Abstract
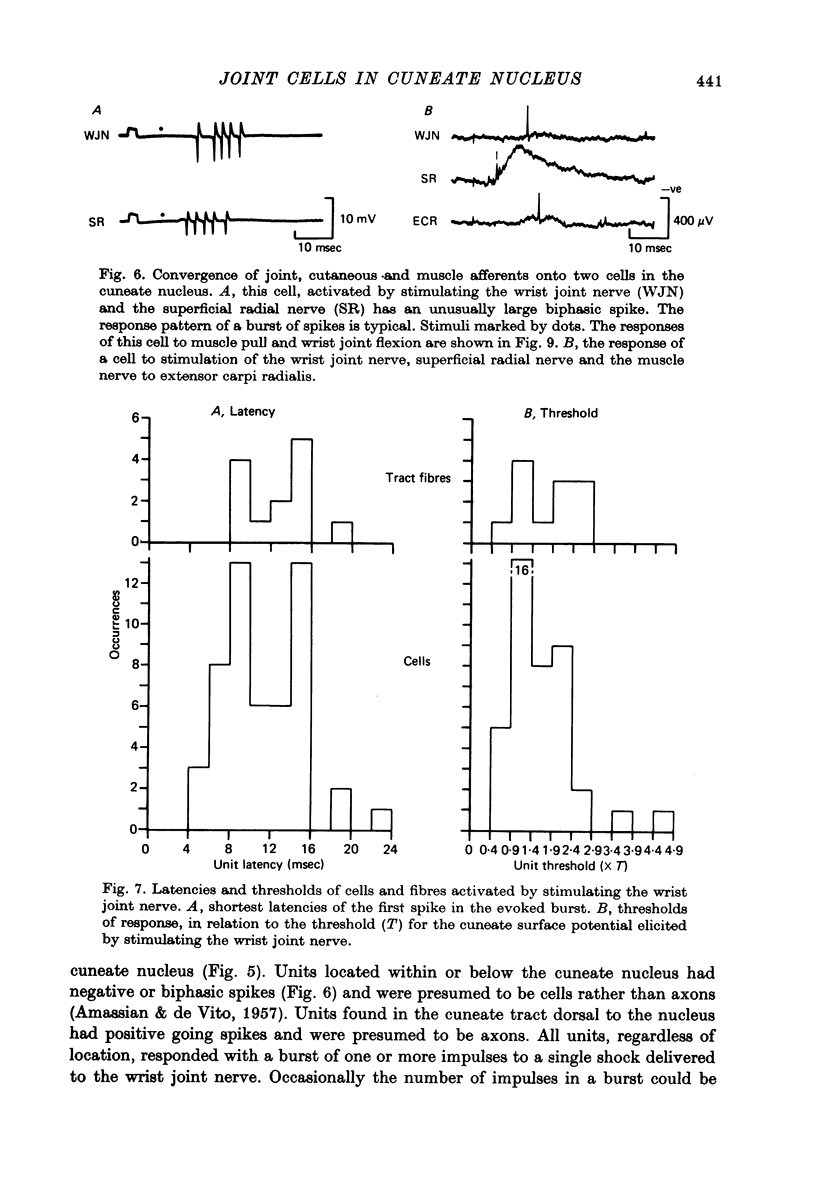
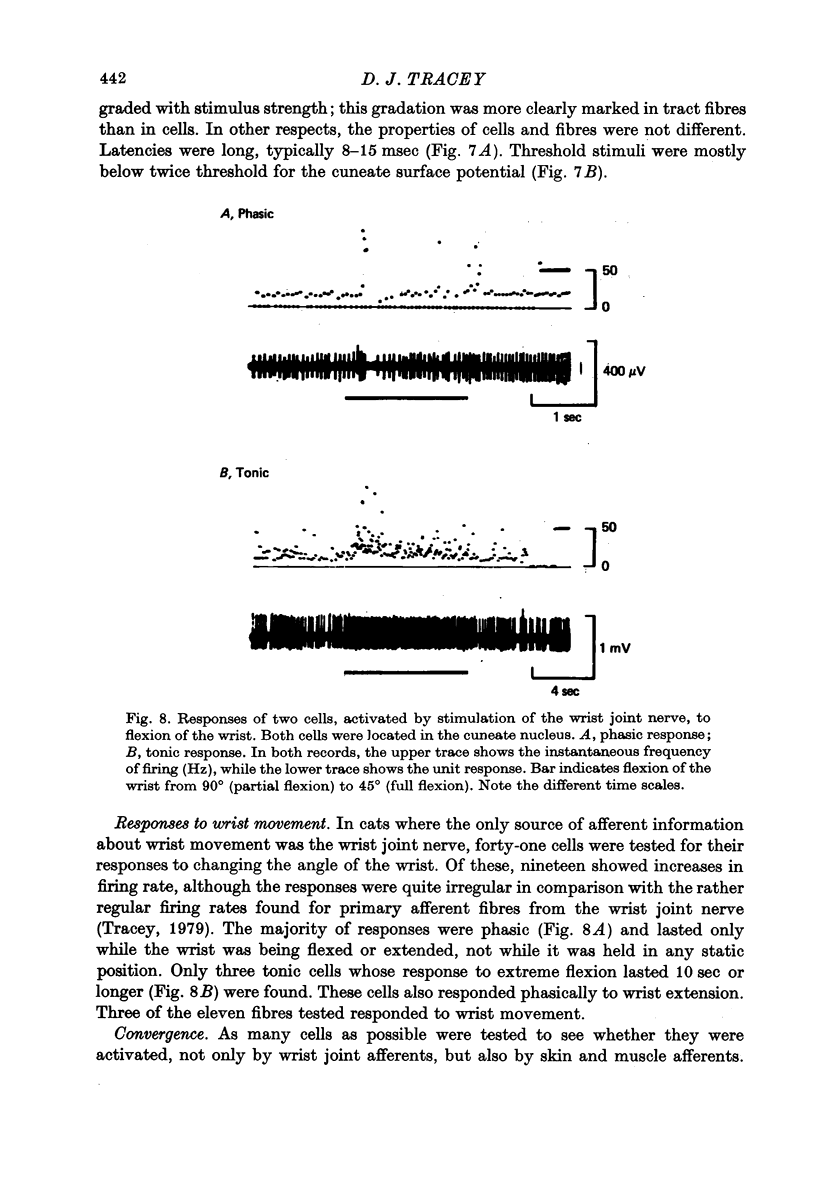
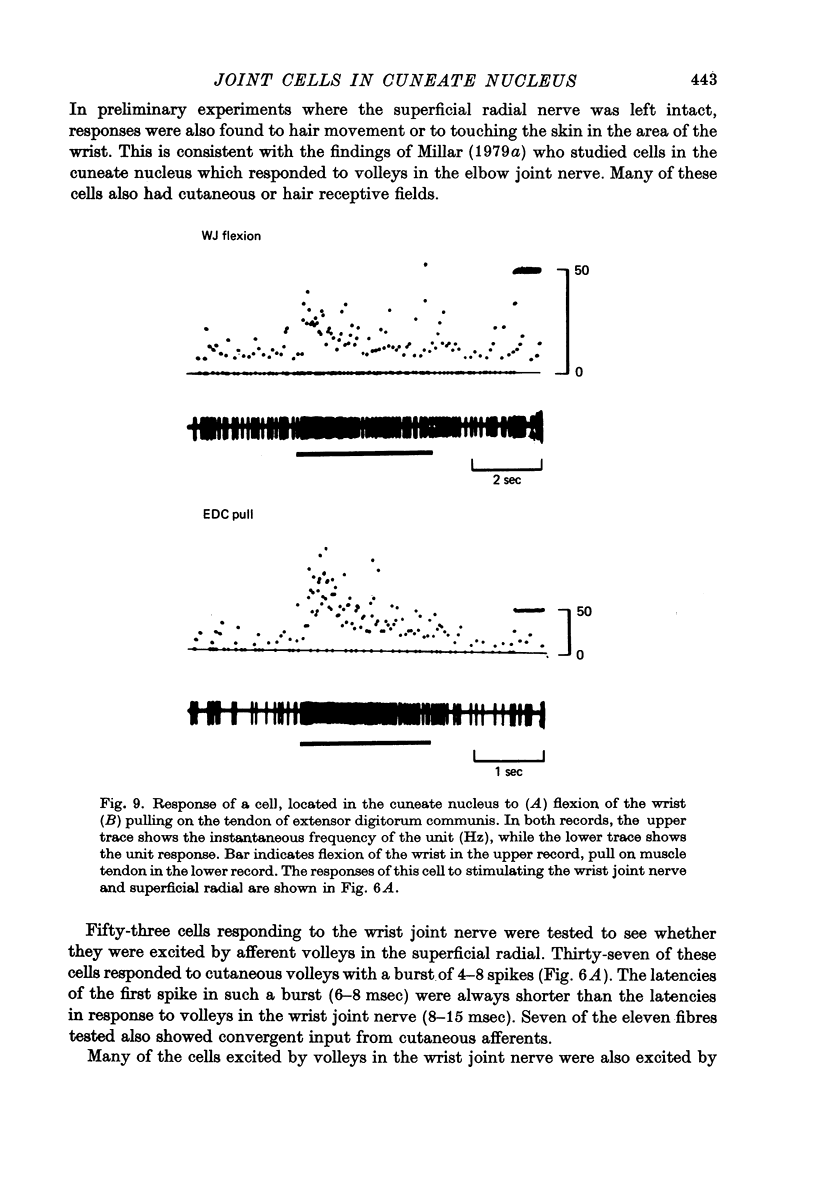
1. Records were made from axons in the dorsal columns and cells in the cuneate nucleus which responded to stimulation of the wrist joint nerve. 2. A sample of twenty-five axons activated by the wrist joint nerve was recorded in the dorsal columns at the level of the third cervical segment. All twenty-five were post-synaptic fibres as judged by response latency, burst length, and maximum frequency of following. Nineteen of the twenty-five had convergent inputs from the wrist joint nerve and the cutaneous superficial radial nerve. 3. While no primary wrist joint afferent fibres were recorded in the dorsal columns, their presence was demonstrated by recording single units in the wrist joint nerve which were antidromically activated by microstimulation in the cuneate fasciculus. 4. The majority of cells recorded in the cuneate nucleus were activated not only by stimulation of joint afferents, but also by skin and muscle afferent fibres. 5. About half of the cells in the cuneate nucleus responded to wrist movement in animals with partially denervated forelimbs, where the intact wrist joint nerve was the only afferent channel providing information about natural, imposed wrist movements. The majority of the cells had phasic responses, which were weak and irregular in comparison with the responses of primary wrist joint afferents to the same movements. 6. Only two of thirty-four cells tested could be shown to project directly to the ventrobasal thalamus, using collision of antidromic and peripherally activated impulses as the criterion.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., ECCLES J. C., SCHMIDT R. F., YOKOTA T. IDENTIFICATION OF RELAY CELLS AND INTERNEURONS IN THE CUNEATE NUCLEUS. J Neurophysiol. 1964 Nov;27:1080–1095. doi: 10.1152/jn.1964.27.6.1080. [DOI] [PubMed] [Google Scholar]
