Abstract
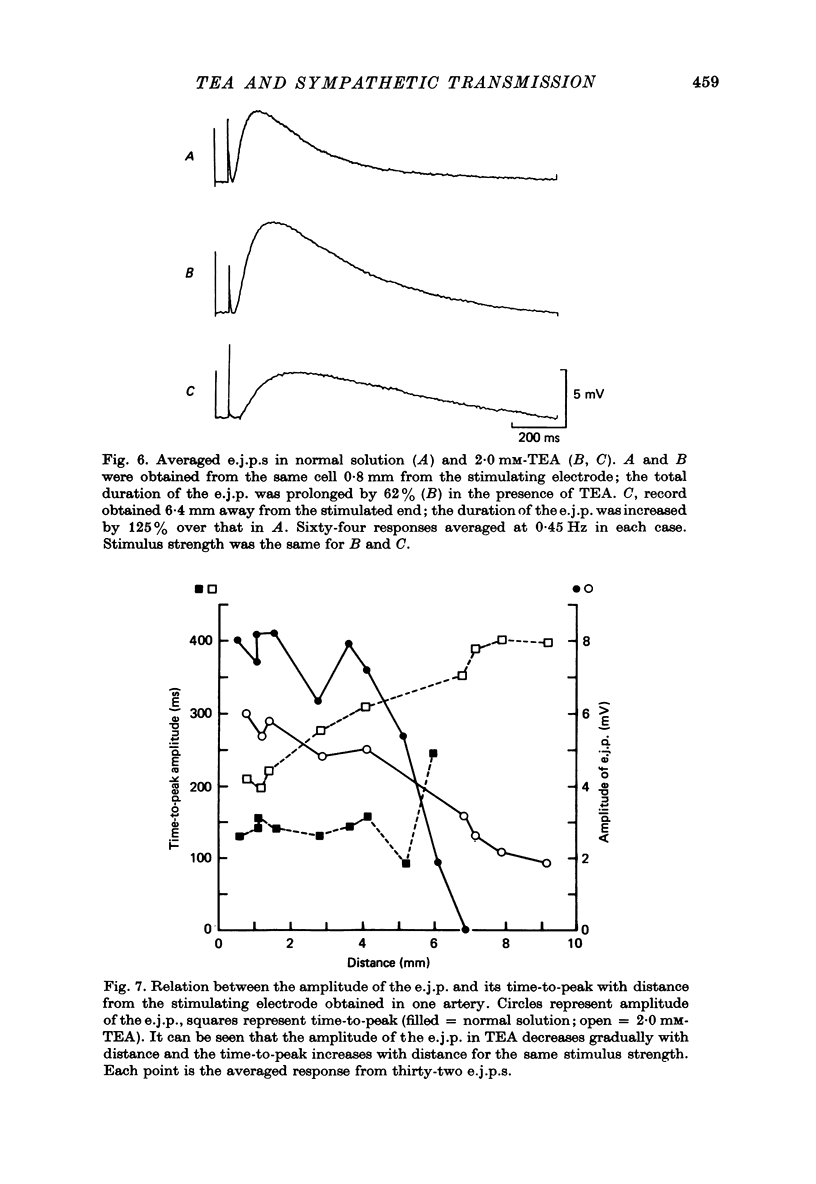
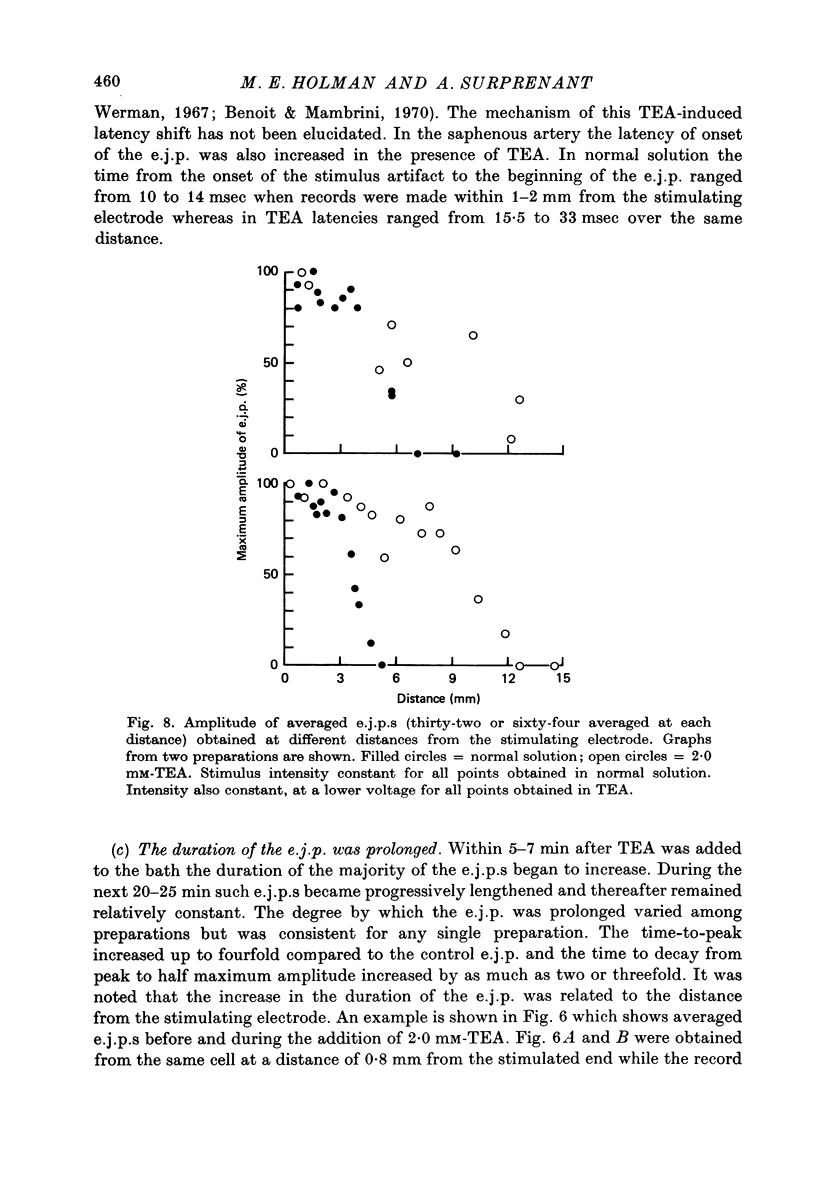
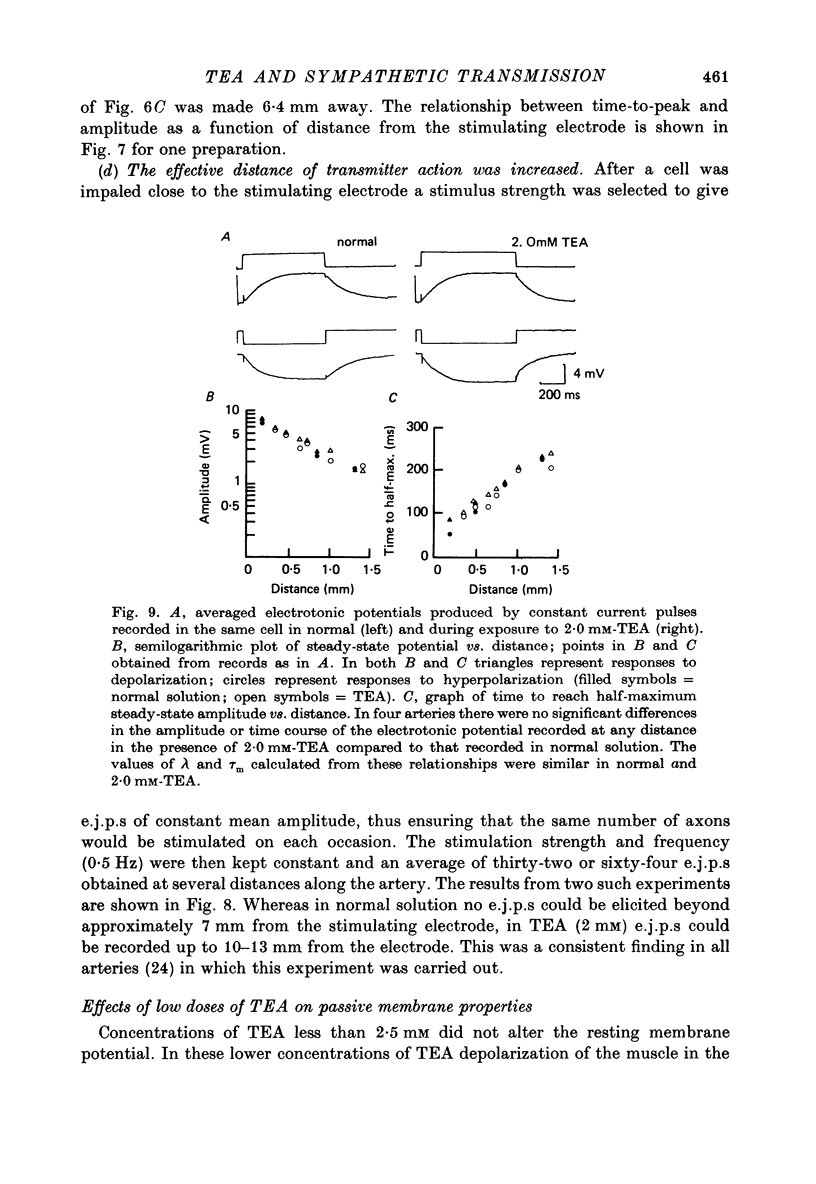
1. Excitatory junction potentials and electrotonic potentials were recorded from the smooth muscle of the rabbit saphenous artery using intracellular electrodes. 2. Tetraethylammonium chloride (TEA) in concentrations greater than 3.5 mM caused depolarization. Concentrations greater than 5 mM caused spontaneous electrical activity in the form of excitatory junction potentials (e.j.p.s) and all-or-nothing action potentials which were associated with spontaneous mechanical activity. 3. Concentrations of TEA less than 2.5 mM did not alter the resting potential nor the passive membrane properties of the smooth muscle over a range of +/- 15 mV. 4. The following effects were observed in 2.0 mM-TEA. (a) The minimum stimulus strength required for the initiation of an e.j.p. fell by three to fivefold. (b) Single stimuli that elicited only a small e.j.p. in normal solution evoked an all-or-nothing action potential of up to 70 mV amplitude. (c) Whereas in normal solution e.j.p.s could only be recorded up to 7 mm away from the perivascular stimulating electrode e.j.p.s could be recorded at distances of up to 13 mm. (d) The duration of the e.j.p. was prolonged. 5. Based on these results and the effects of TEA reported for other synapses it is proposed that TEA may act to increase the amount of transmitter released per axon, to increase the duration of release and to cause an increased invasion throughout the autonomic ground plexus by nerve impulses. This would imply that in normal solution, in vitro, the action potential may not propagate throughout the whole length of the terminal axon and its many branches due to failure of conduction at one or more points along the terminal portion of the axon.
