Abstract
1. Using single barrel pipettes, intracellular records were obtained from surface neurones of isolated rat dorsal root ganglia (DRG) impaled under microscopic vision.
2. Responses to γ-aminobutyric acid (GABA) were elicited either by ionophoresis or by placing drops of concentrated GABA solutions directly into the flow of superfusing Ringer. Using this latter method it was estimated that the GABA concentration eliciting threshold (≃ 1 mV) responses was 3-20 μM.
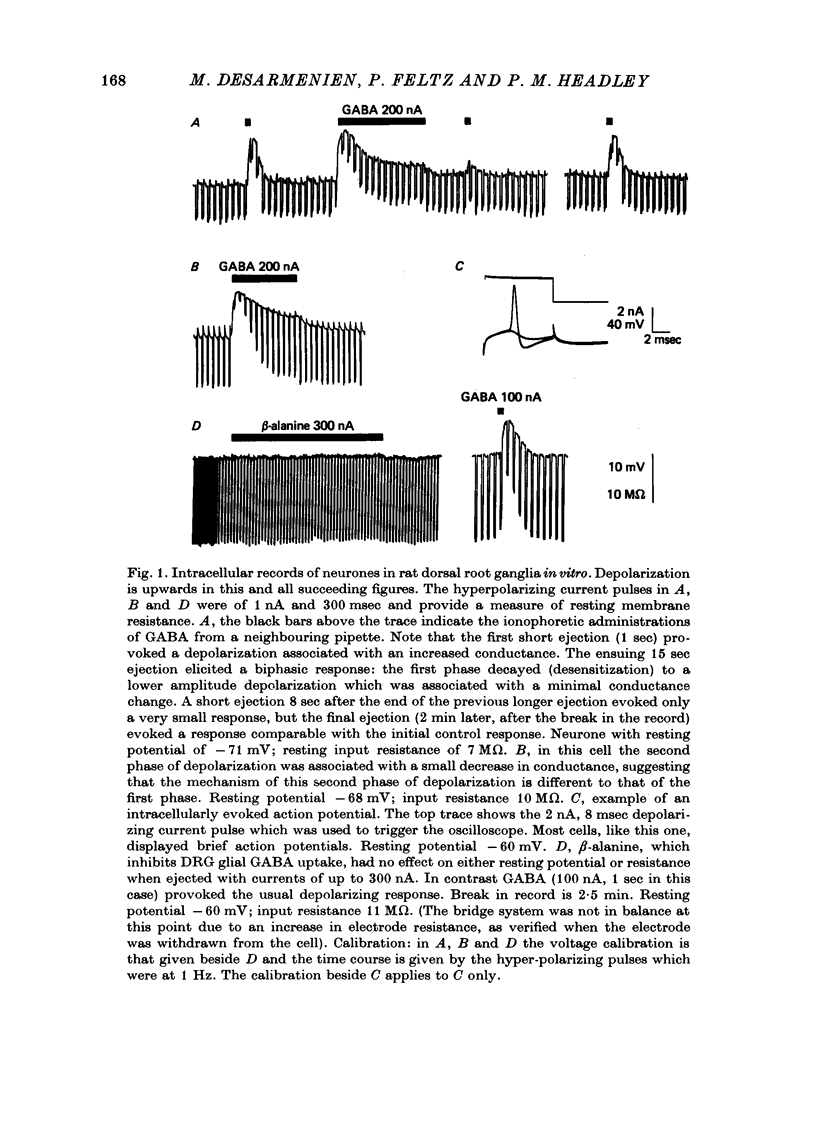
3. Short (≤ 1 sec) ionophoretic or drop administrations of GABA elicited depolarizing responses associated with an increased membrane conductance. With longer applications the initial depolarization was not sustained but decayed to a lower plateau level (desensitization) associated with a minimal conductance change.
4. Low chloride superfusions did not affect subsequent responses to GABA unless GABA was also administered during the low chloride superfusion, in which case responses declined markedly. This suggests that GABA caused appreciable chloride fluxes when it was administered regularly (e.g. for 1 sec every minute).
