Abstract
1. The efflux of labelled phosphate was measured in desheathed rabbit vagus nerve at rest and during activity.
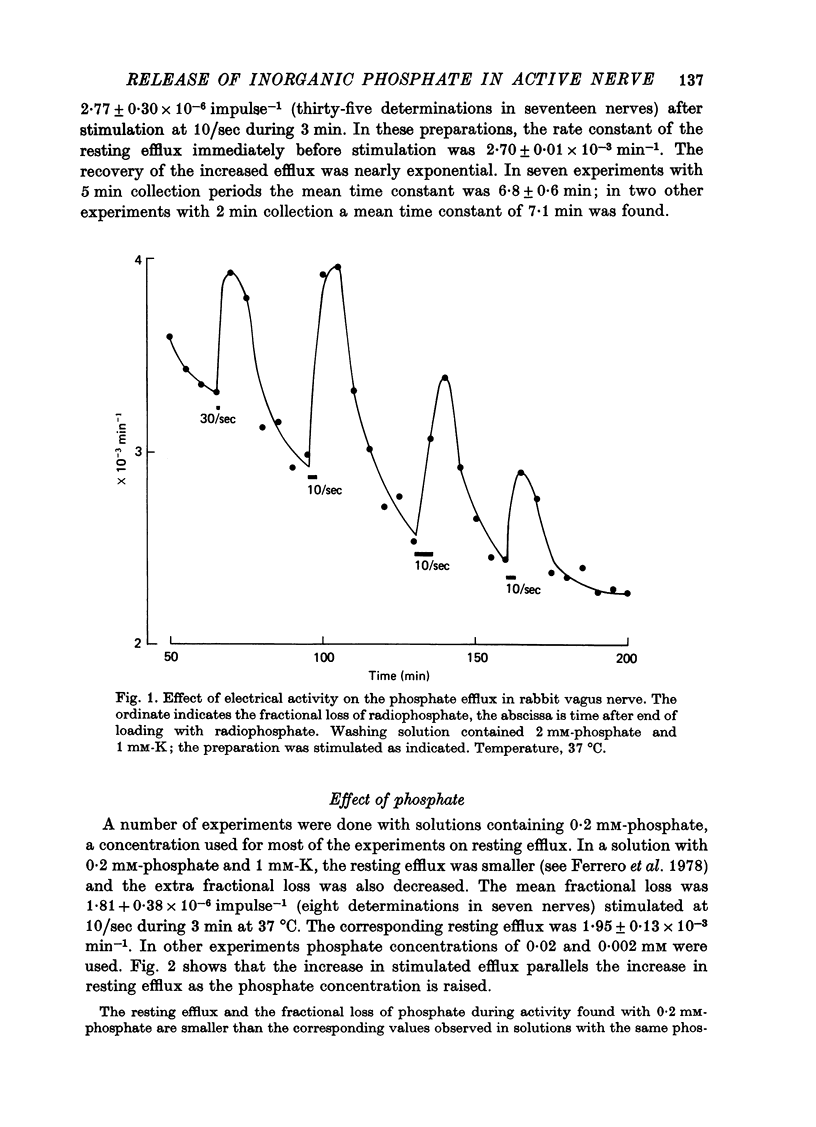
2. In solutions with 2 mM-phosphate and 1 mM-K the rate constant of the resting efflux was 2·7 × 10-3 min-1; stimulation caused an extra fractional loss of 2·8 × 10-6 impulse-1.
