Abstract
Helicobacter pylori must be motile or display chemotaxis to be able to fully infect mammals, but it is not known how this chemotaxis is directed. We disrupted two genes encoding predicted chemoreceptors, tlpA and tlpC. H. pylori mutants lacking either of these genes are fully motile and chemotactic in vitro and are as able as the wild type to infect mice when they are the sole infecting strains. In contrast, when mice are coinfected with the H. pylori SS1 tlpA or tlpC mutant and the wild type, we find more wild type than mutant after 2 weeks of colonization. Neither strain has an in vitro growth defect. These results suggest that the tlpA- and tlpC-encoded proteins assist colonization of the stomach environment.
Many bacterial pathogens have the ability to move via flagella, but little is known about how microbes use this ability inside animal hosts. A bacterium presumably benefits if it regulates its flagellar activity so as to direct its motility, a process called chemotaxis. One pathogen that requires flagella and chemotaxis is the ulcer-causing bacterium Helicobacter pylori (11, 14).
Infection by H. pylori causes chronic gastritis and gastric and duodenal ulcers and can lead to the development of adenocarcinoma of the distal stomach and gastric mucosa-associated lymphoid tissue lymphoma (8, 24, 30). This bacterium infects approximately 50% of the world's human population (5), but only a subset of those infected develop disease.
Previous studies have identified a number of H. pylori factors that contribute to animal infection, including flagellum-directed motility (8, 10, 28). H. pylori carries five sheathed flagella located at one pole. Mutational loss of any of the flagellar filament components or of the ability to move renders H. pylori motility deficient and less able to infect a variety of animal models (11, 15, 18, 25). Many flagellated bacteria perform chemotactic motility by coupling the recognition of environmental conditions, such as amino acids or pH, with regulation of swimming behavior, a process that has been studied extensively in Escherichia coli (reviewed in references 2 and 13). In E. coli, environmental cues are sensed first by transmembrane chemoreceptor proteins that regulate a kinase cascade composed of the CheA kinase, the CheW coupling protein, and the flagellar-motor regulator CheY. The output of these events alters the direction of flagellar rotation to allow the bacteria to respond to environmental conditions.
H. pylori contains orthologues of several E. coli chemotaxis proteins, including CheW, CheY, and a CheA-CheY hybrid (1, 29), suggesting that this portion of the chemotactic signaling pathway is similar in these two bacteria. In support of this, elimination of the cheY, cheA, or cheW gene in H. pylori yields bacteria that have a reduced chemotactic response in vitro (14, 27). Furthermore, the cheA or cheY mutants are unable to infect mouse stomachs (14). Although chemotaxis is needed for mouse stomach colonization by H. pylori, neither its in vivo targets nor the chemoreceptors that direct it are known. Scrutiny of the H. pylori genomes identified four genes predicted to code for putative chemoreceptors (1, 29). These chemoreceptors were identified on the basis of the presence of a conserved domain in the chemoreceptor that interacts with CheW (1, 29). To begin to understand how H. pylori chemotaxis is controlled in vivo, we constructed mutations that eliminated two of these potential chemoreceptors, TlpA and TlpC, and analyzed their behavior.
Construction of tlpA and tlpC mutants in H. pylori strains SS1 and G27.
