Abstract
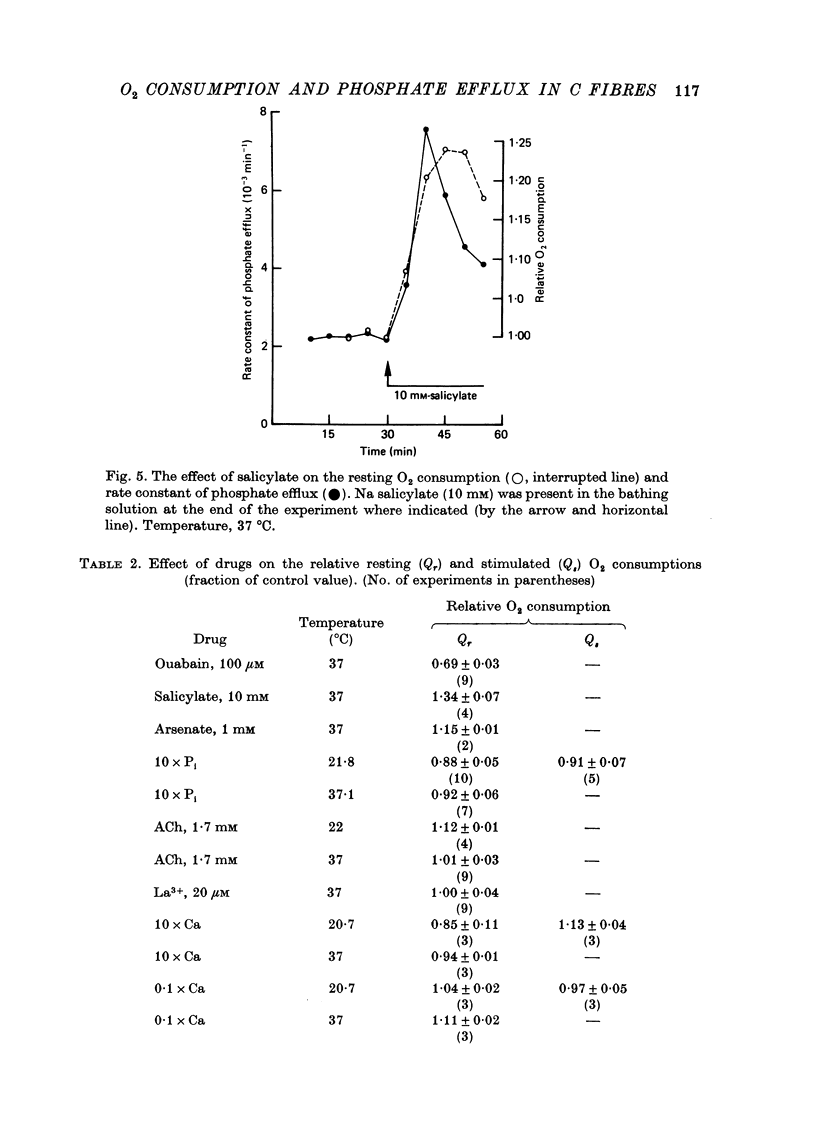
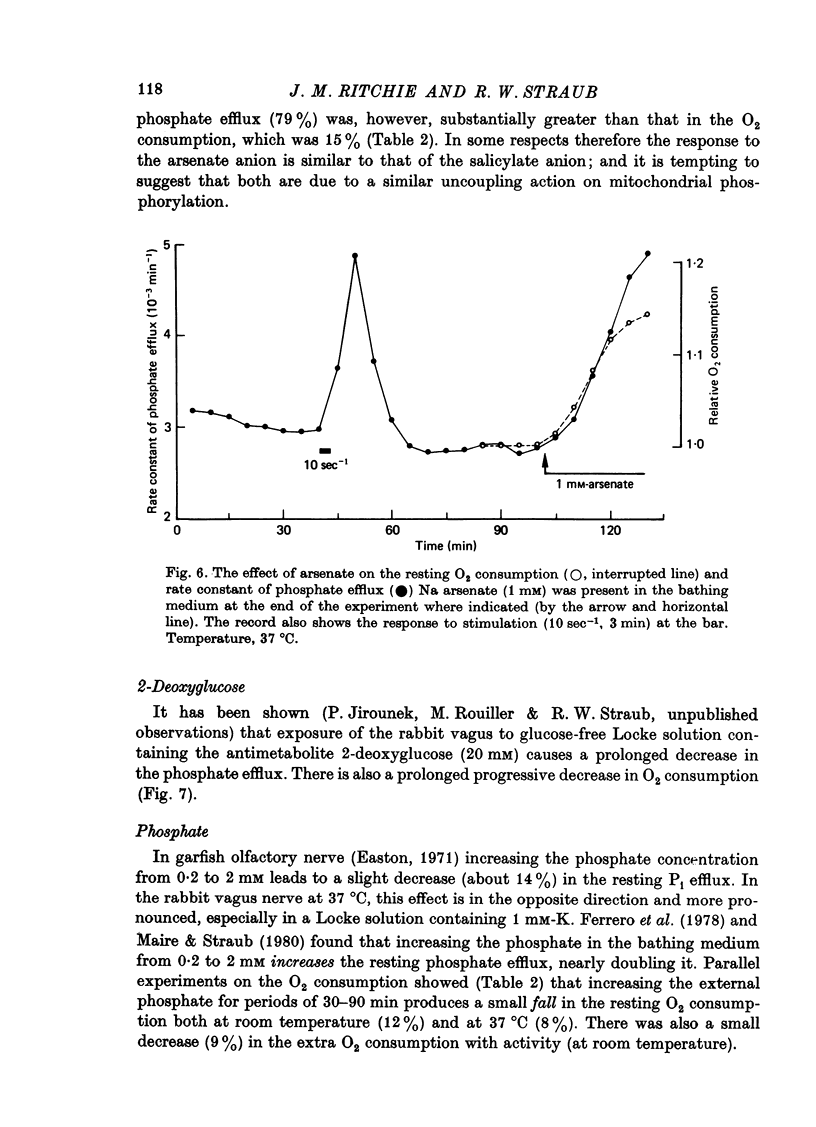
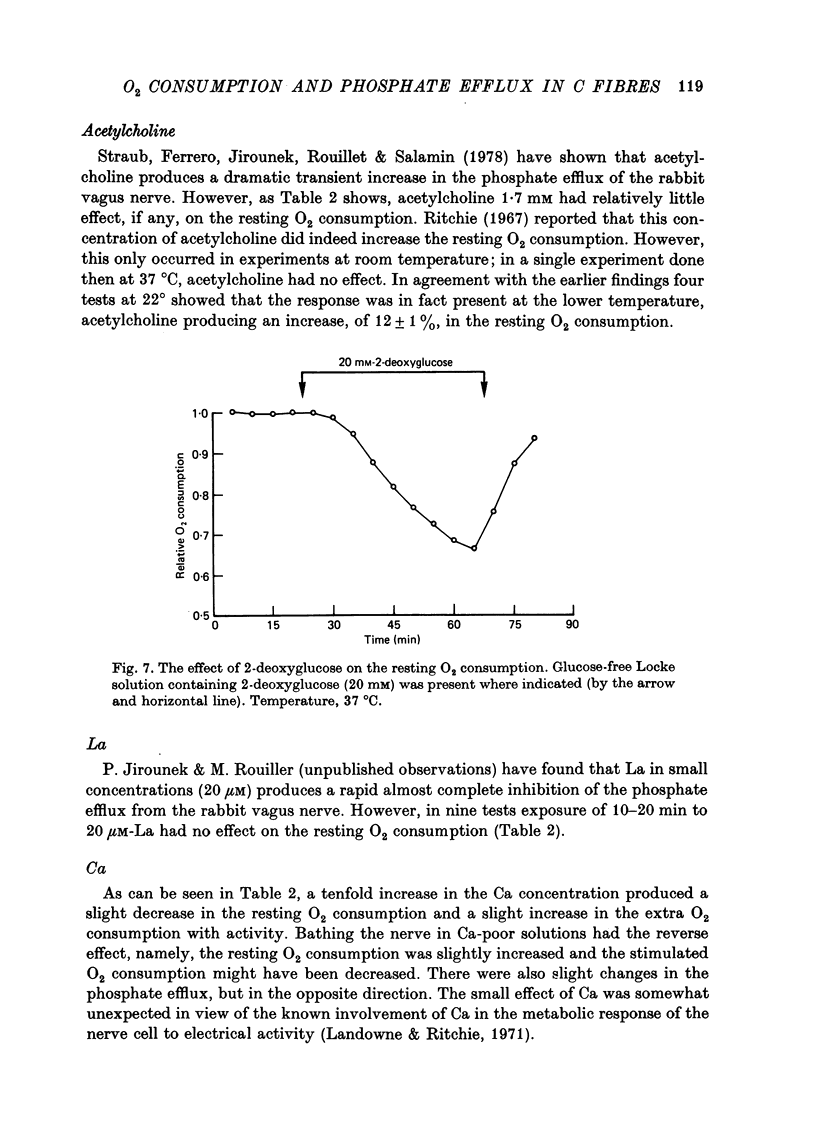
1. A comparison has been made between the efflux of labelled phosphate from the non-myelinated fibres of the desheathed rabbit vagus nerve at 37 degrees C and the corresponding O2 consumption at rest and during activity, and during a variety of experimental interventions. 2. The resting rate constant of phosphate efflux was 2.61 X 10(-3) min-1: electrical stimulation (10 sec-1, 3 min) produced an extra fractional loss of 6.75 X 10(-6) impulse-1. 3. The corresponding resting O2 consumption was 0.484 m-mole x kg-1 impulse-1. 4. Ouabain (100 microM) produced a sustained depression (of about 40%) of the resting O2 consumption, accompanied by a transient fall (of about 14%) in the rate constant of phosphate efflux. 