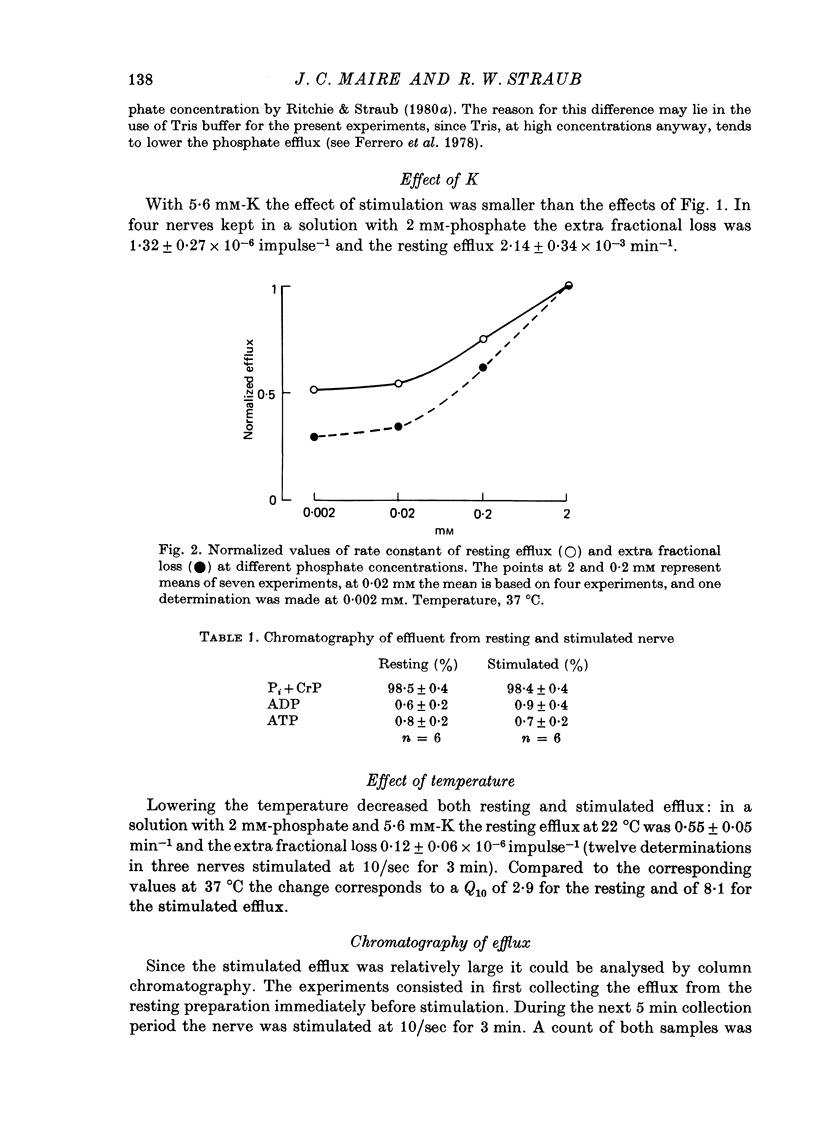
3. Lowering the phosphate concentration decreased the resting and the stimulated efflux; with 0·2 mM-phosphate the corresponding values were 1·9 × 10-3 min-1 and 1·8 × 10-6 impulse-1, respectively.
4. Increasing the K to 5·6 mM decreased both resting and stimulated efflux.
5. Lowering the temperature decreased the resting efflux with a Q10 of 2·9 and the stimulated efflux with a Q10 of 8·1.
6. Chromatography of the effluent showed that at rest and during activity at least 96% of the radiophosphate was in the orthophosphate fraction.