For this study, the human-isolated type I H. pylori strains G27, SPM342f, and SS1 were used (Table 1). H. pylori was grown as previously described on Columbia blood agar plates supplemented with H. pylori-selective antibiotics and 5% defibrinated horse blood (CHBA) under microaerobic conditions (25). Liquid culture was done in brucella broth containing 10% fetal bovine serum (BB10) in anaerobic jars with Campy Paks (Oxoid) and shaking at 37°C. Chloramphenicol was used at concentrations of 5 to 10 μg/ml (H. pylori) or 20 μg/ml (E. coli), while kanamycin was used at 15 μg/ml (H. pylori) or 30 μg/ml (E. coli). Long-term storage of H. pylori was as previously described (25). DNA was amplified by using Pfu-Turbo polymerases (Stratagene) or Taq polymerase (generous gift of D. Kellogg). All DNA sequencing was performed by the sequencing facility at the University of California at Berkeley.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype or description | Antibiotic resistance | Source or reference |
|---|---|---|---|
| E. coli DH10B | Cloning strain | Lab stock | |
| H. pylori | |||
| G27 | Wild type, type I | N. Salama (6) | |
| SS1 | Wild type, type I | J. O'Rourke (19) | |
| SPM342F | Wild type, type I | N. Salama (21) | |
| TAG101 | G27 ΔtlpA::cat-1 | Cmr | This study |
| TAG102 | G27 ΔtlpA::cat-2 | Cmr | This study |
| TAS101 | SS1 ΔtlpA::cat-1 | Cmr | This study |
| TCG568 | G27 ΔtlpC::cat-1 | Cmr | This study |
| TCS565 | SS1 ΔtlpC::cat-1 | Cmr | This study |
| CYG487 | G27 ΔcheY::aphA3-1 | Kmr | This study |
| CYS481 | SS1 ΔcheY::aphA3-1 | Kmr | This study |
| Plasmids | |||
| pBluescript | Cloning vector | Apr | Stratagene |
| pBS-cat | pBluescript::cat (from Campylobacter coli) | Apr Cmr | N. Salama |
| pBS-kan | pBluescript::aphA3 (from C. coli) | Apr Kmr | N. Salama |
| pKO112 | pBluescript with tlpA (SPM342F) | Apr | This study |
| pTA101 | pKO112 ΔtlpA::cat-1 | Apr Cmr | This study |
| pTA102 | pKO112 ΔtlpA::cat-2 | Apr Cmr | This study |
| pTC100 | pBluescript with tlpC (SS1) | Apr | This study |
| pTC200 | pTC100 ΔtlpC::cat-1 | Apr Cmr | This study |
| pKO126 | pBluescript with cheY (SS1) | Apr | This study |
| pKO126K | pKO126 cheY::aphA3-1 | Apr Kmr | This study |
tlpA, tlpC, and cheY were cloned from H. pylori genomic DNA using PCR. Primers to amplify each gene were designed on the basis of the sequence of H. pylori strain 26695 (Table 2) (29). tlpA (HP0099 from the H. pylori 26695 genome [29]) was amplified using primers tlpA1 and tlpA3, H. pylori strain SPM342f genomic DNA, and Pfu polymerase. The resultant 2.3-kb PCR product was ligated with pCR2.1-topo (Invitrogen) to create pKO102. An EcoRI-containing tlpA fragment was subcloned into pBluescript KS to create pKO112. tlpC (HP0082) was amplified from strain SS1 using Pfu-Turbo polymerase and oligonucleotides tlpC4 and tlpC5 to obtain a 2.8-kb PCR product. This PCR product was ligated into EcoRV-cut pBluescript KS to create pTC100. cheY (HP1067) was cloned using SS1 genomic DNA and primers cheY6 and cheY7. This reaction yielded a 2.6-kb fragment that was cloned to create pKO126, using an approach similar to that used for tlpC. We used restriction enzyme analysis, DNA sequencing, and database comparison to confirm the construction of each plasmid.
TABLE 2.
PCR primers used in this study
| Primer | Sequence |
|---|---|
| Colicat1 | 5′-GTATAGTCTGCTGTAAACTCAGTCC-3′ |
| tlpA1 | 5′-TGAAAGATCTGCCTTTTGGGGCGTTG-3′ |
| tlpA3 | 5′-CCGCAAGCTTGAAACTGCTTTTTATTCACATC-3′ |
| tlpA4 | 5′-GCTGTTTAAGGACACCCC-3′ |
| tlpA5 | 5′-TTAGCGAACGATAGCGCG-3′ |
| tlpC-4 | 5′-ACCCCCAACTAACTCCCCTTAAG-3′ |
| tlpC-5 | 5′-CAGAGCTTGAATCAATGGTTGGG-3′ |
| pTC2-1 | 5′-CAAGAAAGGAGTCTGAAAAC-3′ |
| pTC2-2 | 5′-TTGATTCTTACCCATTAGGGG-3′ |
| cheY6 | 5′-ATCGCACAAGATAGAAACGG-3′ |
| cheY7 | 5′-GCTGTTCTAAAACCTCAAATCCATT-3′ |
Next, a portion of each gene was deleted, using inverse PCR. For tlpA in pKO112, we used primers tlpA4 and tlpA5 to delete 1,374 bp (encoding amino acids 29 to 487). For tlpC, we used primers pTC2-1 and pTC2-2 and pTC100 DNA to remove 1,005 bp (encoding amino acids 175 to 510). After the deletion mutants were created, the cat gene was excised from pBS-cat by digestion with either HincII alone or KpnI and SacI with additional blunting. This cat fragment was ligated with the tlpA inverse PCR product to create two plasmids, pTA101 and pTA102, that contained both orientations of cat within the deleted tlpA gene (Table 1). Similarly, the tlpC inverse PCR product was ligated with the cat gene to create pTC200. A cheY insertion mutant was created by digesting pKO126 with Bpu1102I, which cuts at the DNA sequence corresponding to amino acid 46. The aphA3 gene was prepared by restriction digestion of pBS-kan with XhoI and EagI, and blunt ends were created with T4 polymerase. These two DNA pieces were ligated together to create pKO126K.
