Abstract
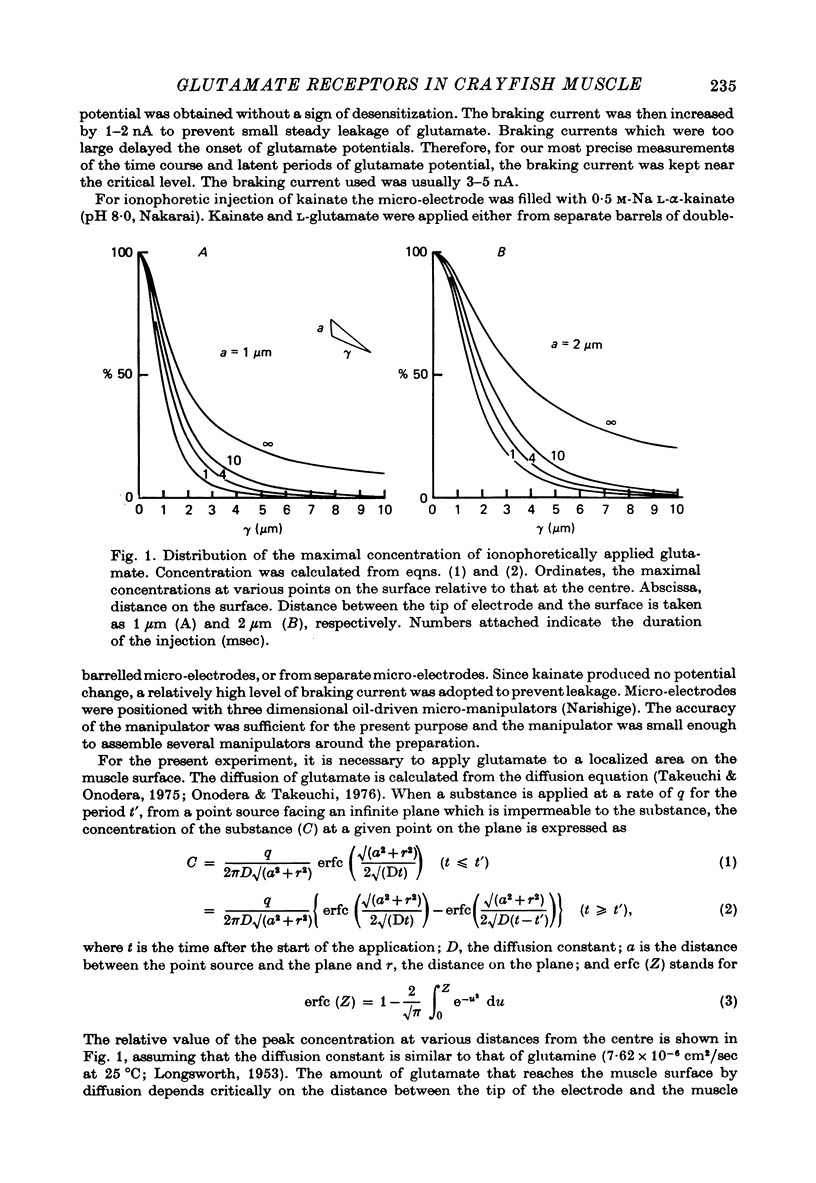
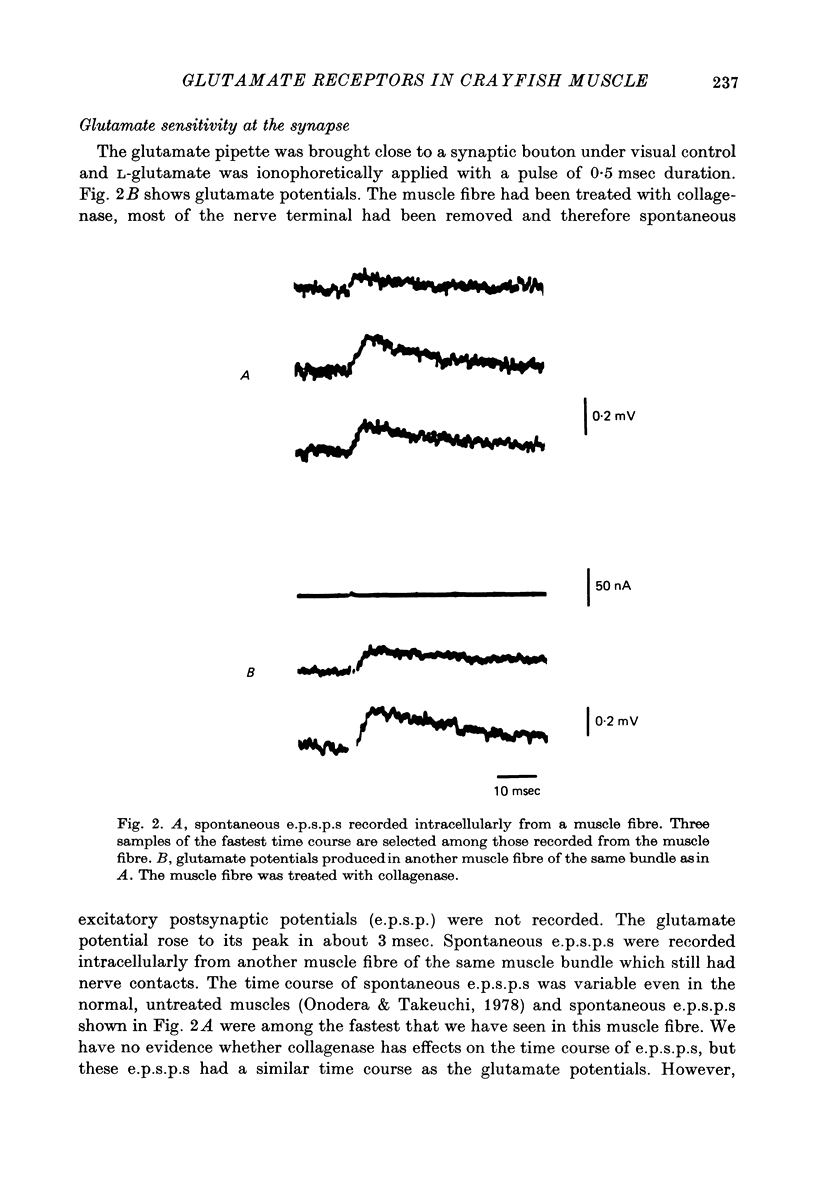
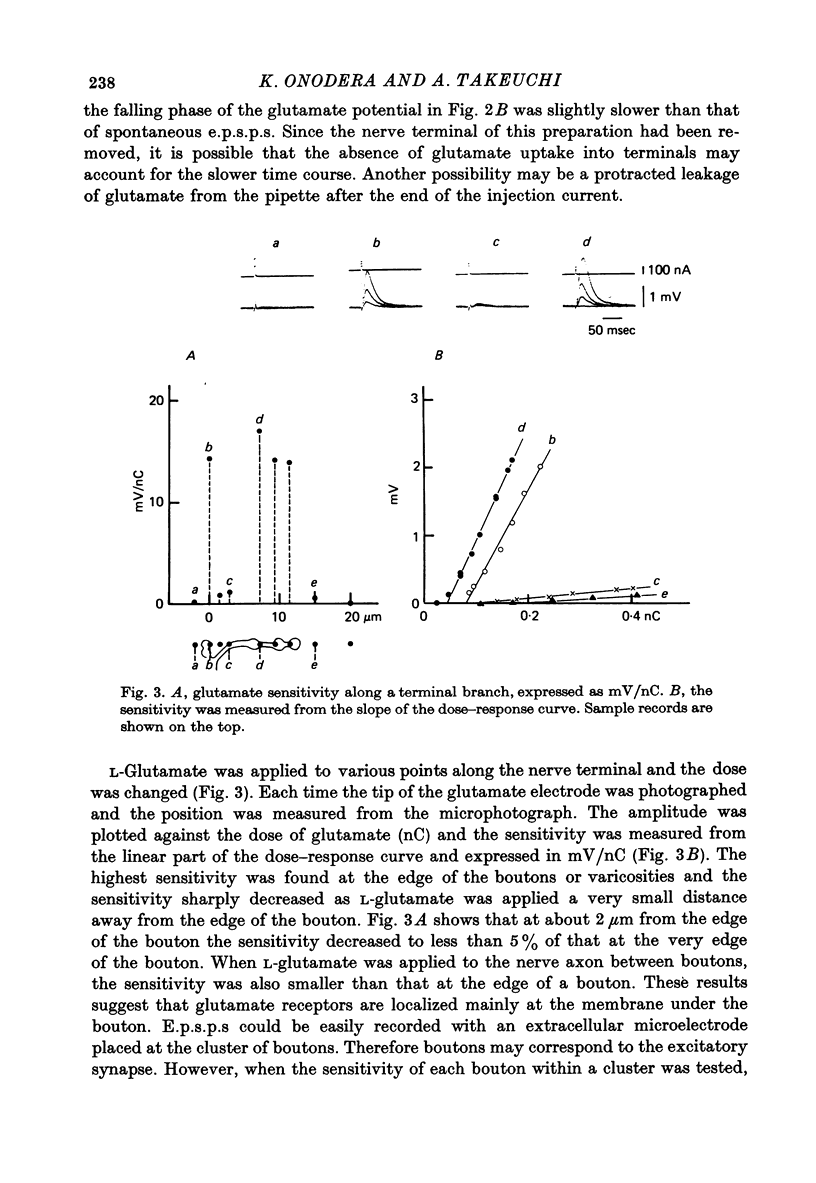
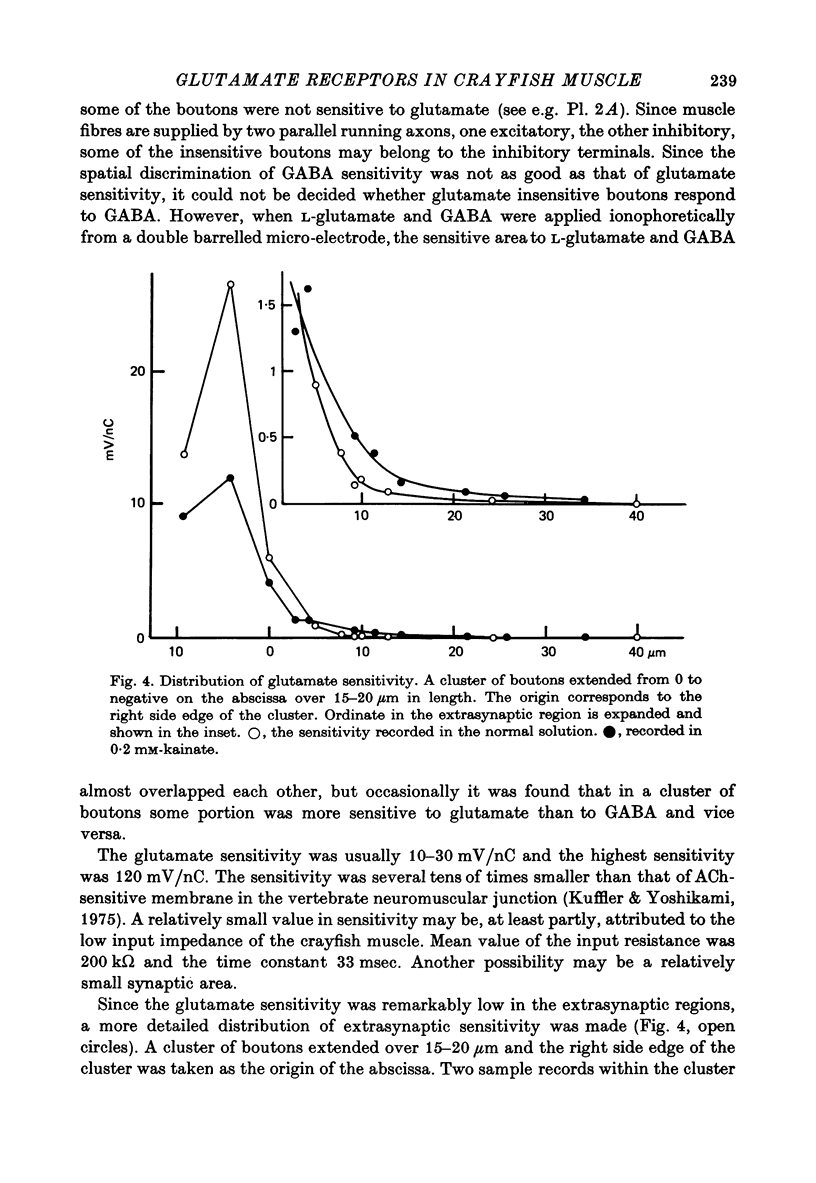
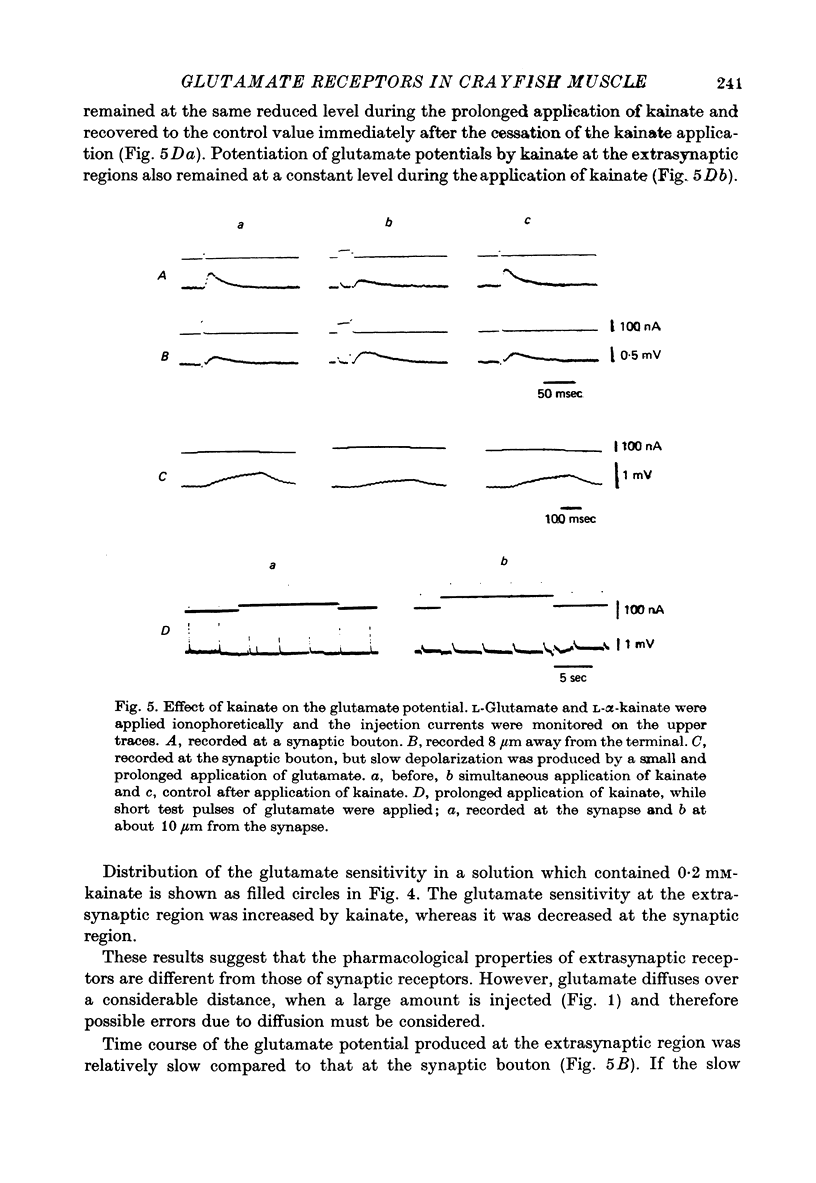
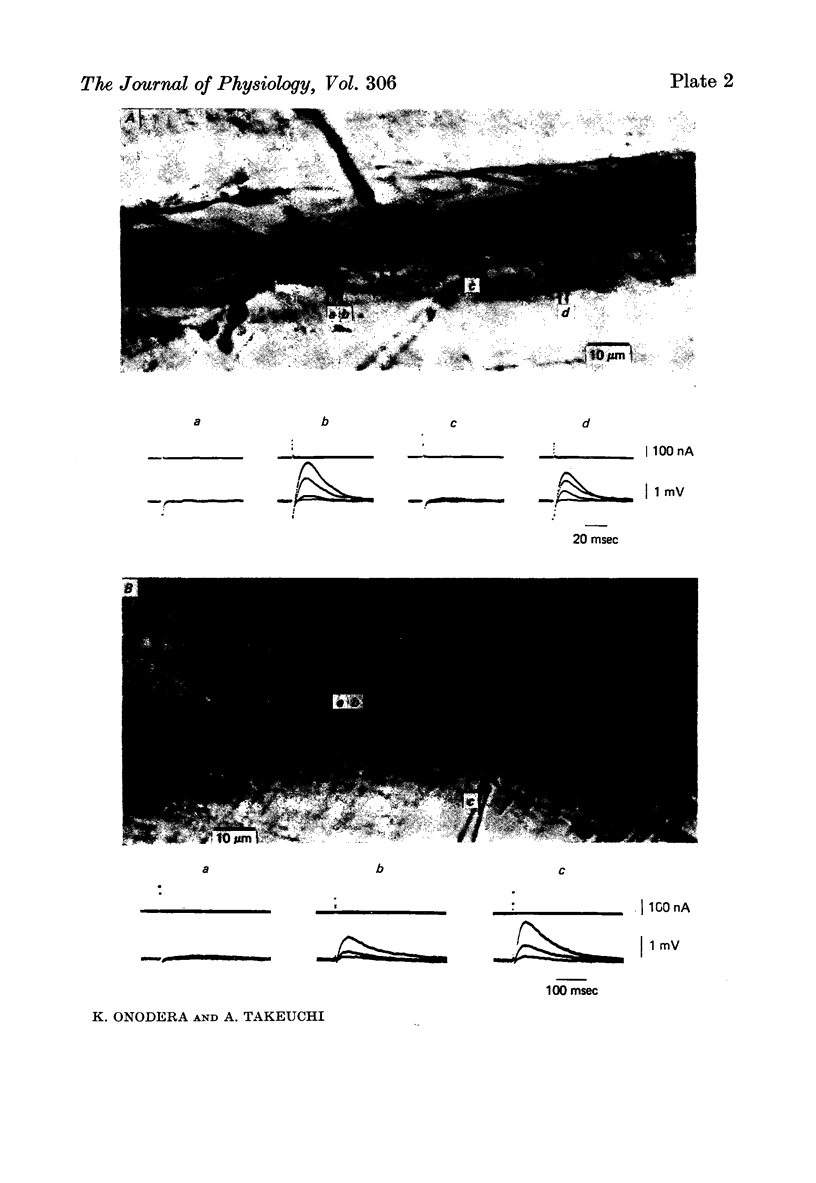
1. The distribution of glutamate sensitivity was measured in the opener muscle in the walking legs of the crayfish (Cambarus