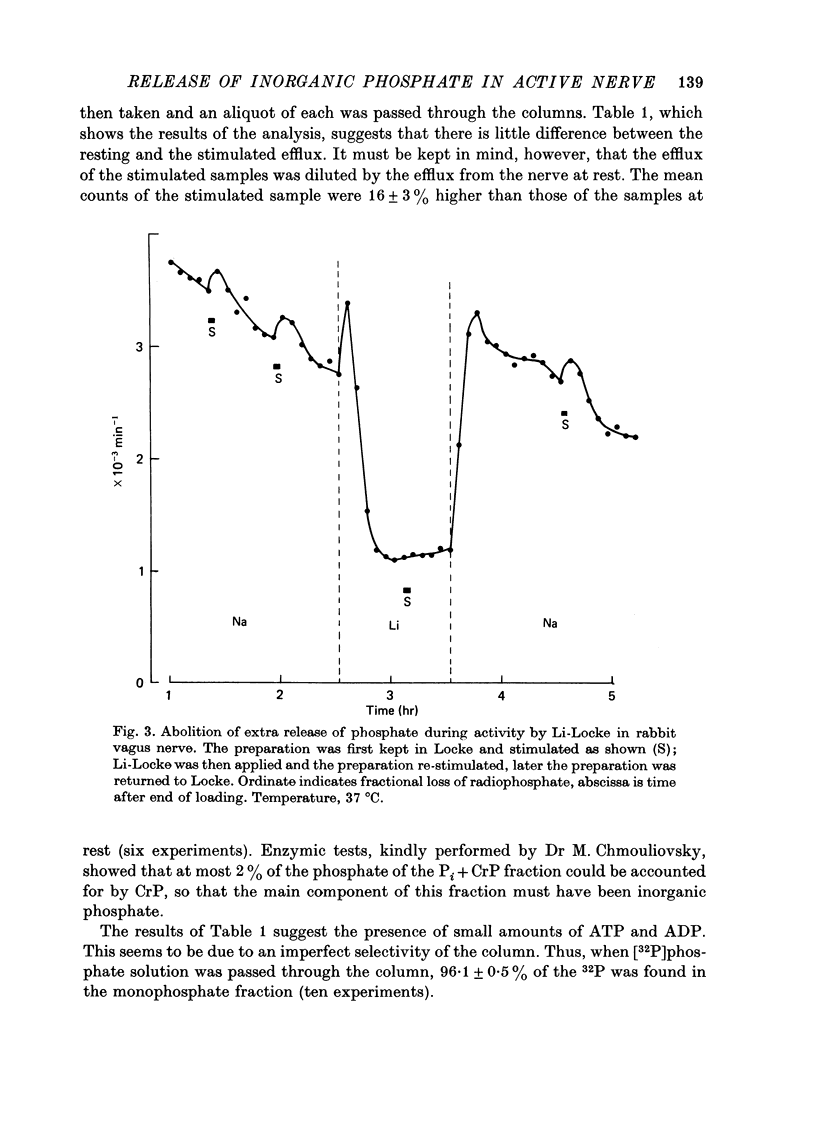
7. Replacing the Na of the solution by Li lowered the rate constant of the resting efflux to 0·8 × 10-3 min-1 and abolished the extra release during activity, without reduction of the action potential.
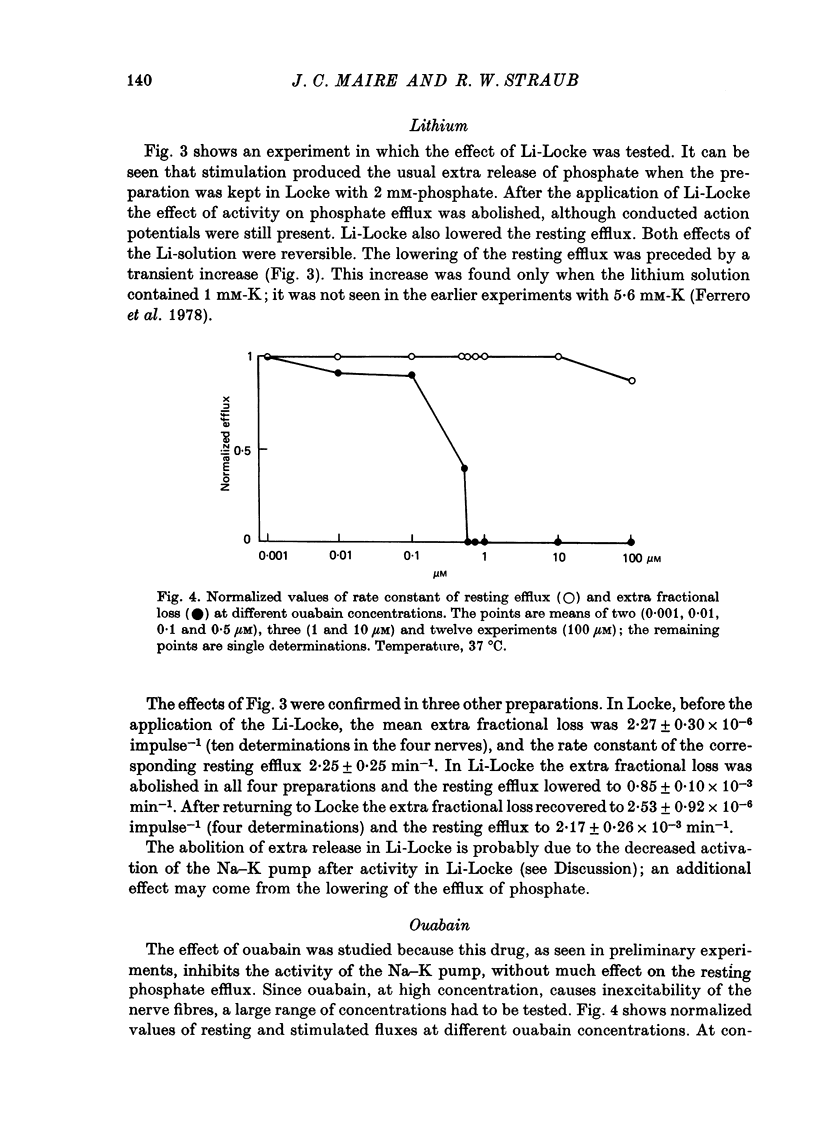
8. The presence of ouabain did not affect the resting efflux, except at 100 μM, when a transient reduction was found. The extra fractional loss was not affected with 0·001 μM; with 0·01-0·5 μM, it was reduced without much change in the action potential, and abolished at higher concentrations.