- Angaut-Petit D. The dorsal column system: I. Existence of long ascending postsynaptic fibres in the cat's fasciculus gracilis. Exp Brain Res. 1975 May 22;22(5):457–470. doi: 10.1007/BF00237348. [DOI] [PubMed] [Google Scholar]
- Angaut-Petit D. The dorsal column system: II. Functional properties and bulbar relay of the postsynaptic fibres of the cat's fasciculus gracilis. Exp Brain Res. 1975 May 22;22(5):471–493. doi: 10.1007/BF00237349. [DOI] [PubMed] [Google Scholar]
- Burgess P. R., Clark F. J. Dorsal column projection of fibres from the cat knee joint. J Physiol. 1969 Aug;203(2):301–315. doi: 10.1113/jphysiol.1969.sp008865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark F. J., Landgren S., Silfvenius H. Projections to the cat's cerebral cortex from low threshold joint afferents. Acta Physiol Scand. 1973 Dec;89(4):504–521. doi: 10.1111/j.1748-1716.1973.tb05544.x. [DOI] [PubMed] [Google Scholar]
- DARIAN-SMITH I., PHILLIPS G., RYAN R. D. FUNCTIONAL ORGANIZATION IN THE TRIGEMINAL MAIN SENSORY AND ROSTRAL SPINAL NUCLEI OF THE CAT. J Physiol. 1963 Aug;168:129–146. doi: 10.1113/jphysiol.1963.sp007182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDNER E., NOER R. Projection of afferent fibers from muscles and joints to the cerebral cortex of the cat. Am J Physiol. 1952 Feb;168(2):437–441. doi: 10.1152/ajplegacy.1952.168.2.437. [DOI] [PubMed] [Google Scholar]
- GORDON G., LANDGREN S., SEED W. A. The functional characteristics of single cells in the caudal part of the spinal nucleus of the trigeminal nerve of the cat. J Physiol. 1961 Oct;158:544–559. doi: 10.1113/jphysiol.1961.sp006784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON G., SEED W. A. An investigation of nucleus gracilis of the cat by antidromic stimulation. J Physiol. 