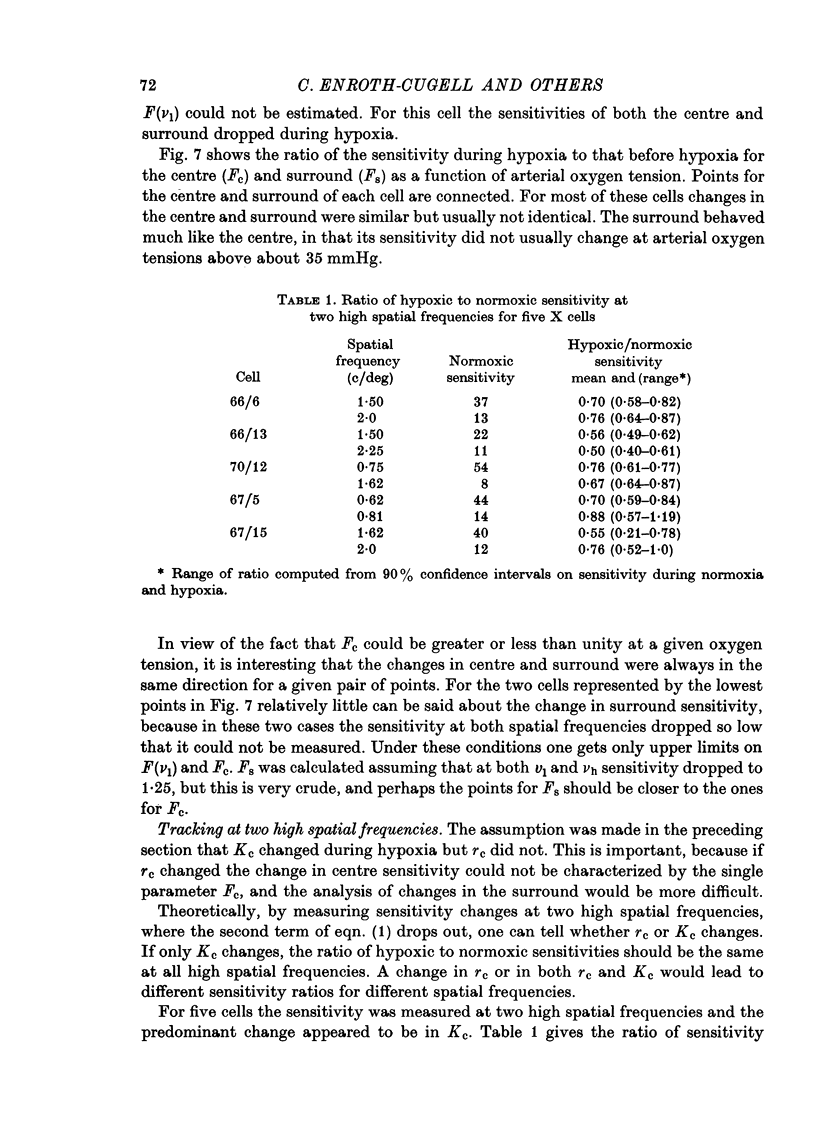
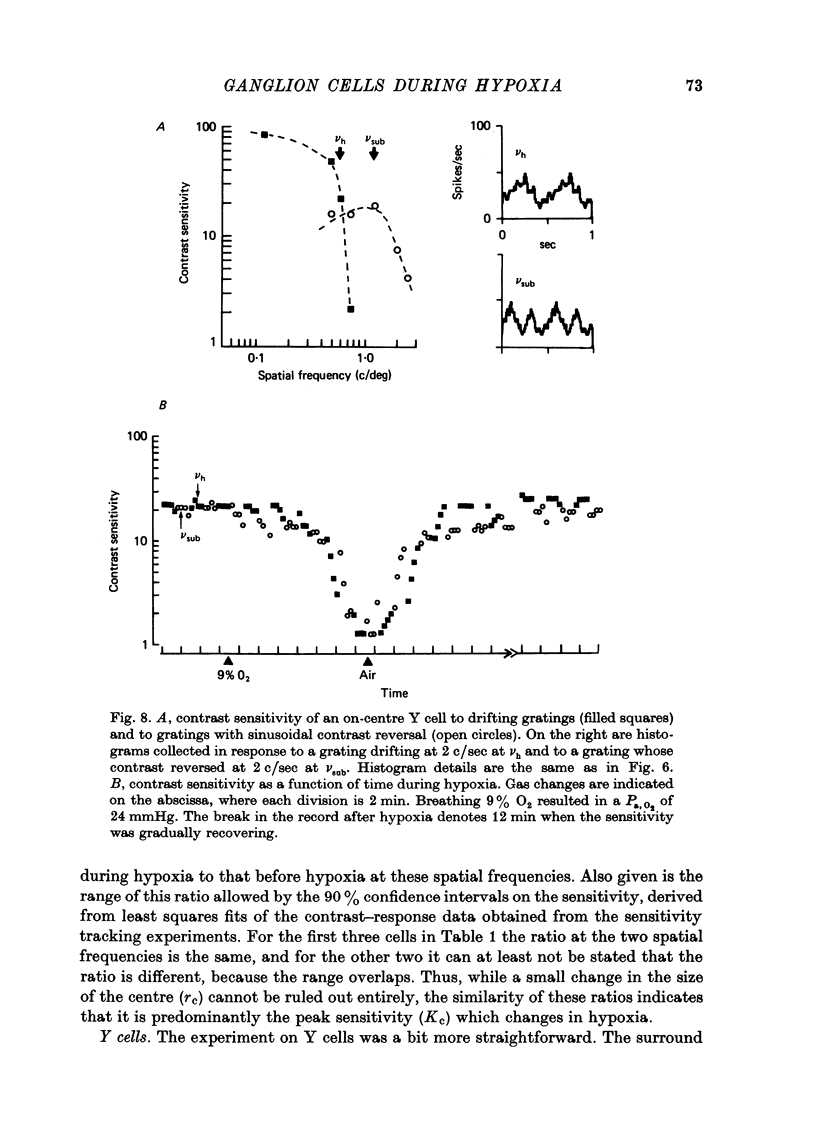
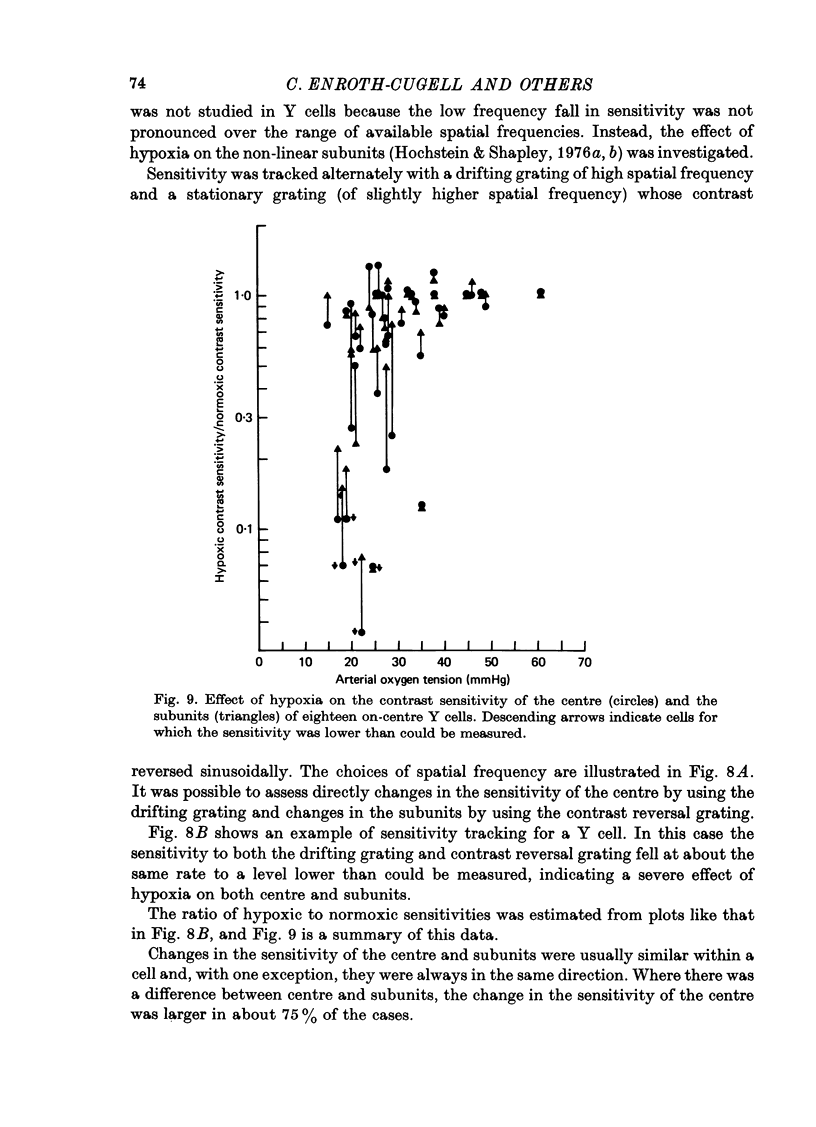
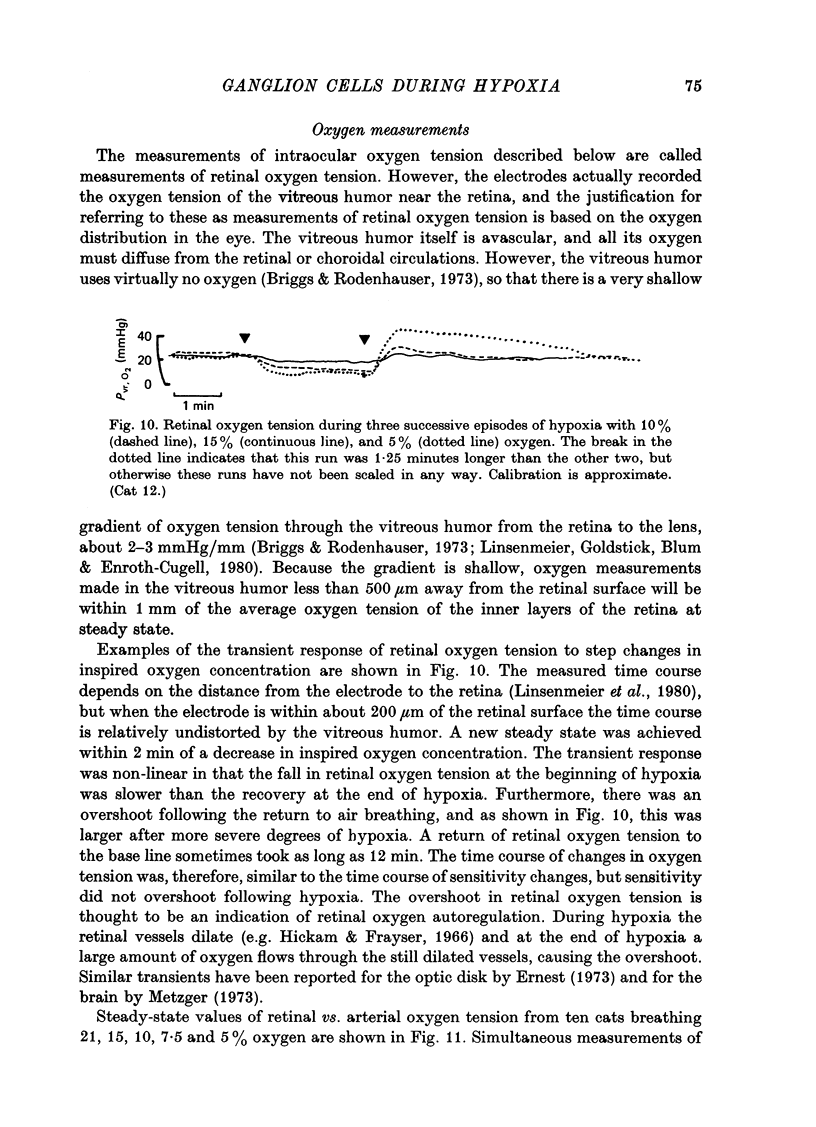
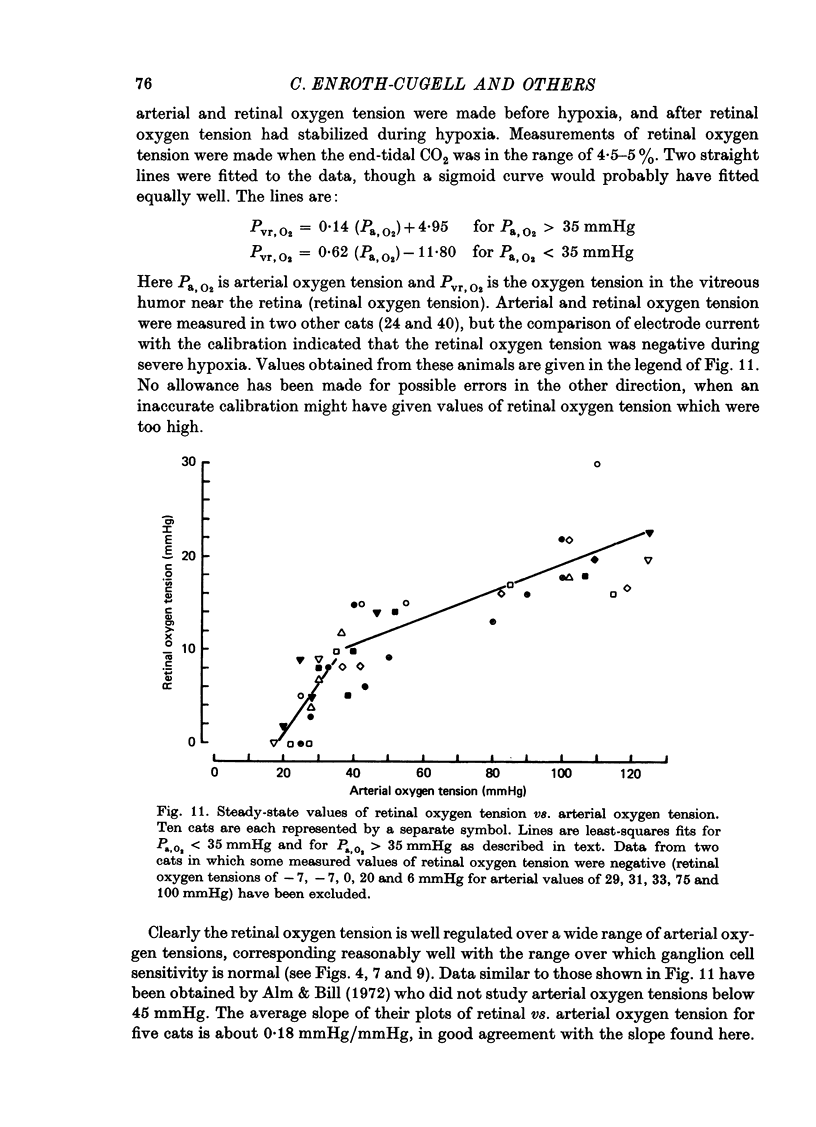
1. These experiments were done to investigate the effect of various degrees of hypoxia on the function of retinal ganglion cells (recorded in the optic tract) and on retinal oxygen tension. 2. The contrast sensitivity of the centre of X and Y cells, the surround of X cells and the non-linear subunits of Y cells were measured separately by choosing appropriate spatial and temporal parameters of a sinusoidal grating pattern. 3. Retinal oxygen tension was measured with a bipolar polarographic oxygen electrode positioned in the vitreous humor close to the retina. 4. The time course of changes in ganglion cell sensitivity and retinal oxygen tension was similar. However, oxygen tension frequently overshot the prehypoxic value at the end of hypoxia, while sensitivity did not. 5. The cat retina was rather resistant to hypoxia. Contrast sensitivity and mean firing rate did not change provided the arterial oxygen tension was above about 35 mmHg, but usually dropped precipitously at lower arterial values. 6. The apparent reason for this resistance is that retinal oxygen tension was well regulated, falling only 0.14 mmHg per mmHg of arterial oxygen tension for arterial values above about 35 mmHg, which corresponds to a retinal oxygen tension of about 10 mmHg. Retinal oxygen tension decreased more sharply (0.62 mmHg per mmHg) at lower values of arterial oxygen tension, where sensitivity also decreased. 