clarkii). L-Glutamate was ionophoretically applied under visual control. 2. Bundles of a few muscle fibres were isolated and viewed with Nomarski optics. Two axons, presumably excitatory and inhibitory, branched widely over the surface of individual muscle fibres, forming numerous clusters of boutons or varicosities. 3. Glutamate sensitivity was measured from the slope of the dose-response curves obtained by ionophoretic application of L-glutamate and expressed as mV/nC. The highest sensitivities were about 100 mV/nC, obtained at the edge of synaptic boutons. The sensitivity declined to less than 5% about 2 micrometer away from the synaptic terminal. The time course of glutamate potentials was approximately the same as that of spontaneous synaptic potentials. 4. Glutamate depolarization started within 300 microsec after ionophoretic release of glutamate. This time lag was shorter than the synaptic delay of the nerve-evoked synaptic potential measured with an extracellular micro-electrode. This indicates that glutamate depolarization results from a direct action on the post-synaptic receptor. 5. Application of L-alpha-kainic acid decreased the amplitude of the glutamate potential produced at the synaptic region, whereas kainate increased the amplitude of the glutamate potential at the extrasynaptic region. It is suggested that the pharmacological properties of the extrasynaptic receptor differ from those of the synaptic receptor. Possible mechanisms for the different actions of kainate are discussed.