pTA101, pTA102, pTC200, and pKO126K were used individually to transform H. pylori G27 and SS1 to chloramphenicol or kanamycin resistance using natural transformation as previously described (9, 28). PCR and Southern blotting verified the correct nature of all these mutations and the insertion of only one cat or aphA3 gene (data not shown).
Analysis of in vitro and mouse-infecting characteristics of H. pylori mutants lacking TlpA or TlpC. We used phase-contrast microscopy of wet mounts (grown in CHBA for 6 to 9 h) of G27 and SS1 ΔtlpA::cat or ΔtlpC::cat mutants suspended in BB10 to analyze motility and found no observable defect of these mutants compared to their wild-type parents. Swim rates in soft agar were comparable between the wild-type and tlpA or tlpC mutant H. pylori, suggesting that the mutants retained wild-type chemotaxis (Table 3). In contrast, a mutant lacking chemotaxis entirely by virtue of loss of cheY was motile but did not migrate in soft agar, as shown previously (3, 14).
TABLE 3.
In vitro motility of H. pylori tlpA, tlpC, and cheY mutantsa
| Strain | Swim rate (mm/day) |
|---|---|
| SS1 | 4.5 ± 0.3 |
| SS1 ΔtlpA::cat-2 | 4.1 ± 0.8 |
| SS1 ΔtlpC::cat-1 | 4.1 ± 0.1 |
| SS1 cheY::aphA3-1 | 0.4 ± 0.1 |
| G27 | 5.2 ± 0.3 |
| G27 ΔtlpA::cat-1 | 5.1 ± 0.9 |
| G27 ΔtlpA::cat-2 | 5.0 ± 0.7 |
| G27 ΔtlpC::cat-1 | 4.7 ± 0.4 |
| G27 cheY::aphA3-1 | 0.75 ± 0.15 |
Colonial expansion (swim) rates were determined by measuring the colony diameter each day for 7 days of cells grown in brucella broth, 2.5 to 5% fetal bovine serum, and 0.35% agar.
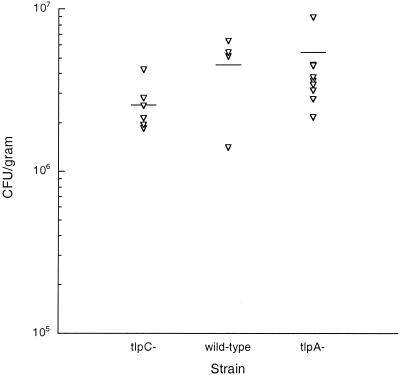
To determine whether TlpA or TlpC is required to colonize animal stomachs, we infected 4- to 6-week-old male FVB/N mice with SS1 ΔtlpA::cat or ΔtlpC::cat mutants as described previously (25) and compared levels of colonization to that of the wild-type strain. For these experiments, we used two separate isolates of the H. pylori SS1 tlpA mutants and one SS1 tlpC mutant. After 2 weeks of infection, the mice were sacrificed, and the numbers of H. pylori in their stomachs were determined by plating the homogenized stomach onto CHBA plates supplemented with nalidixic acid and bacitracin as described previously (25). Infection with wild-type SS1 resulted in mice carrying an average of 5.85 × 106 CFU/g of stomach (Fig. 1). Infection with either the tlpA or tlpC mutant resulted in similar levels of H. pylori in mouse stomachs (Fig. 1). Statistical analysis using the paired t test (available at http://www.graphpad.com/calculators/ttest1.cfm) revealed no significant difference (P > 0.05) in the colonization abilities of the wild type and the ΔtlpA::cat or ΔtlpC::cat mutant.
FIG. 1.