7. The centre, surround and subunits reacted similarly to hypoxia. This suggests that lateral pathways (i.e. surround) and pathways which might be expected to use more synapses than the centre (i.e. surround and subunits) are not more susceptible to hypoxia.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES A., 3rd, GURIAN B. S. Effects of glucose and oxygen deprivation on function of isolated mammalian retina. J Neurophysiol. 1963 Jul;26:617–634. doi: 10.1152/jn.1963.26.4.617. [DOI] [PubMed] [Google Scholar]
- Acker H., Keller H. P., Lübbers D. W., Bingmann D., Schulze H., Caspers H. The relationship between neuronal activity of chemoreceptor fibers and tissue PO2 of the carotid body of the cat during changes in arterial PO2 and blood pressure. Pflugers Arch. 1973 Nov 8;343(4):287–296. doi: 10.1007/BF00595816. [DOI] [PubMed] [Google Scholar]
- Alm A., Bill A. The oxygen supply to the retina. I. Effects of changes in intraocular and arterial blood pressures, and in arterial P O2 and P CO2 on the oxygen tension in the vitreous body of the cat. Acta Physiol Scand. 1972 Feb;84(2):261–274. doi: 10.1111/j.1748-1716.1972.tb05177.x. [DOI] [PubMed] [Google Scholar]
- BORNSCHEIN H. Spontan- und Belichtungsaktivität in Einzelfasern des N. opticus der Katze. I. Der Einfluss kurzdauernder retinaler Ischämie. Z Biol. 1958 May;110(3):210–222. [PubMed] [Google Scholar]