Full text
PDF







Images in this article
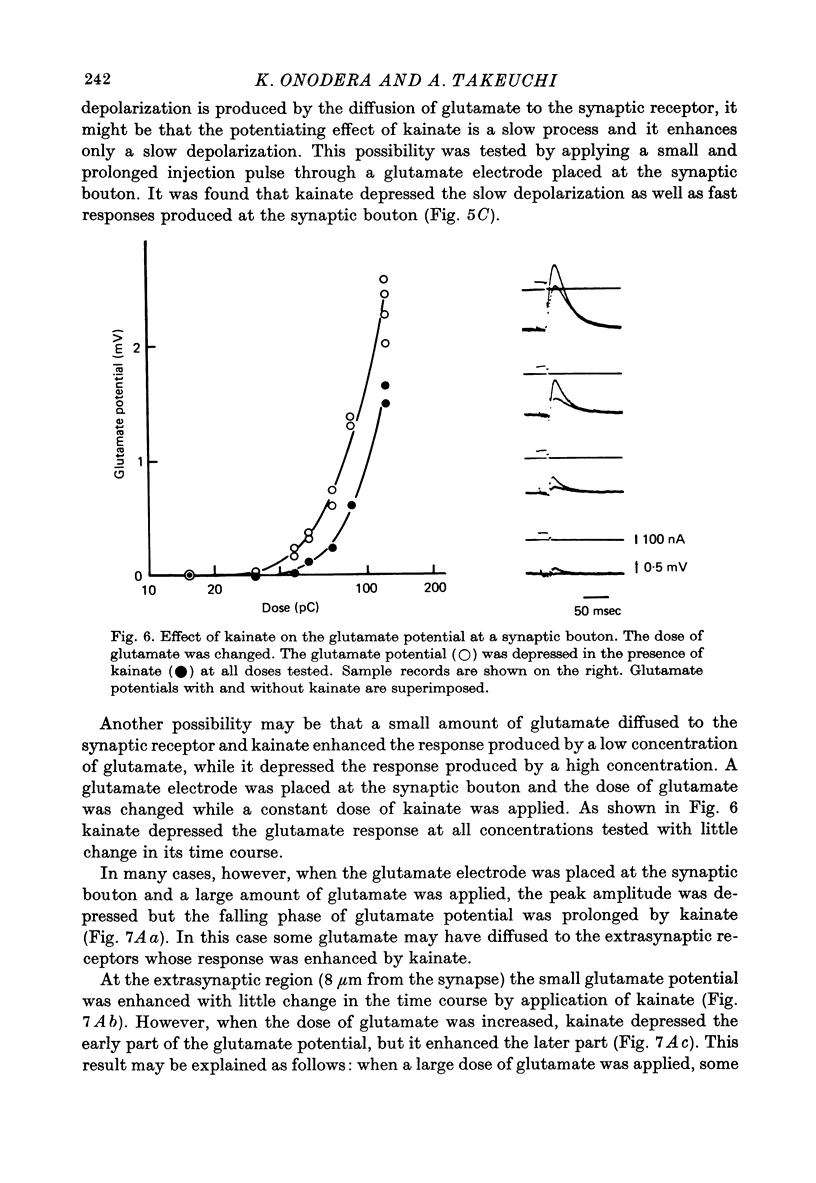
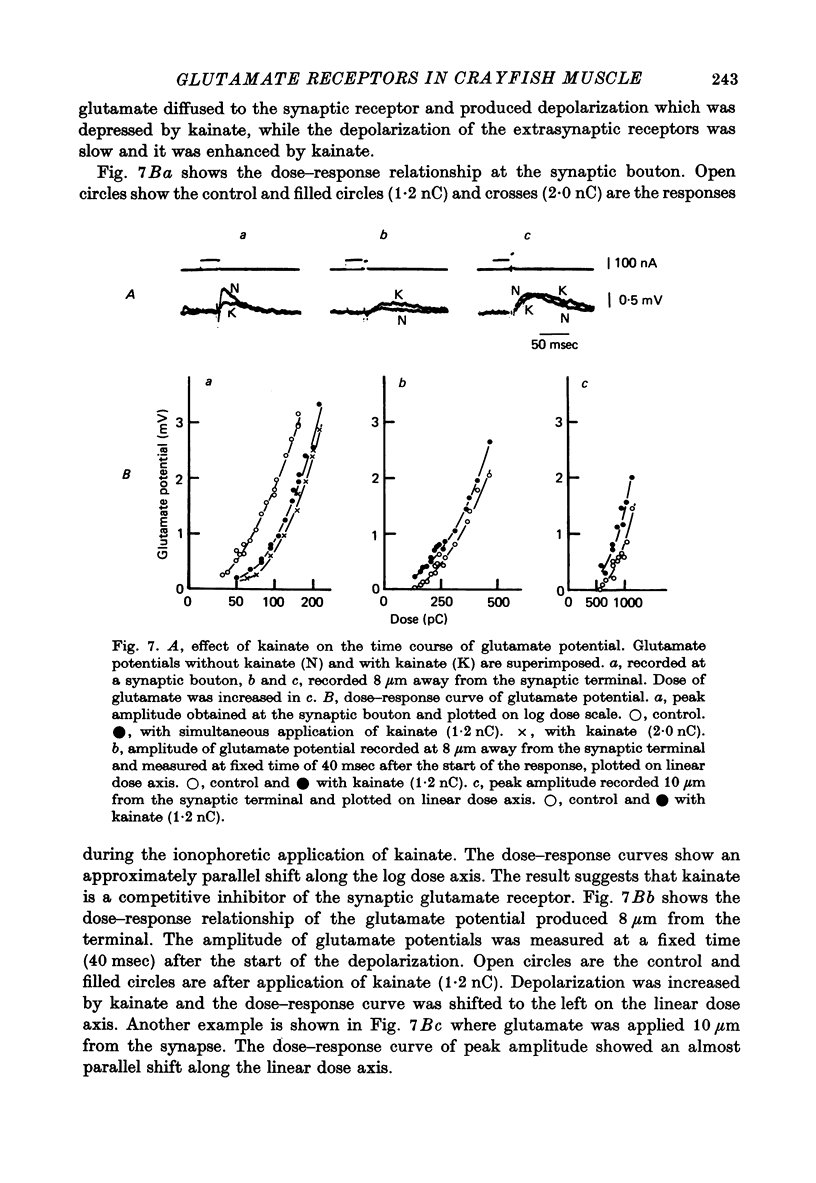
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albuquerque E. X., Gage P. W. Differential effects of perhydrohistrionicotoxin on neurally and iontophoretically evoked endplate currents. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1596–1599. doi: 10.1073/pnas.75.3.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley C. C., Campbell A. K. Free-calcium and tension responses in single barnacle muscle fibres following the application of L-glutamate. Biochim Biophys Acta. 1978 Sep 22;512(2):429–435. doi: 10.1016/0005-2736(78)90265-1. [DOI] [PubMed] [Google Scholar]
- Bracho H., Orkand R. K. Effect of calcium on excitatory neuromuscular transmission in the crayfish. J Physiol. 1970 Jan;206(1):61–71. doi: 10.1113/jphysiol.1970.sp008997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton C. K., Freeman A. R. La3+ blockage of glutamate action at the lobster neuromuscular junction. Comp Biochem Physiol C. 1975 Aug 1;51(2):285–289. doi: 10.1016/0306-4492(75)90075-1. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G. Two types of extrajunctional L-glutamate receptors in locust muscle fibres. J Physiol. 1976 Feb;255(2):449–464. doi: 10.1113/jphysiol.1976.sp011289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gration K. A., Clark R. B., Usherwood P. N. Three types of L-glutamate receptor on junctional membrane of locust muscle fibres. Brain Res. 1979 Aug 3;171(2):360–364. doi: 10.1016/0006-8993(79)90343-3. [DOI] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE MEASUREMENT OF SYNAPTIC DELAY, AND THE TIME COURSE OF ACETYLCHOLINE RELEASE AT THE NEUROMUSCULAR JUNCTION. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:483–495. doi: 10.1098/rspb.1965.0016. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of temperature on the synaptic delay at the neuromuscular junction. J Physiol. 1965 Dec;181(3):656–670. doi: 10.1113/jphysiol.1965.sp007790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler S. W., Yoshikami D. The distribution of acetylcholine sensitivity at the post-synaptic membrane of vertebrate skeletal twitch muscles: iontophoretic mapping in the micron range. J Physiol. 1975 Jan;244(3):703–730. doi: 10.1113/jphysiol.1975.sp010821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Postsynaptic entry of calcium induced by transmitter action. Proc R Soc Lond B Biol Sci. 1975 Apr 29;189(1094):49–56. doi: 10.1098/rspb.1975.0040. [DOI] [PubMed] [Google Scholar]
- Lang F., Atwood H. L., Morin W. A. Innervation and vascular supply of the crayfish opener muscle: scanning and transmission electron microscopy. Z Zellforsch Mikrosk Anat. 1972;127(2):189–200. doi: 10.1007/BF00306801. [DOI] [PubMed] [Google Scholar]
- MILEDI R. Junctional and extra-junctional acetylcholine receptors in skeletal muscle fibres. J Physiol. 1960 Apr;151:24–30. [PMC free article] [PubMed] [Google Scholar]
- Matsumura M. Electro-mechanical coupling in crayfish muscle fibers examined by the voltage clamp method. Jpn J Physiol. 1972 Feb;22(1):53–69. doi: 10.2170/jjphysiol.22.53. [DOI] [PubMed] [Google Scholar]
- McMahan U. J., Kuffler S. W. Visual identification of synaptic boutons on living ganglion cells and of varicosities in postganglionic axons in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):485–508. doi: 10.1098/rspb.1971.0044. [DOI] [PubMed] [Google Scholar]
- Miyamoto M. D. The actions of cholinergic drugs on motor nerve terminals. Pharmacol Rev. 1977 Sep;29(3):221–247. [PubMed] [Google Scholar]