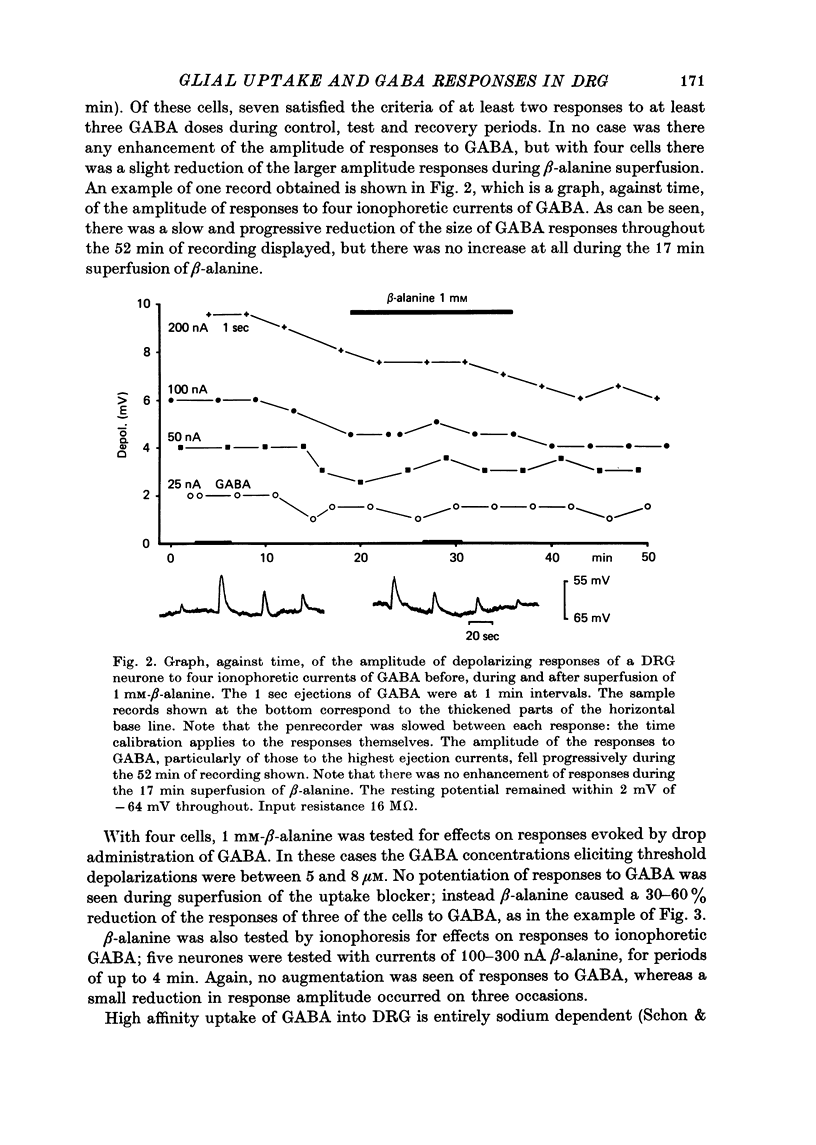
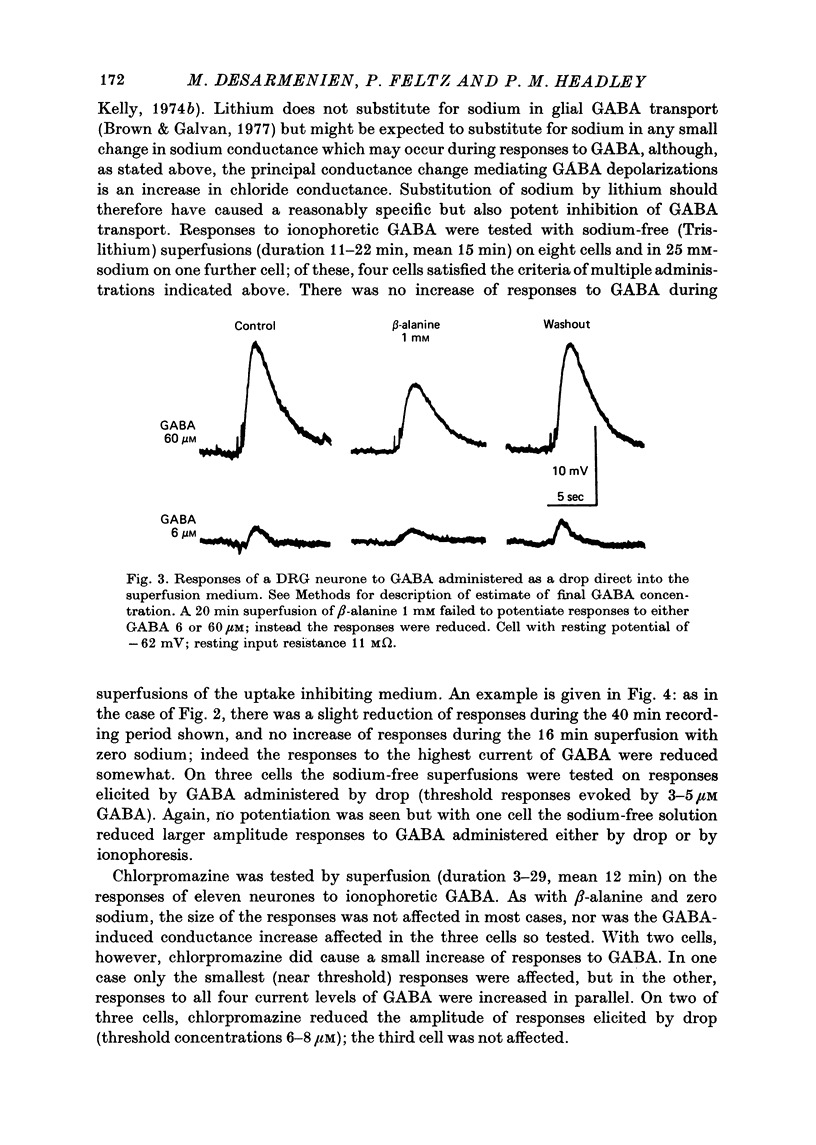
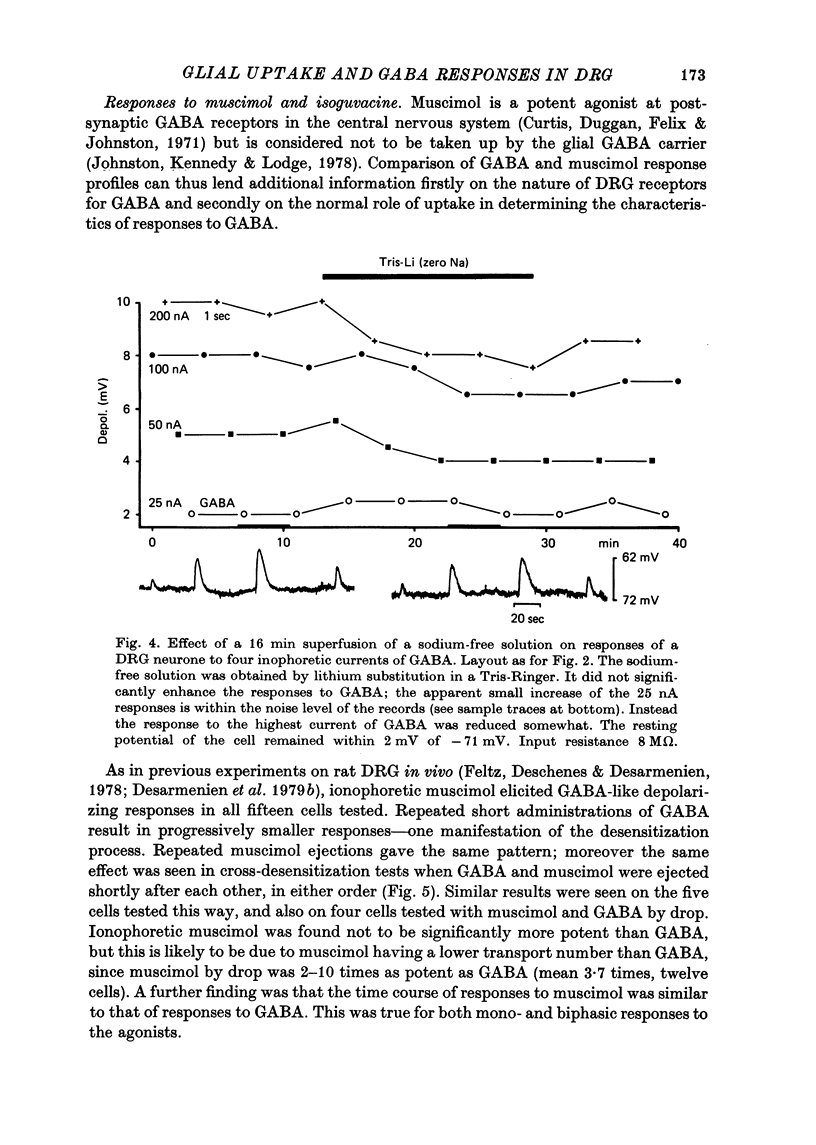
5. Glial GABA uptake was inhibited by adding 1 mM-β-alanine or 0·25 mM-chlorpromazine to the bicarbonate-Ringer superfusate or by