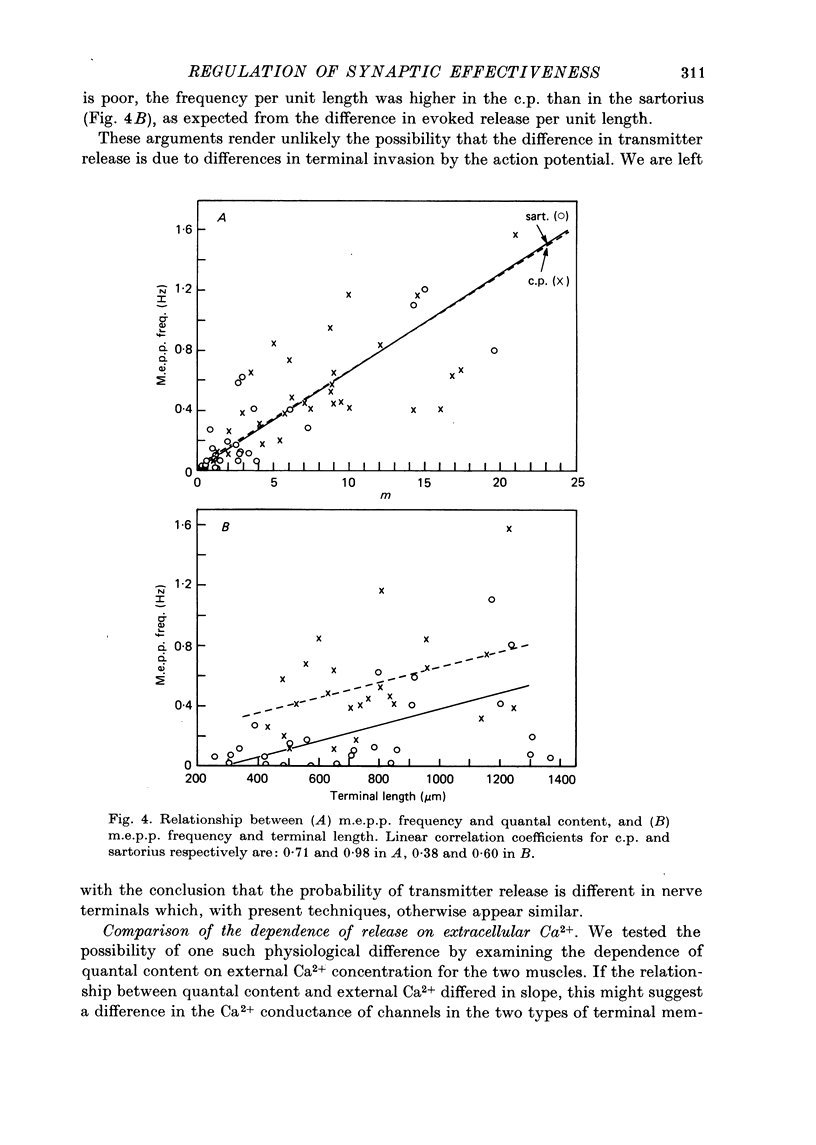
Abstract
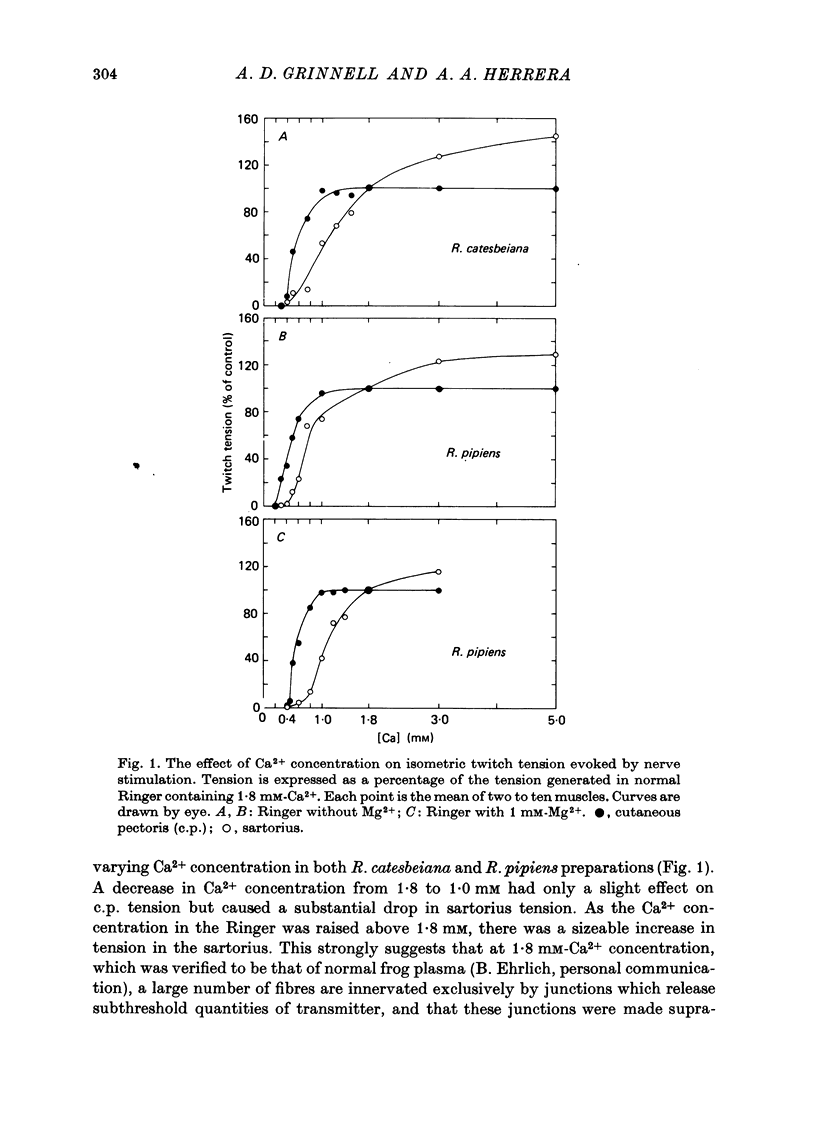
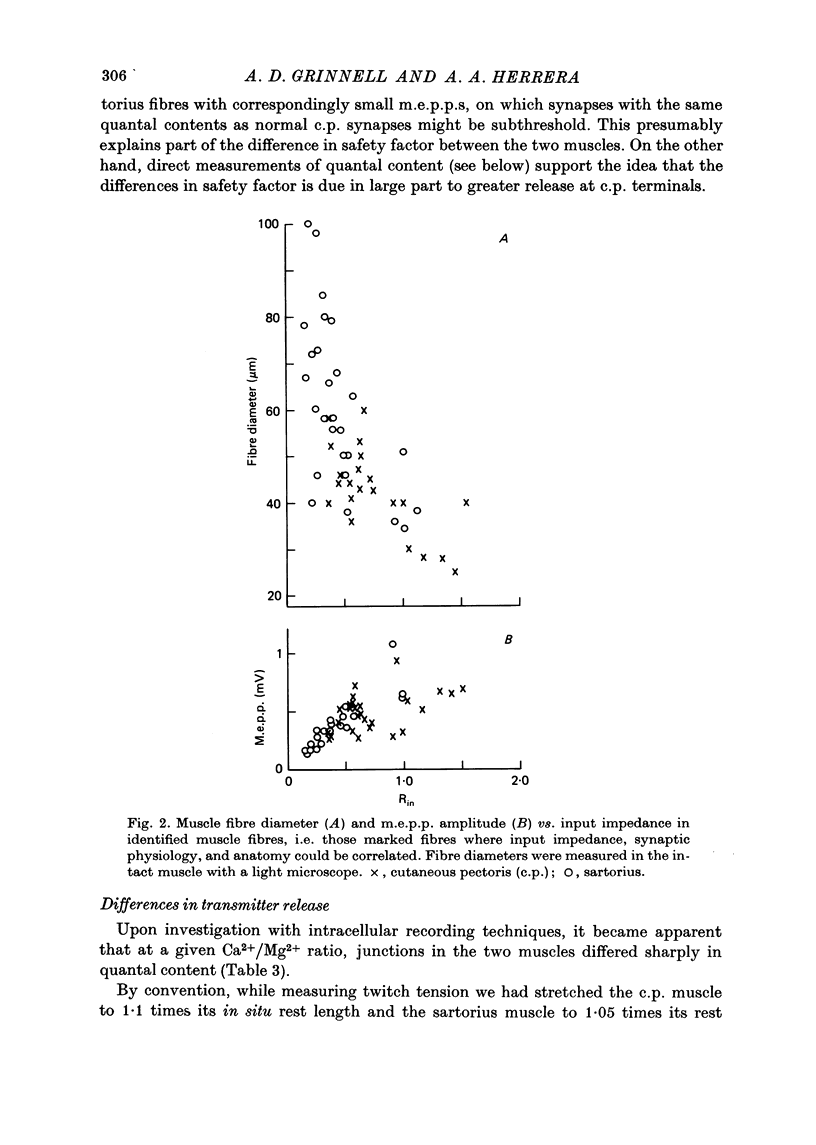
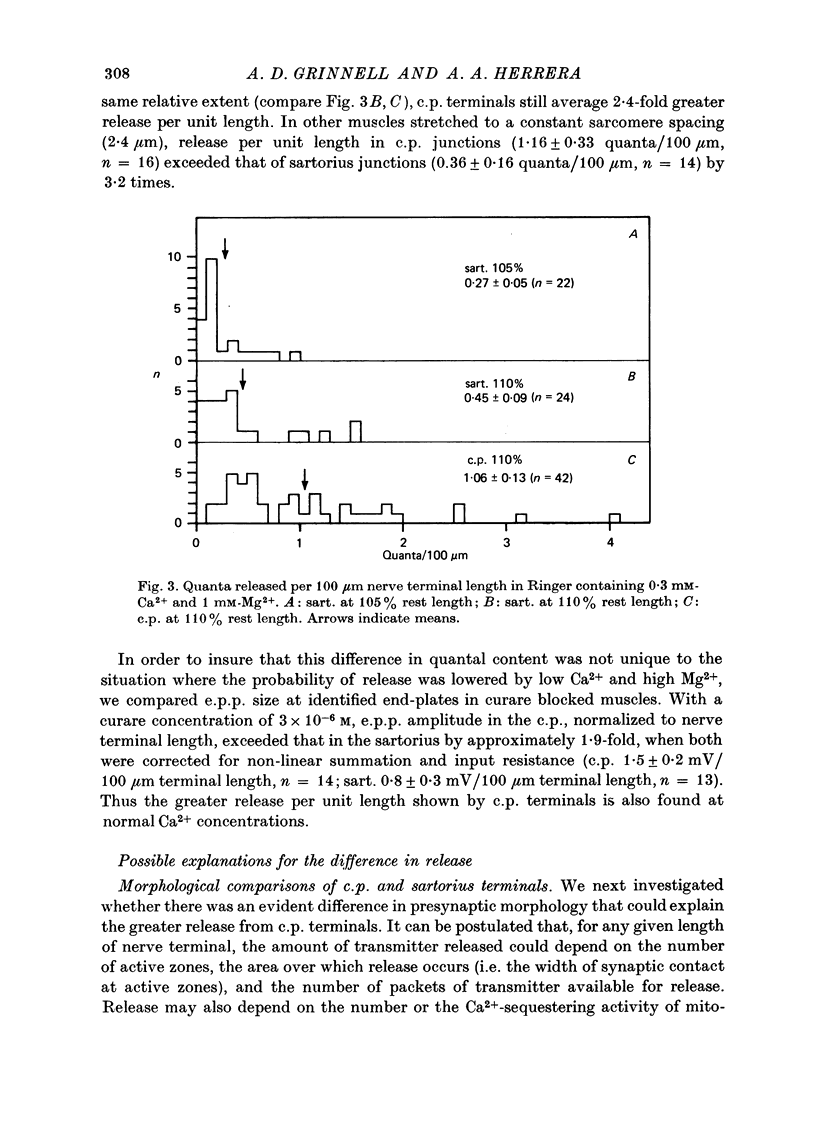
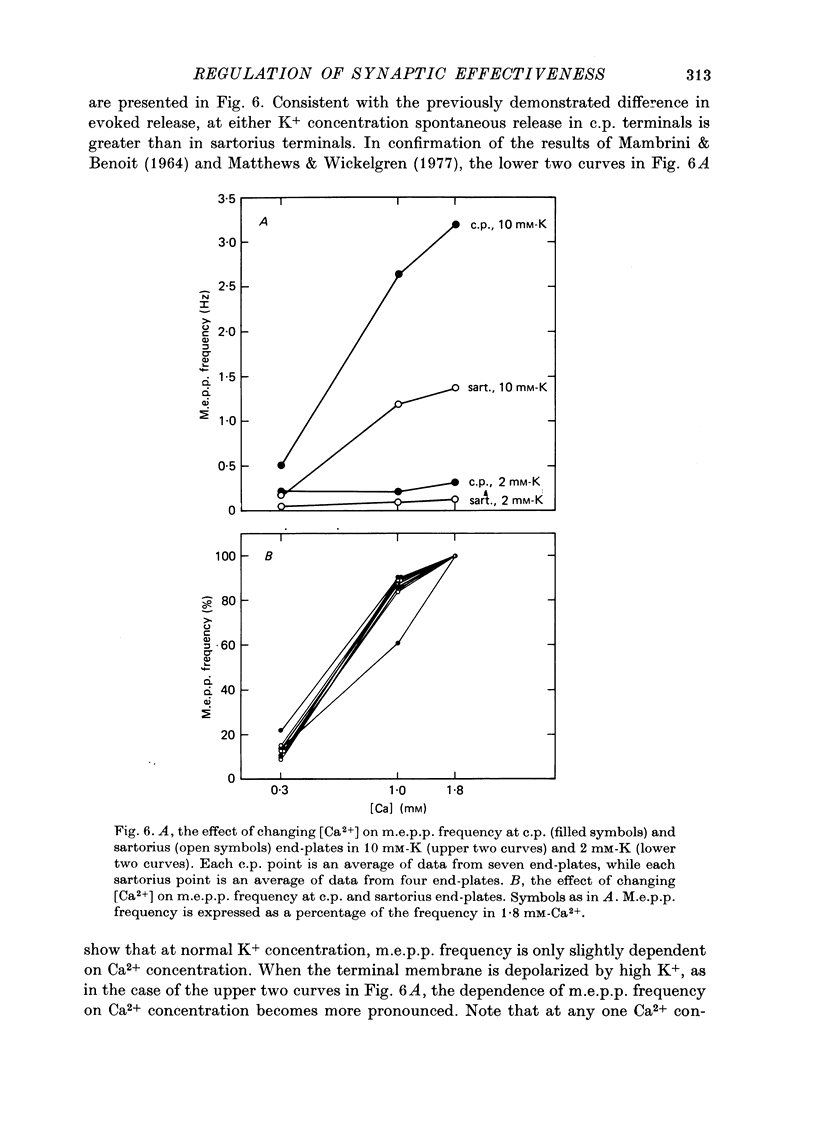
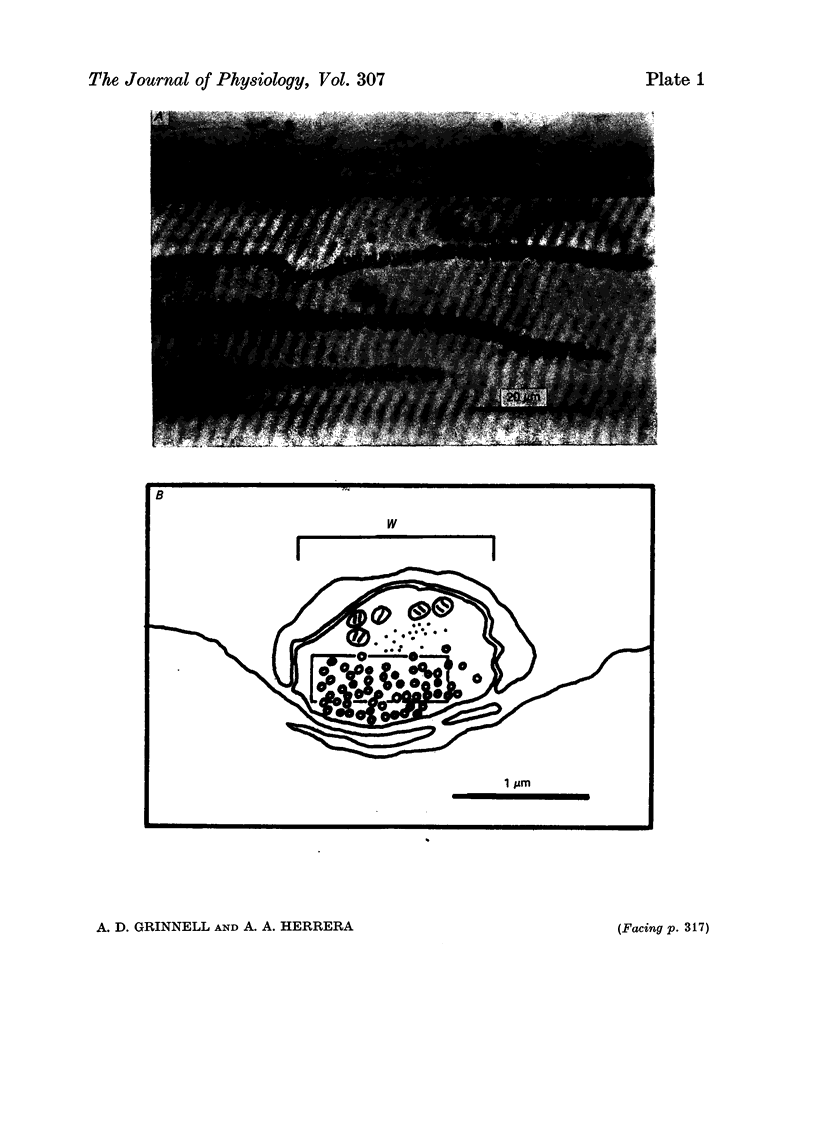
1. Nerve terminals in two different muscles of the frog, the sartorius and cutaneous pectoris (c.p.), have been found to differ sharply in safety factor. This difference is shown to be attributable to corresponding disparities in the amount of transmitter released, without evident correlated morphological differences. 2. In Ringer containing 0.3 mM-Ca2+ and 1 mM-Mg2+, quantal content of c.p. junctions exceeded that of sartorius junctions by 3-4 times. 3. When quantal content was corrected for nerve terminal size, c.p. terminals still released 2-4 times more transmitter per unit terminal length. 