- BROWN J. L., HILL J. H., BURKE R. E. The effect of hypoxia on the human electroretinogram. Am J Ophthalmol. 1957 Jul;44(1):57–67. doi: 10.1016/0002-9394(57)91955-4. [DOI] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Changes in the maintained discharge with adaptation level in the cat retina. J Physiol. 1969 Jun;202(3):699–718. doi: 10.1113/jphysiol.1969.sp008836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branston N. M., Symon L., Crockard H. A., Pasztor E. Relationship between the cortical evoked potential and local cortical blood flow following acute middle cerebral artery occlusion in the baboon. Exp Neurol. 1974 Nov;45(2):195–208. doi: 10.1016/0014-4886(74)90112-5. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R. Brisk and sluggish concentrically organized ganglion cells in the cat's retina. J Physiol. 1974 Jul;240(2):421–456. doi: 10.1113/jphysiol.1974.sp010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R. Properties of rarely encountered types of ganglion cells in the cat's retina and an overall classification. J Physiol. 1974 Jul;240(2):457–492. doi: 10.1113/jphysiol.1974.sp010618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollery C. T., Bulpitt C. J., Kohner E. M. Oxygen supply to the retina from the retinal and choroidal circulations at normal and increased arterial oxygen tensions. Invest Ophthalmol. 1969 Dec;8(6):588–594. [PubMed] [Google Scholar]