The numbers of H. pylori recovered from mice infected with mutants lacking tlpA or tlpC do not differ from those from mice infected with the wild type. Each point represents the bacterial numbers per gram of stomach in mice infected for 2 weeks with SS1 ΔtlpC::cat-1 mutant (left), wild-type SS1 (middle), or SS1 ΔtlpA::cat-1 mutant (right). Mice infected by wild-type SS1 were given 1 × 108 CFU (n = 4), mice receiving SS1 ΔtlpA::cat (n = 9) were inoculated with 4 × 107 to 13 × 107 CFU, and mice receiving SS1 ΔtlpC::cat (n = 6) were given 1 × 107 to 6 × 107 CFU. Two different isolates of ΔtlpA::cat-1 were used but their colonization levels did not differ, so their data are shown together in one column. The short horizontal lines represent the geometric mean CFUs per gram. The levels of colonization do not differ significantly from each other (P > 0.05).
tlpA and tlpC mutants are defective for mouse colonization when they are coinfected with wild type.
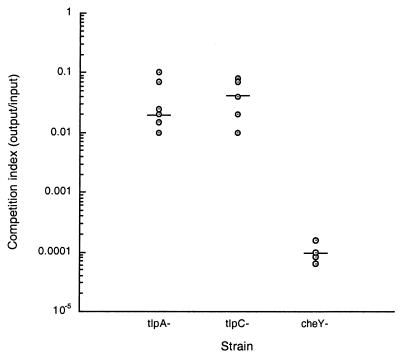
We examined mice infected with a mixture of approximately equal amounts of wild-type SS1 and either SS1 ΔtlpA::cat or SS1 ΔtlpC::cat mutant, because previous studies had shown that some H. pylori mutants appear to have wild-type colonization ability when they are the sole infecting strain but have a colonization defect when coinfected with the wild type (28). After 2 weeks, we collected the stomachs and determined the amounts of each of the two strains that remained. The wild-type strain was better able to colonize the mouse stomach than either the tlpA or tlpC mutant strain (Fig. 2). After dividing by the input ratio, there was 52-fold more wild type than the tlpA mutant and 25-fold more than the tlpC mutant. In contrast, when wild-type SS1 and SS1 ΔtlpA::cat or SS1 ΔtlpC::cat were grown together in vitro, the ratio of wild type to mutant did not change over 48 h (Table 4). Consistent with this, loss of tlpA or tlpC did not change the growth rate of either SS1 or G27 ΔtlpA::cat or ΔtlpC::cat compared to their wild-type parents when grown singly (data not shown). These results suggest that both TlpA and TlpC confer an advantage in vivo in a competitive setting but are not needed in vitro for growth, motility, or chemotaxis.
FIG. 2.
Number of bacteria in the stomachs of mice infected with mixtures of wild-type and mutant H. pylori after 2 weeks. Each point represents one mouse. The competitive index (CI) is the mutant/wild-type ratio for output divided by the same ratio for input. The short horizontal lines represent the geometric means. The number of mice (n) used follows: for ΔtlpA::cat mutant (left), n = 10; for ΔtlpC::cat mutant (middle), n = 8; and for cheY::aphA3 mutant (right), n = 4. We were unable to detect any cheY mutants in the output and used 250 CFU/g (the estimated detection limit) for these calculations. Statistical analysis using the Wilcoxon matched-pair signed-rank test (available at http://www.fon.hum.uva.nl/Service/Statistics/Signed_Rank_Test.html) showed that all strains are significantly different from a strain with no competition defect (CI = 1) (P < 0.01). On the basis of observations with other mutants and wild-type strains, two strains with equal abilities yield a CI of 1 (data not shown).
TABLE 4.
In vitro competition of wild-type SS1 with its tlpA, tlpC, or cheY mutantsa
| Mutant | Mutant/wild-type strain ratio after growth for the following time:
|
||||||
|---|---|---|---|---|---|---|---|
| 0 h | 12 h | 21-24 h | 31-32 h | 36 h | 40 h | 48 h | |
| tlpA | 0.8 | -b | 1.05 | 1.0 | - | - | 1.1 |
| tlpC | 0.91 | 0.47 | 0.47 | - | 0.45 | - | 0.47 |
| cheY | 0.5 | - | 0.39 | - | - | 0.41 | - |
Bacteria were mixed together and grown in BB10 as described in the text. At the time points indicated, a sample was withdrawn and plated onto CHBA and CHBA supplemented with chloramphenicol or kanamycin. Data are representative of two separate experiments.