1961 Mar;155:589–601. doi: 10.1113/jphysiol.1961.sp006649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand P. J., Van Winkle T. The efferent connections of the feline nucleus cuneatus. J Comp Neurol. 1977 Jan 1;171(1):83–109. doi: 10.1002/cne.901710107. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Rastad J., Zarzecki P. Segmental and supraspinal input to cells of origin of non-primary fibres in the feline dorsal columns. J Physiol. 1979 May;290(2):185–200. doi: 10.1113/jphysiol.1979.sp012767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRUGER L., SIMINOFF R., WITKOVSKY P. Single neuron analysis of dorsal column nuclei and spinal nucleus of trigeminal in cat. J Neurophysiol. 1961 Jul;24:333–349. doi: 10.1152/jn.1961.24.4.333. [DOI] [PubMed] [Google Scholar]
- Lindström S., Takata M. Monosynaptic excitation of dorsal spinocerebellar tract neurones from low threshold joint afferents. Acta Physiol Scand. 1972 Mar;84(3):430–432. [PubMed] [Google Scholar]
- Matsumoto A., Mori S. Number and diameter distribution of myelinated afferent fibers innervating the paws of the cat and monkey. Exp Neurol. 1975 Aug;48(2):261–274. doi: 10.1016/0014-4886(75)90156-9. [DOI] [PubMed] [Google Scholar]
- Millar J. Loci of joint cells in the cuneate and external cuneate nuclei of the cat. Brain Res. 1979 May 11;167(2):385–390. doi: 10.1016/0006-8993(79)90832-1. [DOI] [PubMed] [Google Scholar]
- Roberts W. J., Smith D. O. Analysis of threshold currents during microstimulation of fibres in the spinal cord. Acta Physiol Scand. 1973 Nov;89(3):384–394. doi: 10.1111/j.1748-1716.1973.tb05533.x. [DOI] [PubMed] [Google Scholar]