- Drujan B. D., Svaetichin G., Negishi K. Retinal aerobic metabolism as reflected in S-potential behavior. Vision Res. 1971;Suppl 3:151–159. doi: 10.1016/0042-6989(71)90036-8. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernest J. T. In vivo measurement of optic-disk oxygen tension. Invest Ophthalmol. 1973 Dec;12(12):927–931. [PubMed] [Google Scholar]
- Ernest J. T., Krill A. E. The effect of hypoxia on visual function. Psychophysical studies. Invest Ophthalmol. 1971 May;10(5):323–328. [PubMed] [Google Scholar]
- Goldstick T. K., Fry W. A., Caprini J. A., Wagner E. P., Jr, Ellwein R. W. Study of oxygen transport in skeletal muscle using an unsteady state method. Bibl Anat. 1973;12:386–392. [PubMed] [Google Scholar]
- Granit R. The components of the retinal action potential in mammals and their relation to the discharge in the optic nerve. J Physiol. 1933 Feb 8;77(3):207–239. doi: 10.1113/jphysiol.1933.sp002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert D. A., Mitchell R. A. Blood gas tensions and acid-base balance in awake cats. J Appl Physiol. 1971 Mar;30(3):434–436. doi: 10.1152/jappl.1971.30.3.434. [DOI] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Linear and nonlinear spatial subunits in Y cat retinal ganglion cells. J Physiol. 1976 Nov;262(2):265–284. doi: 10.1113/jphysiol.1976.sp011595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Quantitative analysis of retinal ganglion cell classifications. J Physiol. 