5. Na salicylate (10 mM) or Na arsenate (1 mM) produced a much larger increase in phosphate efflux than in resting O2 consumption. 6. Changing the external phosphate concentration (between 0.02 and 2 mM), addition of acetylcholine (1.7 mM), and addition of lanthanum (20 microM)--all of which are known to affect markedly the phosphate efflux in rabbit non-myelinated fibres--had little or no effect on the resting O2 consumption or, where tested, on the extra O2 consumption with electrical stimulation. 7. Changing the external Ca concentration (between 0.09 and 9 mM) had only minor effects on the O2 consumption (resting and stimulated) and on the rate constant of resting phosphate efflux. 8. It is concluded that although changes in metabolism of the nerve produce changes in the phosphate efflux expected on the basis of the concomitant changes in the internal concentration of inorganic phosphate, the converse is not true; and increases and decreases in the rate constant of phosphate efflux do not necessarily signal the corresponding metabolic changes.
Full text
PDF

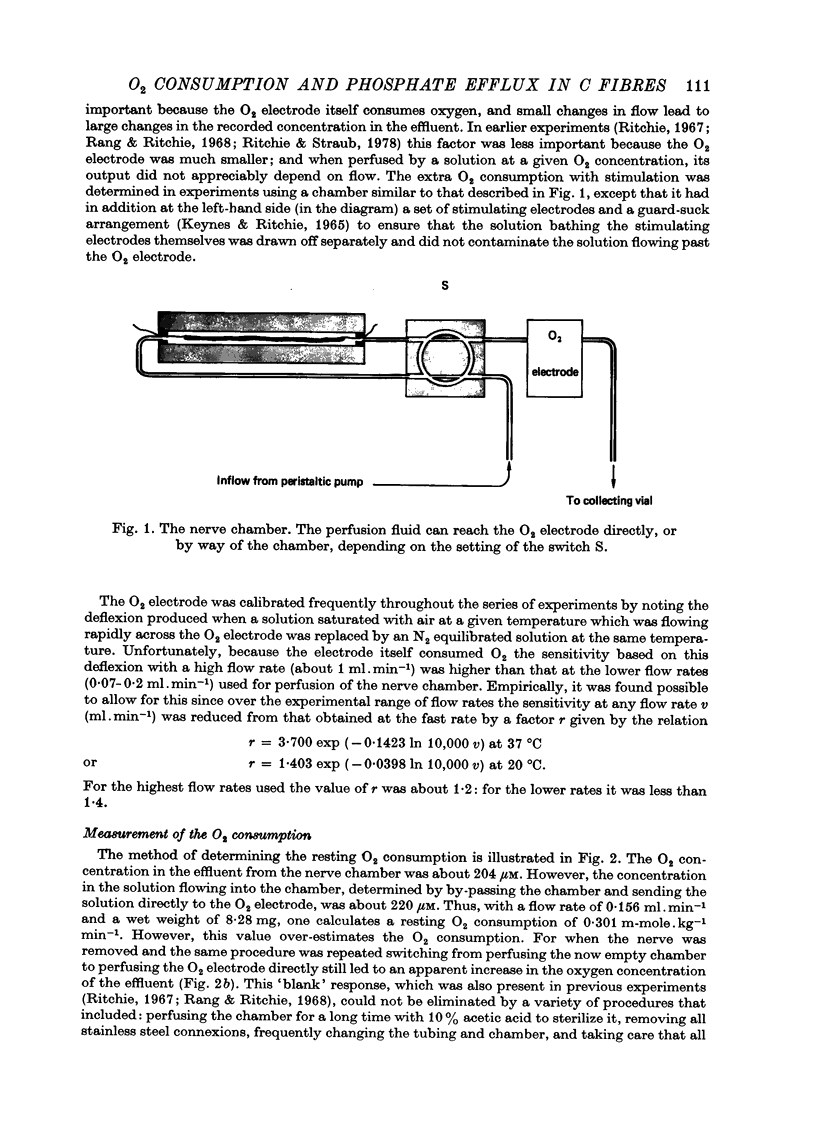
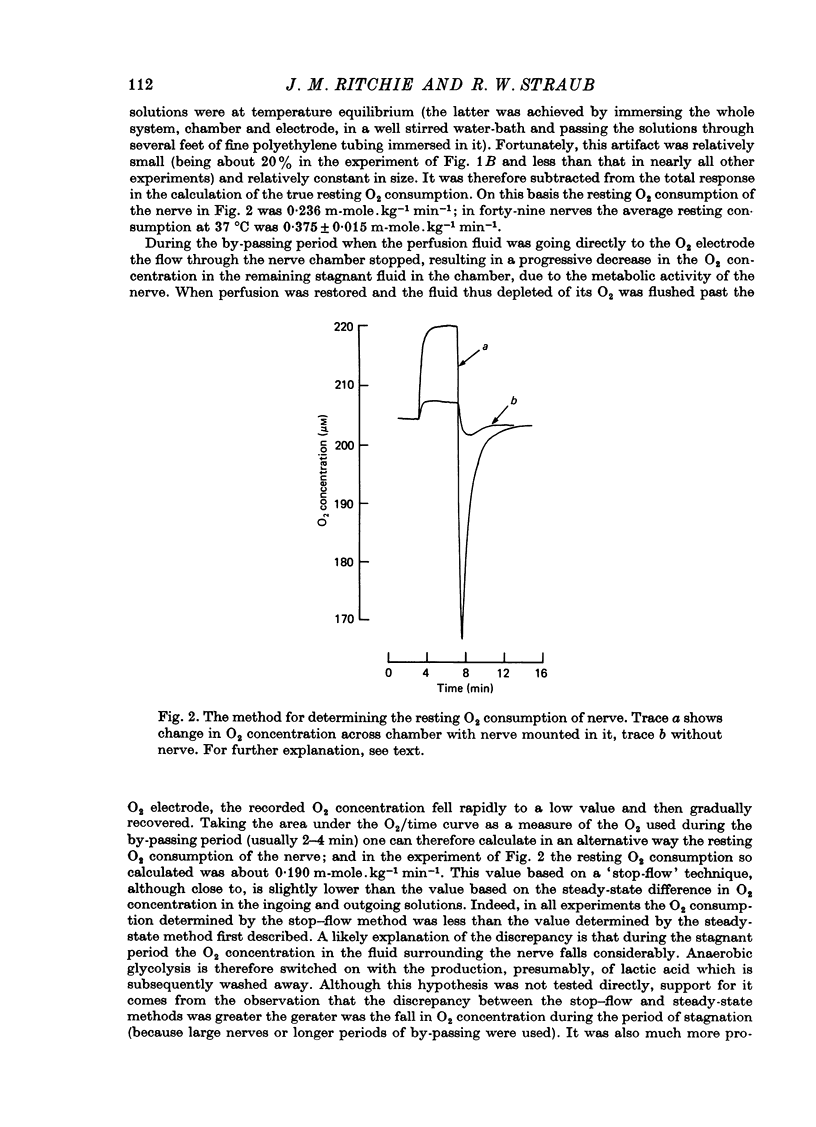

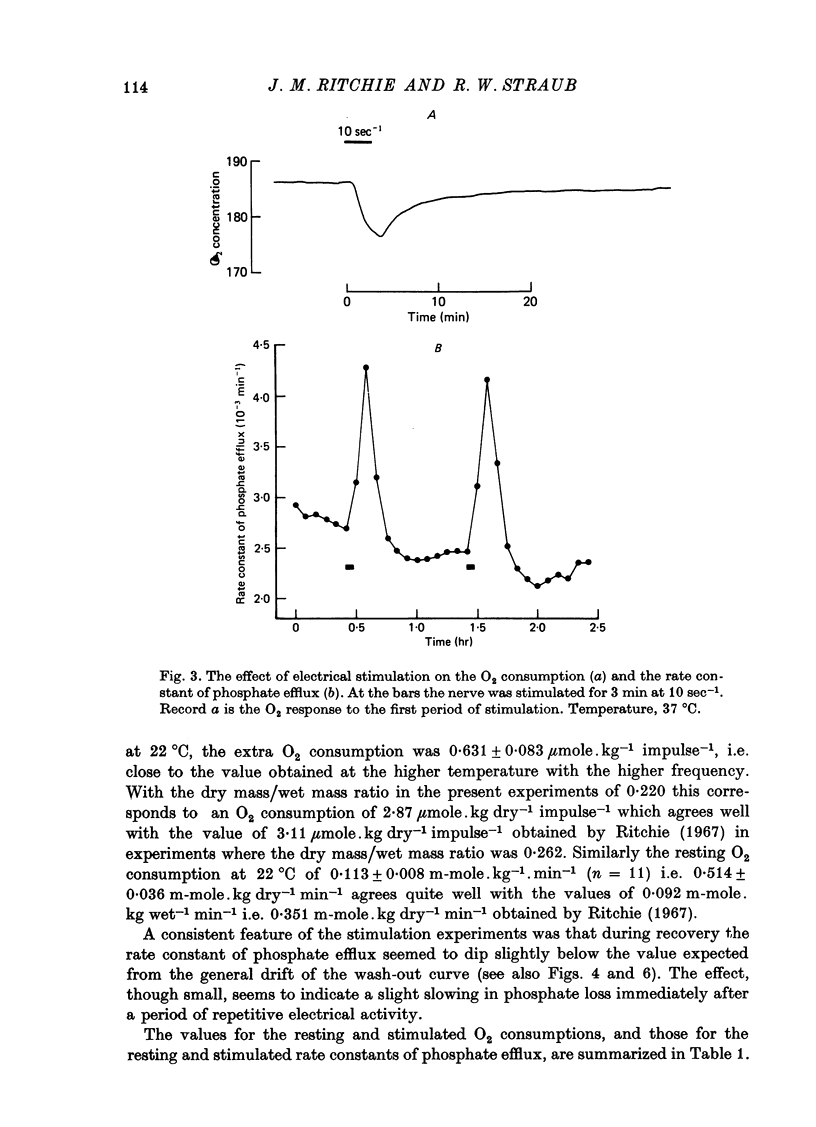

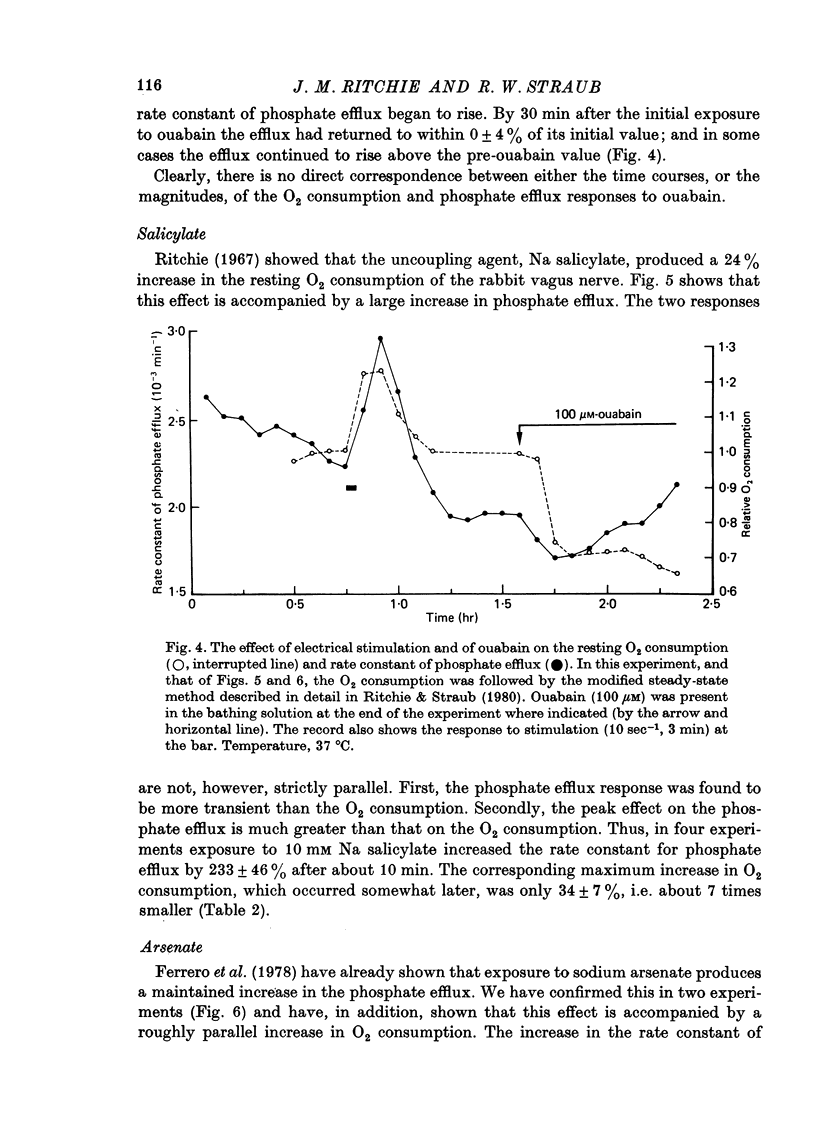


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F. Phosphorus metabolism of intact crab nerve and its relation to the active transport of ions. J Physiol. 1965 Sep;180(2):383–423. doi: 10.1113/jphysiol.1965.sp007709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M. J., Gadian D. G., Wilkie D. R. Contraction and recovery of living muscles studies by 31P nuclear magnetic resonance. J Physiol. 