-, not determined.
Because loss of either the tlpA- or tlpC-encoded chemoreceptors resulted in H. pylori that were not completely outcompeted by their wild-type parent, we competed a totally nonchemotactic mutant (cheY mutant) with the wild type for comparison. After coinfection of this mutant with wild-type SS1, we found that there were no cheY mutants after 13 days (Fig. 2). Similar to the results with the tlpA or tlpC mutants, when this cheY mutant was grown in vitro together with wild-type SS1, the ratio of the two strains did not appreciably change (Table 4), nor was the growth rate of either G27 or SS1 cheY::aphA3 mutant different from those of their parents (data not shown). These findings are consistent with the notion that loss of cheY does not affect in vitro growth, but its function (chemotaxis) is necessary in vivo.
Conclusions.
We have created and characterized H. pylori mutants that lack the tlpA- or tlpC-encoded gene products which are predicted to encode chemoreceptors. We found that bacteria lacking either of these proteins were less fit than their wild-type parents for mouse stomach colonization but have no in vitro growth or chemotaxis defects. Because chemotactic motility has been shown to be required for full stomach colonization (14), an interpretation of these findings is that tlpA and tlpC encode chemoreceptors that direct the chemotactic response of the bacterium upon sensing mouse stomach conditions.
Chemoreceptor-encoding genes are usually identified on the basis of a highly conserved domain (HCD) of the encoded protein sequence, a portion that interacts with CheW (1, 29). Although the HCD allows identification of putative chemoreceptors, it does not indicate to what those chemoreceptors respond, because sensing of environmental cues occurs outside the HCD, commonly in the extracellular or periplasmic domains. The periplasmic portions of both TlpA and TlpC are not homologous to any proteins with known functions but are homologous to each other (data not shown). This homology suggests they sense similar compounds or contain similar structures, although this remains to be determined. Furthermore, this similarity may suggest redundancy and may explain why loss of either one of these proteins has only a minor colonization defect: the remaining (TlpA or TlpC) protein can substitute to some degree.
Other groups have found that H. pylori moves in vitro towards several chemicals and conditions, including urea, several urea analogs including fluorofamide and hydroxyurea, sodium and potassium bicarbonate, sodium chloride, glutamate, and methionine (22). In addition, strains with functional urease respond positively to viscosity (23). Although several of these chemicals are found in vivo, their relevance for H. pylori chemotaxis in vivo has not been shown. In addition, it remains to be determined if TlpA or TlpC participate in sensing these chemicals. We have been unable to reproduce the capillary assay as reported by Mizote and colleagues (22) and so have not analyzed our mutants in this fashion.
Mutation of either tlpC or tlpA resulted in H. pylori that behaved as their wild-type parent with regard to chemotaxis in vitro. This is not surprising, given that loss of any one E. coli chemoreceptor does not change its behavior in rich media such as used here. Furthermore, although both TlpA and TlpC contain a chemoreceptor signature, the HCD, it remains to be proven that these proteins function as chemoreceptors. While the vast majority of HCD-containing proteins are chemoreceptors, at least two appear to regulate gene expression (4, 7; J. Kirby and D. Zusman, unpublished data). It seems likely that the TlpA and TlpC proteins participate in chemotaxis, but this remains to be shown.
Most characterized HCD-containing proteins (referred to as chemoreceptors) participate in directed motility, and some of these proteins from pathogenic bacteria have been shown to participate in colonization or virulence. For example, the legume-nodulating bacterium Rhizobium leguminosarum contains several chemoreceptors, two of which (McpB and McpC) appear to be important for plant colonization (31). Mutants lacking either of these proteins had a 10-fold plant colonization defect only when coinoculated with the wild type. The causative agent of cholera, Vibrio cholerae, is predicted to contain 43 HCD-containing proteins (17), some of which have been implicated in virulence using a variety of approaches. Peterson and Mekalanos (26) identified acfB, a gene that encodes a chemoreceptor. This gene product is coordinately expressed with known V. cholerae virulence factors and is required for full colonization (12, 26). Using a different approach, Lee and coworkers looked for genes that affected cholera toxin expression in the mouse intestine and found that several of these genes encoded proteins involved in chemotaxis, including one gene that coded for a HCD-containing protein, McpX (20). Loss of this gene created bacteria that outcompeted their parents, a phenotype that is common to V. cholerae chemotaxis mutants (16, 20). Taken together, these studies are consistent with the notion that chemoreceptors are important for infection in V. cholerae, R. leguminosarum, and as we show here, for H. pylori.