1976 Nov;262(2):237–264. doi: 10.1113/jphysiol.1976.sp011594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H. Tungsten Microelectrode for Recording from Single Units. Science. 1957 Mar 22;125(3247):549–550. doi: 10.1126/science.125.3247.549. [DOI] [PubMed] [Google Scholar]
- Kayama Y. Evoked potentials of the central visual system during and after hypoxia in cats. Electroencephalogr Clin Neurophysiol. 1974 Jun;36(6):619–628. doi: 10.1016/0013-4694(74)90228-4. [DOI] [PubMed] [Google Scholar]
- Kogure K., Scheinberg P., Utsunomiya Y., Kishikawa H., Busto R. Sequential cerebral biochemical and physiological events in controlled hypoxemia. Ann Neurol. 1977 Oct;2(4):304–310. doi: 10.1002/ana.410020408. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEWIS C. The quantitative histochemistry of the retina. J Biol Chem. 1956 Jun;220(2):879–892. [PubMed] [Google Scholar]
- Lahiri S. Blood oxygen affinity and alveolar ventilation in relation in body weight in mammals. Am J Physiol. 1975 Aug;229(2):529–536. doi: 10.1152/ajplegacy.1975.229.2.529. [DOI] [PubMed] [Google Scholar]
- Levick W. R. Another tungsten microelectrode. Med Biol Eng. 1972 Jul;10(4):510–515. doi: 10.1007/BF02474199. [DOI] [PubMed] [Google Scholar]
- Linsenmeier R. A., Hertz B. G. Eye movements in paralyzed cats induced by drugs and sympathetic stimulation. Vision Res. 1979;19(11):1249–1252. doi: 10.1016/0042-6989(79)90191-3. [DOI] [PubMed] [Google Scholar]