substituting lithium for sodium in a Tris-Ringer superfusate. Uptake inhibition had no consistent effect on any of the parameters studied, namely membrane potential, input resistance, amplitude and time course of responses to GABA, and GABA desensitization.
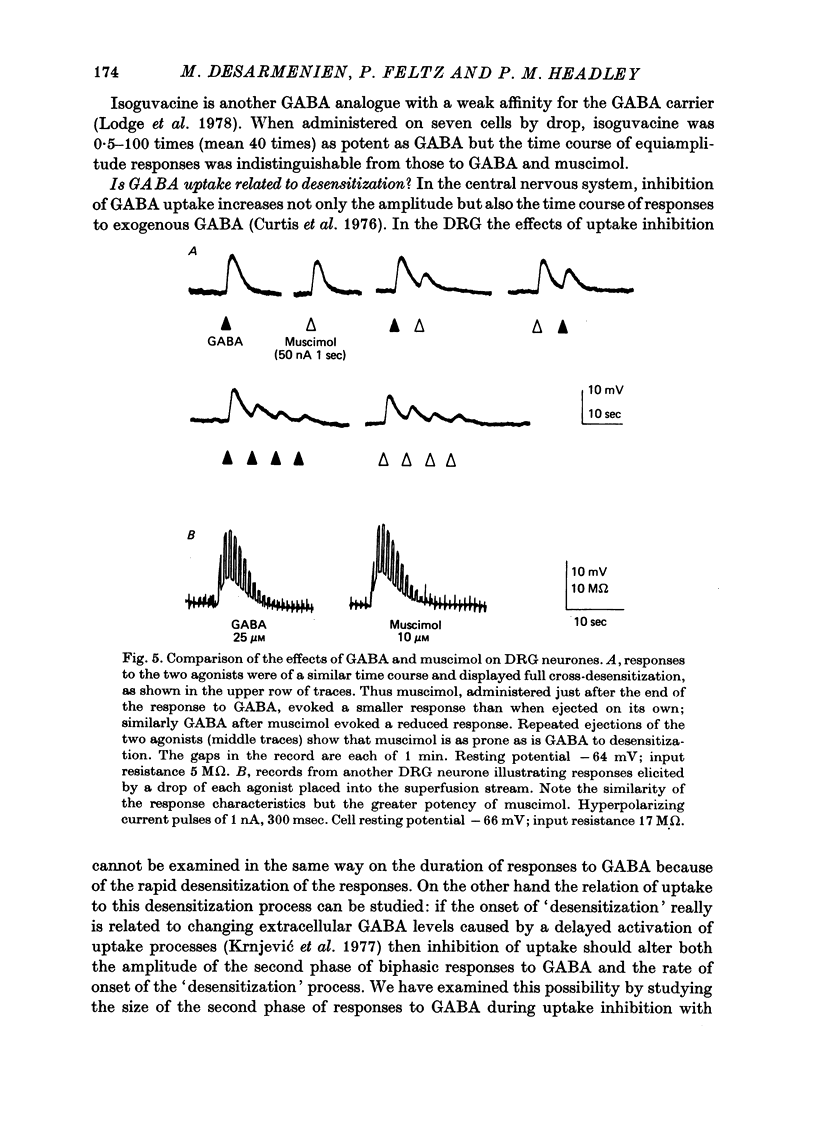
6. Muscimol and isoguvacine, which are probably not substrates for the glial GABA carrier, elicited responses with time course and desensitization characteristics indistinguishable from those of responses to GABA.