Acknowledgments
Tessa M. Andermann and Yu-Ting Chen contributed equally to this work.
This work was funded in part by a Burroughs Wellcome Career Award to K.M.O.
We thank Nina Salama and Glen Otto for H. pylori advice, Nina Salama for providing strains and plasmids, Andy Camilli for statistical suggestions, and Susan Williams and Alvin Go for comments on the manuscript.
Editor: V. J. DiRita
REFERENCES
- 1.Alm, R. A., L.-S. L. Ling, D. T. Moir, B. L. King, E. D. Brown, P. C. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. Dejonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, C. Ives, R. Gibson, D. Merber, S. D. Mills, Q. Jiang, D. E. Taylor, G. F. Vovis, and T. J. Trast. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176-180. [DOI] [PubMed] [Google Scholar]
- 2.Armitage, J. P. 1999. Bacterial tactic responses. Adv. Microb. Physiol. 41:229-289. [DOI] [PubMed] [Google Scholar]
- 3.Beier, D., G. Spohn, R. Rappuoli, and V. Scarlato. 1997. Identification and characterization of an operon of Helicobacter pylori that is involved in motility and stress adaptation. J. Bacteriol. 179:4676-4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhaya, D., A. Takahashi, and A. R. Grossman. 2001. Light regulation of type IV pilus-dependent motility by chemosensor-like elements in Synechocystis PCC6803. Proc. Natl. Acad. Sci. USA 98:7540-7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaser, M. J. 1999. Where does Helicobacter pylori come from and why is it going away? JAMA 282:2260-2262. [DOI] [PubMed] [Google Scholar]
- 6.Censini, S., C. Lange, Z. Xiang, J. E. Crabtree, P. Ghiara, M. Borodovsky, R. Rappuoli, and A. Covacci. 1996. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. USA 93:14648-14653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung, Y. H., M. S. Cho, Y. J. Moon, J. S. Choi, Y. C. Yoo, Y. I. Park, K. M. Lee, K. W. Kang, and Y. M. Park. 2001. ctr1, a gene involved in a signal transduction pathway of the gliding motility in the cyanobacterium Synechocystis sp. PCC 6803. FEBS Lett. 492:33-38. [DOI] [PubMed] [Google Scholar]
- 8.Covacci, A., J. L. Telford, G. Del Giudice, J. Parsonnet, and R. Rappuoli. 1999. Helicobacter pylori virulence and genetic geography. Science 284:1328-1333. [DOI] [PubMed] [Google Scholar]
- 9.Donahue, J. P., D. A. Israel, R. M. J. Peek, M. J. Blaser, and G. G. Miller. 2000. Overcoming the restriction barrier to plasmid transformation of Helicobacter pylori. Mol. Microbiol. 37:1066-1074. [DOI] [PubMed] [Google Scholar]
- 10.Eaton, K. A., D. R. Morgan, and S. Krakowka. 1992. Motility as a factor in the colonisation of gnotobiotic piglets by Helicobacter pylori. J. Med. Microbiol. 37:123-127. [DOI] [PubMed] [Google Scholar]
- 11.Eaton, K. A., S. Suerbaum, C. Josenhans, and S. Krakowka. 1996. Colonization of gnotobiotic piglets by Helicobacter pylori deficient in two flagellin genes. Infect. Immun. 64:2445-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Everiss, K. D., K. J. Hughes, M. E. Kovach, and K. M. Peterson. 1994. The Vibrio cholerae acfB colonization determinant encodes an inner membrane protein that is related to a family of signal-transducing proteins. Infect. Immun. 62:3289-3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falke, J. J., and G. L. Hazelbauer. 2001. Transmembrane signaling in bacterial chemoreceptors. Trends Biochem. Sci. 26:257-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foynes, S., N. Dorrell, S. J. Ward, R. A. Stabler, A. A. McColm, A. N. Rycroft, and B. W. Wren. 2000. Helicobacter pylori possesses two CheY response regulators and a histidine kinase sensor, CheA, which are essential for chemotaxis and colonization of the gastric mucosa. Infect. Immun. 68:2016-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foynes, S., N. Dorrell, S. J. Ward, Z. W. Zhang, A. A. McColm, M. J. G. Farthing, and B. W. Wren. 1999. Functional analysis of the roles of FliQ and FlhB in flagellar expression in Helicobacter pylori. FEMS Microbiol. Lett. 174:33-39. [DOI] [PubMed] [Google Scholar]