- Onodera K., Takeuchi A. Effects of membrane potential and temperature on the excitatory post-synaptic current in the crayfish muscle. J Physiol. 1978 Mar;276:183–192. doi: 10.1113/jphysiol.1978.sp012227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera K., Takeuchi A. Permeability changes produced by L-glutamate at the excitatory post-synaptic membrane of the crayfish muscle. J Physiol. 1976 Mar;255(3):669–685. doi: 10.1113/jphysiol.1976.sp011302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki H., Ishida M. Pharmacological distinction between the excitatory junctional potential and the glutamate potential revealed by concanavalin A at the crayfish neuromuscular junction. Brain Res. 1979 Feb 9;161(3):493–501. doi: 10.1016/0006-8993(79)90678-4. [DOI] [PubMed] [Google Scholar]
- Shinozaki H., Shibuya I. Potentiation of glutamate-induced depolarization by kainic acid in the crayfish opener muscle. Neuropharmacology. 1974 Nov;13(10-11):1057–1065. doi: 10.1016/0028-3908(74)90096-3. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. THE EFFECT ON CRAYFISH MUSCLE OF IONTOPHORETICALLY APPLIED GLUTAMATE. J Physiol. 1964 Mar;170:296–317. doi: 10.1113/jphysiol.1964.sp007332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Onodera K. Effects of kainic acid on the glutamate receptors of the crayfish muscle. Neuropharmacology. 1975 Sep;14(9):619–625. doi: 10.1016/0028-3908(75)90084-2. [DOI] [PubMed] [Google Scholar]
- Thieffry M., Bruner J. Direct evidence for a presynaptic action of glutamate at a crayfish neuromuscular junction. Brain Res. 1978 Nov 10;156(2):402–406. doi: 10.1016/0006-8993(78)90528-0. [DOI] [PubMed] [Google Scholar]
- de Santis A., Eusebi F., Miledi R. Kainic acid and synaptic transmission in the stellate ganglion of the squid. Proc R Soc Lond B Biol Sci. 1978 Sep 29;202(1149):527–532. doi: 10.1098/rspb.1978.0084. [DOI] [PubMed] [Google Scholar]