7. GABA superfused at concentrations as low as 1 μM could reduce responses to ionophoretic GABA, i.e. cause a desensitization of GABA receptors.
8. It is concluded firstly that in DRG, glial uptake does not affect the amplitude or time course of responses to GABA when the neurone under study is close to the source of GABA; and secondly that desensitization can occur independently of GABA uptake.
9. The findings are discussed in relation to their possible relevance to GABA systems in the central nervous system.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A. Actions of gamma-aminobutyric acid on sympathetic ganglion cells. J Physiol. 1975 Aug;250(1):85–120. doi: 10.1113/jphysiol.1975.sp011044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., Nicoll R. A. The pharmacology and ionic dependency of amino acid responses in the frog spinal cord. J Physiol. 1973 Jan;228(2):259–277. doi: 10.1113/jphysiol.1973.sp010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery N. G., Brown D. A., Collins G. G., Galvan M., Marsh S., Yamini G. Indirect effects of amino-acids on sympathetic ganglion cells mediated through the release of gamma-aminobutyric acid from glial cells. Br J Pharmacol. 1976 May;57(1):73–91. doi: 10.1111/j.1476-5381.1976.tb07658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Galvan M. Influence of neuroglial transport on the action of gamma-aminobutyric acid on mammalian ganglion cells. Br J Pharmacol. 1977 Feb;59(2):373–378. doi: 10.1111/j.1476-5381.1977.tb07502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Marsh S. Axonal GABA-receptors in mammalian peripheral nerve trunks. Brain Res. 1978 Nov 3;156(1):187–191. doi: 10.1016/0006-8993(78)90098-7. [DOI] [PubMed] [Google Scholar]
- CURTIS D. R., PHILLIS J. W., WATKINS J. C. The depression of spinal neurones by gamma-amino-n-butyric acid and beta-alanine. J Physiol. 1959 Apr 23;146(1):185–203. doi: 10.1113/jphysiol.1959.sp006188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowshaw K., Jessup S. J., Ramwell P. W. Thin-layer chromatography of 1-dimethylaminonaphthalene-5-sulphonyl derivatives of amino acids present in superfusates of cat cerebral cortex. Biochem J. 1967 Apr;103(1):79–85. doi: 10.1042/bj1030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Felix D., Johnston G. A. Bicuculline, an antagonist of GABA and synaptic inhibition in the spinal cord of the cat. Brain Res. 1971 Sep 10;32(1):69–96. doi: 10.1016/0006-8993(71)90156-9. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Game C. J., Lodge D. The in vivo inactivation of GABA and other inhibitory amino acids in the cat nervous system. Exp Brain Res. 1976 Jun 30;25(4):413–428. doi: 10.1007/BF00241731. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Johnston G. A. Amino acid transmitters in the mammalian central nervous system. Ergeb Physiol. 1974;69(0):97–188. doi: 10.1007/3-540-06498-2_3. [DOI] [PubMed] [Google Scholar]
- De Groat W. C., Lalley P. M., Saum W. R. Depolarization of dorsal root ganglia in the cat by GABA and related amino acids: antagonism by picrotoxin and bicuculline. Brain Res. 1972 Sep 15;44(1):273–277. doi: 10.1016/0006-8993(72)90383-6. [DOI] [PubMed] [Google Scholar]
- Desarmenien M., Feltz P., Headley P. M. Do glia influence neuronal responses to GABA in the rat? [proceedings]. J Physiol. 1979 Apr;289:58P–59P. [PubMed] [Google Scholar]