Full text
PDF


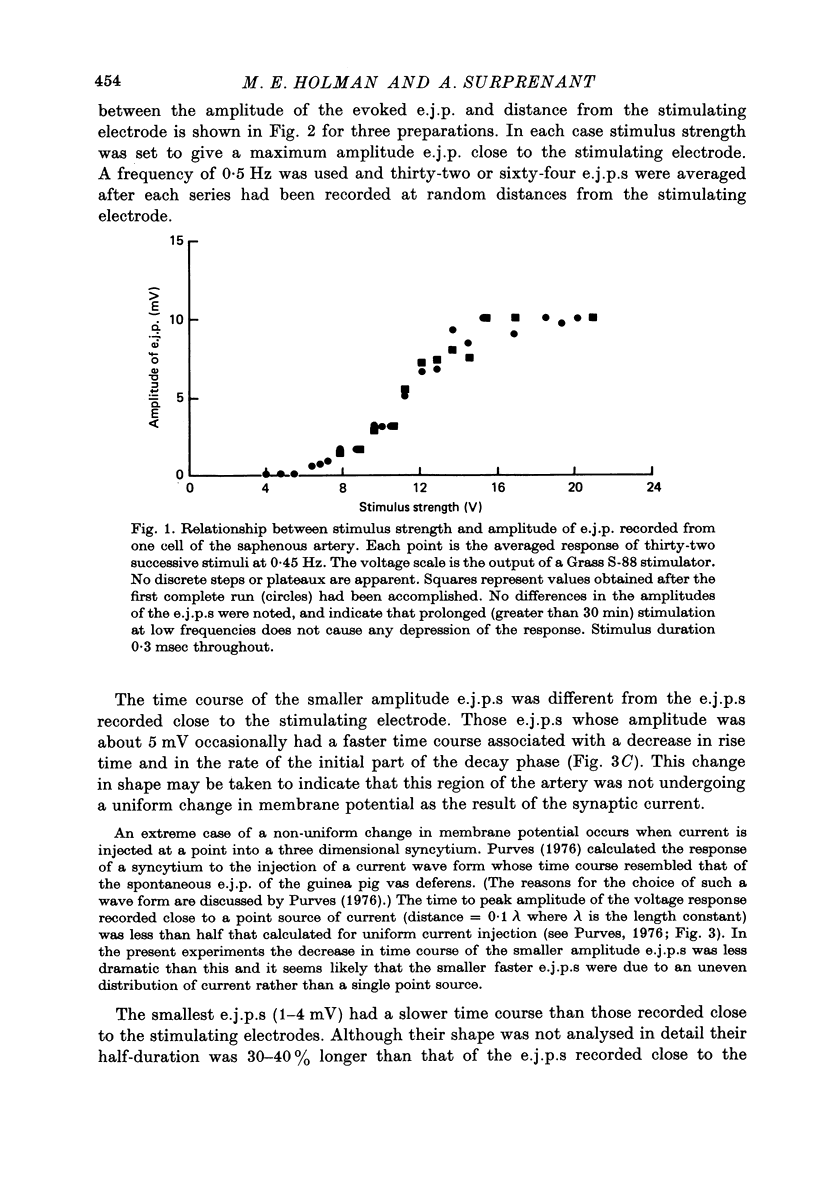
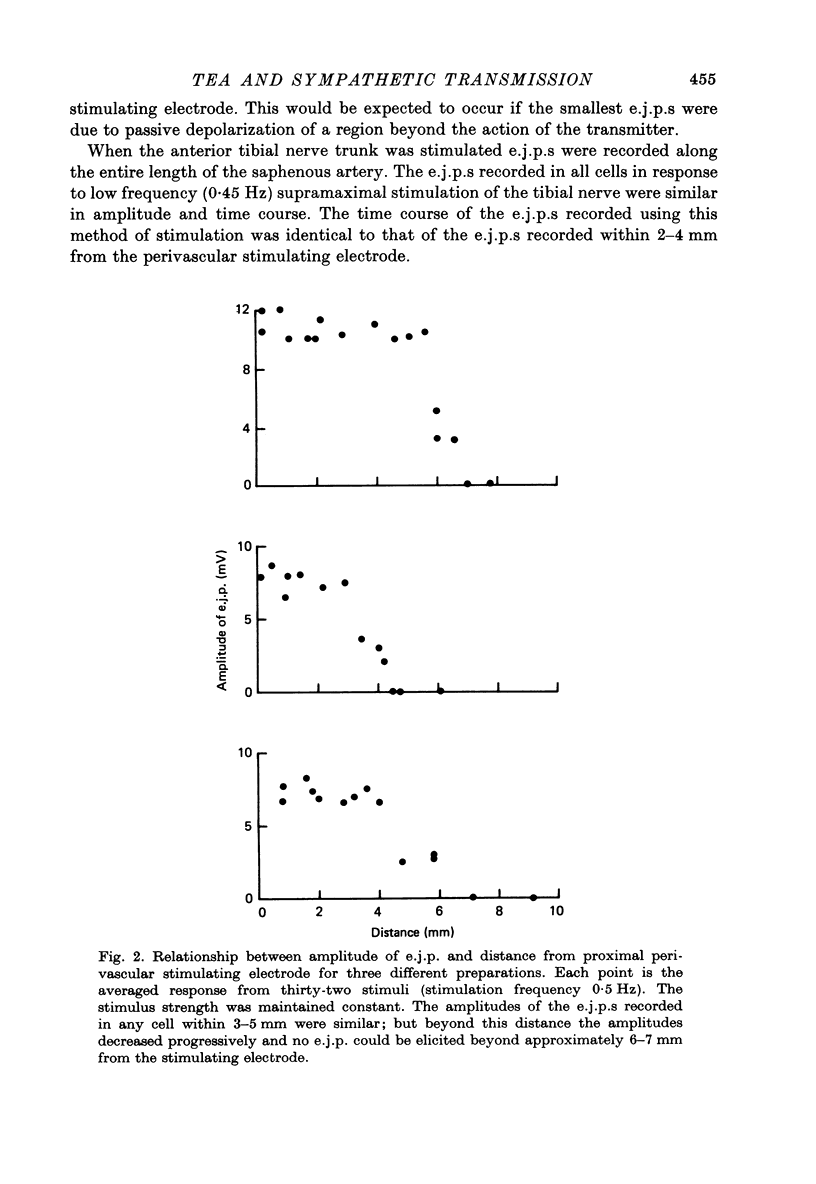
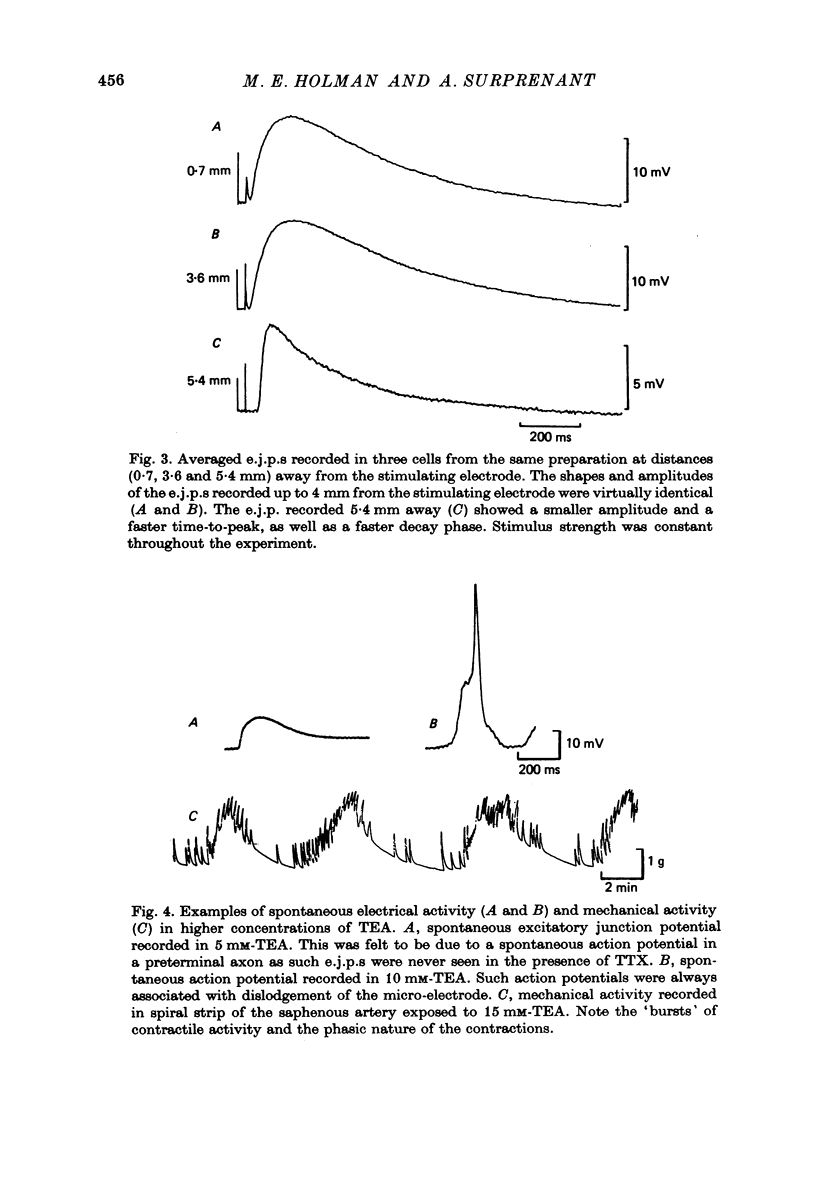

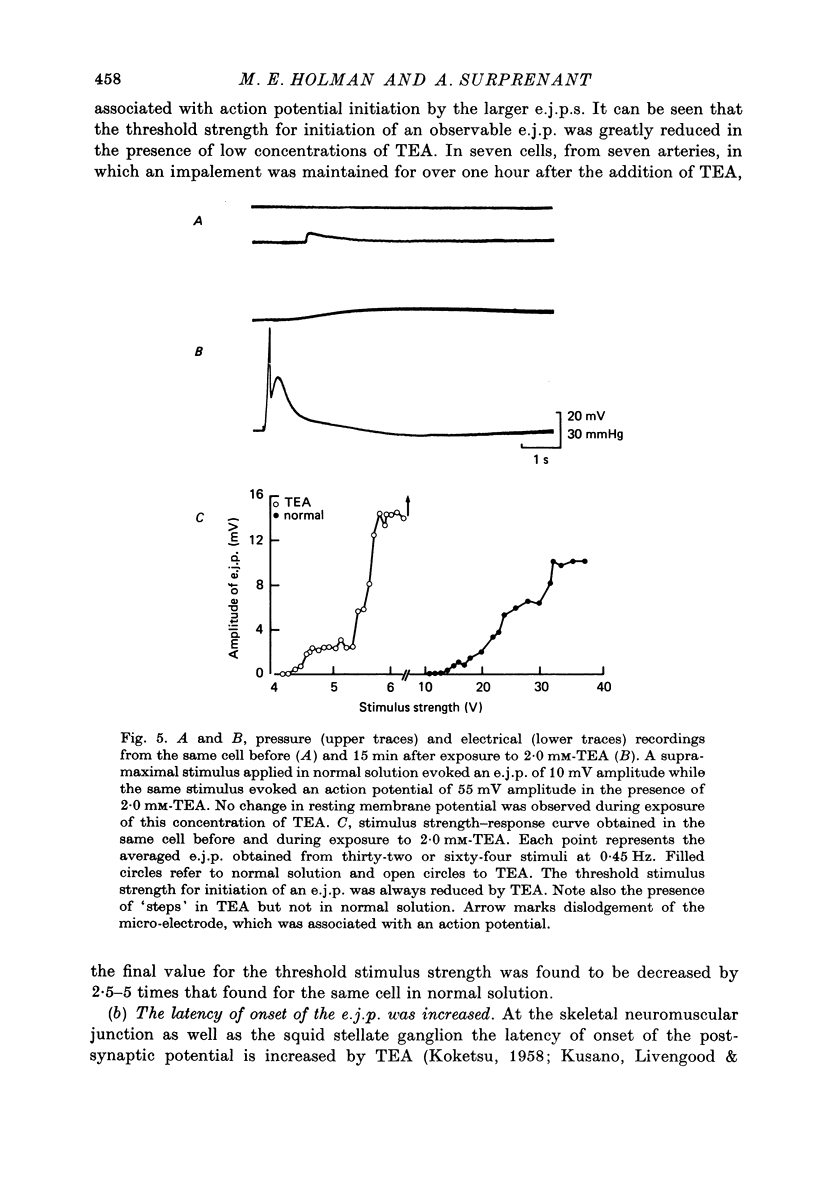




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., HOLMAN M. E. The transmission of excitation from autonomic nerve to smooth muscle. J Physiol. 1961 Jan;155:115–133. doi: 10.1113/jphysiol.1961.sp006617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C. Transmission from vasoconstrictor and vasodilator nerves to single smooth muscle cells of the guinea-pig uterine artery. J Physiol. 1969 Dec;205(3):695–708. doi: 10.1113/jphysiol.1969.sp008991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit P. R., Mambrini J. Modification of transmitter release by ions which prolong the presynaptic action potential. J Physiol. 1970 Oct;210(3):681–695. doi: 10.1113/jphysiol.1970.sp009235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Casteels R., Kitamura K., Kuriyama H., Suzuki H. The membrane properties of the smooth muscle cells of the rabbit main pulmonary artery. J Physiol. 