9. The results agree with the hypothesis that the extra release results from an increase in internal inorganic phosphate caused by increased break-down of ATP during recovery.
10. Comparison with the O2 consumption shows that about 1% of the inorganic phosphate liberated at the inside of the axons escapes to the outside.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anner B., Ferrero J., Jirounek P., Straub R. W. Uptake of orthophosphate by rabbit vagus nerve fibres. J Physiol. 1975 Jun;247(3):759–771. doi: 10.1113/jphysiol.1975.sp010956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmouliovsky M., Schorderet M., Straub R. W. Effect of electrical activity on the concentration of phosphorylated metabolites and inorganic phosphate in mammalian non-myelinated nerve fibres. J Physiol. 1969 Jun;202(2):90P–92P. [PubMed] [Google Scholar]
- Ferrero J., Jirounek P., Rouiller M., Straub R. W. Efflux of inorganic phosphate from mammalian non-myelinated nerve fibres. J Physiol. 1978 Sep;282:507–519. doi: 10.1113/jphysiol.1978.sp012478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth J. V., Keynes R. D., Ritchie J. M. The origin of the initial heat associated with a single impulse in mammalian non-myelinated nerve fibres. J Physiol. 1968 Feb;194(3):745–793. doi: 10.1113/jphysiol.1968.sp008434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RITCHIE J. M., STRAUB R. W. The hyperpolarization which follows activity in mammalian non-medullated fibres. J Physiol. 1957 Apr 3;136(1):80–97. doi: 10.1113/jphysiol.1957.sp005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. The dependence on external cations of the oxygen consumption of mammalian non-myelinated fibres at rest and during activity. J Physiol. 1968 May;196(1):163–181. doi: 10.1113/jphysiol.1968.sp008501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie J. M., Straub R. W. Increase in efflux of inorganic phosphate during electrical activity in small non-myelinated nerve fibres. J Physiol. 1978 Jan;274:539–548. doi: 10.1113/jphysiol.1978.sp012165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie J. M., Straub R. W. Observations on the mechanism for the active extrusion of lithium in mammalian non-myelinated nerve fibres. J Physiol. 1980 Jul;304:123–134. doi: 10.1113/jphysiol.1980.sp013314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie J. M., Straub R. W. Oxygen consumption and phosphate efflux in mammalian non-myelinated nerve fibres. J Physiol. 1980 Jul;304:109–121. doi: 10.1113/jphysiol.1980.sp013313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie J. M., Straub R. W. Phosphate efflux and oxygen consumption in small non-myelinated nerve fibres at rest and during activity. J Physiol. 1979 Feb;287:315–327. doi: 10.1113/jphysiol.1979.sp012661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie J. M. The oxygen consumption of mammalian non-myelinated nerve fibres at rest and during activity. J Physiol. 1967 Feb;188(3):309–329. doi: 10.1113/jphysiol.1967.sp008141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M., Hubbard J. I. Thermal synthesis of amino acids from a simulated primitive atmosphere. Nature. 1973 Jun 15;243(5407):404–405. doi: 10.1038/243404a0. [DOI] [PubMed] [Google Scholar]
- Silinsky E. M. On the association between transmitter secretion and the release of adenine nucleotides from mammalian motor nerve terminals. J Physiol. 1975 May;247(1):145–162. doi: 10.1113/jphysiol.1975.sp010925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wespi H. H., Mevissen D., Straub R. W. The effect of ouabain and ouabagenin on active transport of sodium and potassium in vagus nerve fibres. Arch Int Pharmacodyn Ther. 1969 Oct;181(2):307–315. [PubMed] [Google Scholar]