- Linsenmeier R. A., Jakiela H. G. Non-linear spatial summation in cat retinal ganglion cells at different background levels. Exp Brain Res. 1979 Jul 2;36(2):301–309. doi: 10.1007/BF00238913. [DOI] [PubMed] [Google Scholar]
- Massopust L. C., Jr, Wolin L. R., Barnes H. W. The effect of hypoxia on electrical activity of the visual system in cats. Jpn J Physiol. 1966 Aug 15;16(4):450–461. doi: 10.2170/jjphysiol.16.450. [DOI] [PubMed] [Google Scholar]
- NOELL W. K. Site of asphyxial block in mammalian retinae. J Appl Physiol. 1951 Feb;3(8):489–500. doi: 10.1152/jappl.1951.3.8.489. [DOI] [PubMed] [Google Scholar]
- REINERT H. URETHANE HYPERGLYCAEMIA AND HYPOTHALAMIC ACTIVATION. Nature. 1964 Nov 28;204:889–891. doi: 10.1038/204889a0. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Smith P. S. Slow dark discharge rhythms of cat retinal ganglion cells. J Neurophysiol. 1966 Sep;29(5):942–953. doi: 10.1152/jn.1966.29.5.942. [DOI] [PubMed] [Google Scholar]
- Speckmann E. J., Caspers H., Sokolov W. Aktivittsänderungen spinaler neurone während und nach einer Asphyxie. Pflugers Arch. 1970;319(2):122–138. doi: 10.1007/BF00592491. [DOI] [PubMed] [Google Scholar]
- Stone J., Fukuda Y. Properties of cat retinal ganglion cells: a comparison of W-cells with X- and Y-cells. J Neurophysiol. 1974 Jul;37(4):722–748. doi: 10.1152/jn.1974.37.4.722. [DOI] [PubMed] [Google Scholar]
- van den Bos G. C. L'électrorétinogramme du chat en cas d'hypoxie. J Physiol (Paris) 1968 May-Jun;60(3):199–216. [PubMed] [Google Scholar]