1977 Sep;271(1):41–61. doi: 10.1113/jphysiol.1977.sp011989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droogmans G., Raeymaekers L., Casteels R. Electro- and pharmacomechanical coupling in the smooth muscle cells of the rabbit ear artery. J Gen Physiol. 1977 Aug;70(2):129–148. doi: 10.1085/jgp.70.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeusler G. Relationship between noradrenaline-induced depolarization and contraction in vascular smooth muscle. Blood Vessels. 1978;15(1-3):46–54. doi: 10.1159/000158152. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. Some properties of spontaneous excitatory junction potentials recorded from arterioles of guinea-pigs. J Physiol. 1980 Jun;303:43–60. doi: 10.1113/jphysiol.1980.sp013269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D. Neuromuscular transmission in arterioles of guinea-pig submucosa. J Physiol. 1977 Dec;273(1):263–275. doi: 10.1113/jphysiol.1977.sp012093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman M. E., Surprenant A. M. Some properties of the excitatory junction potentials recorded from saphenous arteries of rabbits. J Physiol. 1979 Feb;287:337–351. doi: 10.1113/jphysiol.1979.sp012663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOKETSU K. Action of tetraethylammonium chloride on neuromuscular transmission in frogs. Am J Physiol. 1958 Apr;193(1):213–218. doi: 10.1152/ajplegacy.1958.193.1.213. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick C. T. Excitation and contraction in bovine tracheal smooth muscle. J Physiol. 1975 Jan;244(2):263–281. doi: 10.1113/jphysiol.1975.sp010796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano K., Livengood D. R., Werman R. Correlation of transmitter release with membrane properties of the presynaptic fiber of the squid giant synapse. J Gen Physiol. 1967 Dec;50(11):2579–2601. doi: 10.1085/jgp.50.11.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekata F. Electrophysiological studies of the smooth muscle cell membrane of the rabbit common carotid artery. J Gen Physiol. 1971 Jun;57(6):738–751. doi: 10.1085/jgp.57.6.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osa T., Kuriyama H. The membrane properties and decremental conduction of excitation in the fundus of the guinea-pig stomach. Jpn J Physiol. 1970 Dec 15;20(6):626–639. doi: 10.2170/jjphysiol.20.626. [DOI] [PubMed] [Google Scholar]
- Purves R. D. Current flow and potential in a three-dimensional syncytium. J Theor Biol. 1976 Jul 21;60(01):147–162. doi: 10.1016/0022-5193(76)90160-0. [DOI] [PubMed] [Google Scholar]
- Szurszewski J. H. A study of the canine gastric action potential in the presence of tetraethylammonium chloride. J Physiol. 1978 Apr;277:91–102. doi: 10.1113/jphysiol.1978.sp012262. [DOI] [PMC free article] [PubMed] [Google Scholar]