1977 Jun;267(3):703–735. doi: 10.1113/jphysiol.1977.sp011835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton D. M. Garfish olfactory nerve: easily accessible source of numerous long, homogeneous, nonmyelinated axons. Science. 1971 May 28;172(3986):952–955. doi: 10.1126/science.172.3986.952. [DOI] [PubMed] [Google Scholar]
- Ferrero J., Jirounek P., Rouiller M., Straub R. W. Efflux of inorganic phosphate from mammalian non-myelinated nerve fibres. J Physiol. 1978 Sep;282:507–519. doi: 10.1113/jphysiol.1978.sp012478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Ritchie J. M. The movements of labelled ions in mammalian non-myelinated nerve fibres. J Physiol. 1965 Jul;179(2):333–367. doi: 10.1113/jphysiol.1965.sp007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landowne D., Ritchie J. M. On the control of glycogenolysis in mammalian nervous tissue by calcium. J Physiol. 1971 Jan;212(2):503–517. doi: 10.1113/jphysiol.1971.sp009338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maire J. C., Straub R. W. Release of inorganic phosphate during activity in mammalian non-myelinated nerve fibres. J Physiol. 1980 Jul;304:135–143. doi: 10.1113/jphysiol.1980.sp013315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. The dependence on external cations of the oxygen consumption of mammalian non-myelinated fibres at rest and during activity. J Physiol. 1968 May;196(1):163–181. doi: 10.1113/jphysiol.1968.sp008501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie J. M., Straub R. W. Increase in efflux of inorganic phosphate during electrical activity in small non-myelinated nerve fibres. J Physiol. 1978 Jan;274:539–548. doi: 10.1113/jphysiol.1978.sp012165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie J. M., Straub R. W. Observations on the mechanism for the active extrusion of lithium in mammalian non-myelinated nerve fibres. J Physiol. 1980 Jul;304:123–134. doi: 10.1113/jphysiol.1980.sp013314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie J. M., Straub R. W. Phosphate efflux and oxygen consumption in small non-myelinated nerve fibres at rest and during activity. J Physiol. 1979 Feb;287:315–327. doi: 10.1113/jphysiol.1979.sp012661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie J. M. The oxygen consumption of mammalian non-myelinated nerve fibres at rest and during activity. J Physiol. 1967 Feb;188(3):309–329. doi: 10.1113/jphysiol.1967.sp008141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi J. P., Garrahan P. J., Rega A. F. Reversal of the calcium pump in human red cells. J Membr Biol. 1978 Dec 8;44(1):37–46. doi: 10.1007/BF01940572. [DOI] [PubMed] [Google Scholar]