- Towe A. L., Harding G. W. Extracellular microelectrode sampling bias. Exp Neurol. 1970 Nov;29(2):366–381. doi: 10.1016/0014-4886(70)90065-8. [DOI] [PubMed] [Google Scholar]
- Tracey D. J. Characteristics of wrist joint receptors in the cat. Exp Brain Res. 1979 Jan 2;34(1):165–176. doi: 10.1007/BF00238349. [DOI] [PubMed] [Google Scholar]
- Uddenberg N. Differential localization in dorsal funiculus of fibres originating from different receptors. Exp Brain Res. 1968;4(4):367–376. doi: 10.1007/BF00235701. [DOI] [PubMed] [Google Scholar]
- Uddenberg N. Functional organization of long, second-order afferents in the dorsal funiculus. Exp Brain Res. 1968;4(4):377–382. doi: 10.1007/BF00235702. [DOI] [PubMed] [Google Scholar]
- WINTER D. L. N. GRACILIS OF CAT. FUNCTIONAL ORGANIZATION AND CORTICOFUGAL EFFECTS. J Neurophysiol. 1965 Jan;28:48–70. doi: 10.1152/jn.1965.28.1.48. [DOI] [PubMed] [Google Scholar]
- Whitehorn D., Howe J. F., Lessler M. J., Burgess P. R. Cutaneous receptors supplied by myelinated fibers in the cat. I. Number of receptors innervated by a single nerve. J Neurophysiol. 1974 Nov;37(6):1361–1372. doi: 10.1152/jn.1974.37.6.1361. [DOI] [PubMed] [Google Scholar]
- Whitsel B. L., Petrucelli L. M., Sapiro G. Modality representation in the lumbar and cervical fasciculus gracilis of squirrel monkeys. Brain Res. 1969 Sep;15(1):67–78. doi: 10.1016/0006-8993(69)90310-2. [DOI] [PubMed] [Google Scholar]
- Williams W. J., BeMent S. L., Yin T. C., McCall W. D., Jr Nucleus gracilis responses to knee joint motion: a frequency response study. Brain Res. 1973 Dec 21;64:123–140. doi: 10.1016/0006-8993(73)90174-1. [DOI] [PubMed] [Google Scholar]
- Yin T. C., Williams W. J. Dynamic response and transfer characteristics of joint neurons in somatosensory thalamus of the cat. J Neurophysiol. 1976 May;39(3):582–600. doi: 10.1152/jn.1976.39.3.582. [DOI] [PubMed] [Google Scholar]