4. Light and electron microscopic examination of junctional morphology in the two muscles revealed no significant difference in the spacing of presynaptic active zones, the width of synaptic contact, or the density of presynaptic vesicles and mitochondria. It seems likely, therefore, that the greater release at c.p. junctions is due to a 'physiological' difference between the two populations of terminals. 5. No evidence could be found that action potential invasion of the terminal was less complete in the sartorius than in the c.p. 6. The dependence of evoked and spontaneous release on Ca2+ concentration was of similar slope for terminals in the two muscles, but of different absolute value, consistent with the observed difference in release.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alnaes E., Rahamimoff R. On the role of mitochondria in transmitter release from motor nerve terminals. J Physiol. 1975 Jun;248(2):285–306. doi: 10.1113/jphysiol.1975.sp010974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Lavidis N. A. The effect of calcium ions on the secretion of quanta evoked by an impulse at nerve terminal release sites. J Gen Physiol. 1979 Oct;74(4):429–456. doi: 10.1085/jgp.74.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Raftos J. The formation and regression of synapses during the re-innervation of axolotl striated muscles. J Physiol. 1977 Feb;265(2):261–295. doi: 10.1113/jphysiol.1977.sp011716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Miledi R. Characteristics of transmitter release at regenerating frog neuromuscular junctions. J Physiol. 1974 Jun;239(3):571–594. doi: 10.1113/jphysiol.1974.sp010583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell A. D., Letinsky M. S., Rheuben M. B. Competitive interaction between foreign nerves innervating frog skeletal muscle. J Physiol. 