- Desarmenien M., Feltz P., Headley P. M. The depolarizing responses to GABA in rat sensory ganglia in vivo and in vitro. A study of the role of glial uptake. J Physiol (Paris) 1979;75(6):661–665. [PubMed] [Google Scholar]
- Deschenes M., Feltz P. GABA-induced rise of extracellular potassium in rat dorsal root ganglia: an electrophysiological study in vivo. Brain Res. 1976 Dec 24;118(3):494–499. doi: 10.1016/0006-8993(76)90319-x. [DOI] [PubMed] [Google Scholar]
- Deschenes M., Feltz P., Lamour Y. A model for an estimate in vivo of the ionic basis of presynaptic inhibition: an intracellular analysis of the GABA-induced depolarization in rat dorsal root ganglia. Brain Res. 1976 Dec 24;118(3):486–493. doi: 10.1016/0006-8993(76)90318-8. [DOI] [PubMed] [Google Scholar]
- Diamond J., Roper S. Analysis of Mauthner cell responses to iontophoretically delivered pulses of GABA, glycine and L-glutamate. J Physiol. 1973 Jul;232(1):113–128. doi: 10.1113/jphysiol.1973.sp010259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreifuss J. J., Kelly J. S., Krnjević K. Cortical inhibition and gamma-aminobutyric acid. Exp Brain Res. 1969;9(2):137–154. doi: 10.1007/BF00238327. [DOI] [PubMed] [Google Scholar]
- Feltz P., Rasminsky M. A model for the mode of action of GABA on primary afferent terminals: depolarizing effects of GABA applied iontophoretically to neurones of mammalian dorsal root ganglia. Neuropharmacology. 1974 Jun;13(6):553–563. doi: 10.1016/0028-3908(74)90145-2. [DOI] [PubMed] [Google Scholar]
- Gallagher J. P., Higashi H., Nishi S. Characterization and ionic basis of GABA-induced depolarizations recorded in vitro from cat primary afferent neurones. J Physiol. 1978 Feb;275:263–282. doi: 10.1113/jphysiol.1978.sp012189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitzemann R. J., Loh H. H. Effects of some conformationally restricted GABA analogues on GABA membrane binding and nerve ending transport. Brain Res. 1978 Apr 7;144(1):63–73. doi: 10.1016/0006-8993(78)90435-3. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Kelly J. S. Uptake and metabolism of gamma-aminobutyric acid by neurones and glial cells. Biochem Pharmacol. 1975 May 1;24(9):933–938. doi: 10.1016/0006-2952(75)90422-0. [DOI] [PubMed] [Google Scholar]
- Johnston G. A., Kennedy S. M., Lodge D. Muscimol uptake, release and binding in rat brain slices. J Neurochem. 1978 Dec;31(6):1519–1523. doi: 10.1111/j.1471-4159.1978.tb06579.x. [DOI] [PubMed] [Google Scholar]
- KRNJEVIC K., PHILLIS J. W. Iontophoretic studies of neurones in the mammalian cerebral cortex. J Physiol. 1963 Feb;165:274–304. doi: 10.1113/jphysiol.1963.sp007057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K., Puil E., Werman R. GABA and glycine actions on spinal motoneurons. Can J Physiol Pharmacol. 1977 Jun;55(3):658–669. doi: 10.1139/y77-090. [DOI] [PubMed] [Google Scholar]
- Lawson S. N., Biscoe T. J., Headley P. M. The effect of electrophoretically applied GABA on cultured dissociated spinal cord and sensory ganglion neurones of the rat. Brain Res. 1976 Dec 3;117(3):493–497. doi: 10.1016/0006-8993(76)90755-1. [DOI] [PubMed] [Google Scholar]