- 16.Freter, R., and P. C. O'Brien. 1981. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: fitness and virulence of nonchemotactic Vibrio cholerae mutants in infant mice. Infect. Immun. 34:222-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heidelberg, J. F., J. A. Eisen, W. C. Nelson, R. A. Clayton, M. L. Gwinn, R. J. Dodson, D. H. Haft, E. K. Hickey, J. D. Peterson, L. Umayam, S. R. Gill, K. E. Nelson, T. D. Read, H. Tettelin, D. Richardson, M. D. Ermolaeva, J. Vamathevan, S. Bass, H. Qin, I. Dragoi, P. Sellers, L. McDonald, T. Utterback, R. D. Fleischmann, W. C. Nierman, and O. White. 2000. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406:477-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim, J. S., J. H. Chang, S. I. Chung, and J. S. Yum. 1999. Molecular cloning and characterization of the Helicobacter pylori fliD gene, an essential factor in flagellar structure and motility. J. Bacteriol. 181:6969-6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, A., J. O'Rourke, M. C. De Ungria, B. Robertson, G. Daskalopoulos, and M. F. Dixon. 1997. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 112:1386-1397. [DOI] [PubMed] [Google Scholar]
- 20.Lee, S. H., S. M. Butler, and A. Camilli. 2001. Selection for in vivo regulators of bacterial virulence. Proc. Natl. Acad. Sci. USA 98:6889-6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marchetti, M., B. Arico, D. Burroni, N. Figura, R. Rappuoli, and P. Ghiara. 1995. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science 267:1655-1658. [DOI] [PubMed] [Google Scholar]
- 22.Mizote, T., H. Yoshiyama, and T. Nakazawa. 1997. Urease-independent chemotactic responses of Helicobacter pylori to urea, urease inhibitors, and sodium bicarbonate. Infect. Immun. 65:1519-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura, H., H. Yoshiyama, H. Takeuchi, T. Mizote, K. Okita, and T. Nakazawa. 1998. Urease plays an important role in the chemotactic motility of Helicobacter pylori in a viscous environment. Infect. Immun. 66:4832-4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.NIH Consensus Development Panel on 1994. Helicobacter pylori in peptic ulcer disease. NIH Consensus Conference. JAMA 272:65-69. [PubMed] [Google Scholar]
- 25.Ottemann, K. M., and A. Lowenthal. 2002. Helicobacter pylori uses motility for both initial colonization and to attain robust infection. Infect. Immun. 70:1984-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterson, K. M., and J. J. Mekalanos. 1988. Characterization of the Vibrio cholerae ToxR regulon: identification of novel genes involved in intestinal colonization. Infect. Immun. 56:2822-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pittman, M. S., M. Goodwin, and D. J. Kelly. 2001. Chemotaxis in the human gastric pathogen Helicobacter pylori: different roles for CheW and the three CheV paralogues, and evidence for CheV2 phosphorylation. Microbiology 147:2493-2504. [DOI] [PubMed] [Google Scholar]
- 28.Salama, N. R., G. Otto, L. Tompkins, and S. Falkow. 2001. The vacuolating cytotoxin of Helicobacter pylori plays a role during colonization of a mouse model of infection. Infect. Immun. 69:730-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomb, J.-F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, E. K. Kichey, D. E. Berg, J. D. Gocayne, T. R. Utterback, J. D. Person, J. Kelley, M. D. Cotton, J. M. Weidman, C. Fujii, C. Bowman, L. Wathey, E. Wallin, W. S. Hayes, M. Borodovsky, P. D. Karp, H. O. Smith, C. M. Fraser, and J. C. Venter. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 30.Uemura, N., S. Okamoto, S. Yamamoto, N. Matsumura, S. Yamaguchi, M. Yamakido, K. Taniyama, N. Sasaki, and R. J. Schlemper. 2001. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 345:784-789. [DOI] [PubMed] [Google Scholar]
- 31.Yost, C. K., P. Rochepeau, and M. F. Hynes. 1998. Rhizobium leguminosarum contains a group of genes that appear to code for methyl-accepting chemotaxis proteins. Microbiology 144:1945-1956. [DOI] [PubMed] [Google Scholar]