1979 Apr;289:241–262. doi: 10.1113/jphysiol.1979.sp012735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell A. D., Rheuben M. B., Letinsky M. S. Mutual repression of synaptic efficacy by pairs of foreign nerves innervating frog skeletal muscle. Nature. 1977 Jan 27;265(5592):368–370. doi: 10.1038/265368a0. [DOI] [PubMed] [Google Scholar]
- HUTTER O. F., TRAUTWEIN W. Neuromuscular facilitation by stretch of motor nerve-endings. J Physiol. 1956 Sep 27;133(3):610–625. doi: 10.1113/jphysiol.1956.sp005613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARNOVSKY M. J. THE LOCALIZATION OF CHOLINESTERASE ACTIVITY IN RAT CARDIAC MUSCLE BY ELECTRON MICROSCOPY. J Cell Biol. 1964 Nov;23:217–232. doi: 10.1083/jcb.23.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. PROPAGATION OF ELECTRIC ACTIVITY IN MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:453–482. doi: 10.1098/rspb.1965.0015. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. On the factors which determine the amplitude of the miniature end-plate potential. J Physiol. 1957 Jul 11;137(2):267–278. doi: 10.1113/jphysiol.1957.sp005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Turkanis S. A., Weakly J. N. Correlation between nerve terminal size and transmitter release at the neuromuscular junction of the frog. J Physiol. 1971 Mar;213(3):545–556. doi: 10.1113/jphysiol.1971.sp009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAMBRINI J., BENOIT P. R. ACTION DU CALCIUM SUR LA JONCTION NEURO-MUSCULAIRE CHEZ LA GRENOUILLE. C R Seances Soc Biol Fil. 1964;158:1454–1458. [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G., Wickelgren W. O. On the effect of calcium on the frequency of miniature end-plate potentials at the frog neuromuscular junction. J Physiol. 1977 Mar;266(1):91–101. doi: 10.1113/jphysiol.1977.sp011757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper K., Dreyer F., Sandri C., Akert K., Moor H. Structure and ultrastructure of the frog motor endplate. A freeze-etching study. Cell Tissue Res. 1974 Jun 24;149(4):437–455. doi: 10.1007/BF00223024. [DOI] [PubMed] [Google Scholar]
- Rahamimoff R. A dual effect of calcium ions on neuromuscular facilitation. J Physiol. 1968 Mar;195(2):471–480. doi: 10.1113/jphysiol.1968.sp008468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkanis S. A. Effects of muscle stretch on transmitter release at end-plates of rat diaphragm and frog sartorius muscle. J Physiol. 1973 Apr;230(2):391–403. doi: 10.1113/jphysiol.1973.sp010194. [DOI] [PMC free article] [PubMed] [Google Scholar]