- Lawson S. N., Caddy K. W., Biscoe T. J. Development of rat dorsal root ganglion neurones. Studies of cell birthdays and changes in mean cell diameter. Cell Tissue Res. 1974;153(3):399–413. doi: 10.1007/BF00229167. [DOI] [PubMed] [Google Scholar]
- Levy R. A. The role of GABA in primary afferent depolarization. Prog Neurobiol. 1977;9(4):211–267. doi: 10.1016/0301-0082(77)90002-8. [DOI] [PubMed] [Google Scholar]
- Lodge D., Curtis D. R., Johnston G. A. Does uptake limit the actions of GABA agonists in vivo? Experiments with muscimol, isoguvacine and THIP in cat spinal cord. J Neurochem. 1978 Dec;31(6):1525–1528. doi: 10.1111/j.1471-4159.1978.tb06580.x. [DOI] [PubMed] [Google Scholar]
- Lodge D., Johnston G. A., Curtis D. R., Brand S. J. Effects of the Areca nut constituents arecaidine and guvacine on the action of GABA in the cat central nervous system. Brain Res. 1977 Nov 18;136(3):513–522. doi: 10.1016/0006-8993(77)90075-0. [DOI] [PubMed] [Google Scholar]
- Minchin M. C. Factors influencing the efflux of [3H]gamma-aminobutyric acid from satellite glial cells in rat sensory ganglia. J Neurochem. 1975 Mar;24(3):571–577. doi: 10.1111/j.1471-4159.1975.tb07676.x. [DOI] [PubMed] [Google Scholar]
- Minchin M. C., Iversen L. L. Release of (3H)gamma-aminobutyric acid from glial cells in rat dorsal root ganglia. J Neurochem. 1974 Sep;23(3):533–540. doi: 10.1111/j.1471-4159.1974.tb06056.x. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. The blockade of GABA mediated responses in the frog spinal cord by ammonium ions and furosemide. J Physiol. 1978 Oct;283:121–132. doi: 10.1113/jphysiol.1978.sp012491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi S., Minota S., Karczmar A. G. Primary afferent neurones: the ionic mechanism of GABA-mediated depolarization. Neuropharmacology. 1974 Mar;13(3):215–219. doi: 10.1016/0028-3908(74)90110-5. [DOI] [PubMed] [Google Scholar]
- Obata K. Transmitter sensitivities of some nerve and muscle cells in culture. Brain Res. 1974 Jun 14;73(1):71–88. doi: 10.1016/0006-8993(74)91008-7. [DOI] [PubMed] [Google Scholar]
- Olsen R. W., Ticku M. K., Van Ness P. C., Greenlee D. Effects of drugs on gamma-aminobutyric acid receptors, uptake, release and synthesis in vitro. Brain Res. 1978 Jan 13;139(2):277–294. doi: 10.1016/0006-8993(78)90929-0. [DOI] [PubMed] [Google Scholar]
- Ransom B. R., Bullock P. N., Nelson P. G. Mouse spinal cord in cell culture. III. Neuronal chemosensitivity and its relationship to synaptic activity. J Neurophysiol. 1977 Sep;40(5):1163–1177. doi: 10.1152/jn.1977.40.5.1163. [DOI] [PubMed] [Google Scholar]
- Schousboe A., Krogsgaard-Larsen P., Svenneby G., Hertz L. Inhibition of the high-affinity, net uptake of GABA into cultured astrocytes by beta-proline, nipecotic acid and other compounds. Brain Res. 1978 Sep 29;153(3):623–626. doi: 10.1016/0006-8993(78)90349-9. [DOI] [PubMed] [Google Scholar]
- Yoshida S., Matsuda Y., Samejima A. Tetrodotoxin-resistant sodium and calcium components of action potentials in dorsal root ganglion cells of the adult mouse. J Neurophysiol. 1978 Sep;41(5):1096–1106. doi: 10.1152/jn.1978.41.5.1096. [DOI] [PubMed] [Google Scholar]
- Yoshida S., Matsuda Y. Studies on sensory neurons of the mouse with intracellular-recording and horseradish peroxidase-injection techniques. J Neurophysiol. 1979 Jul;42(4):1134–1145. doi: 10.1152/jn.1979.42.4.1134. [DOI] [PubMed] [Google Scholar]


