Abstract
1. Responses were recorded from individual tactile afferent fibres isolated by microdissection from the median nerve of pentobarbitone-anaesthetized neonatal kittens (1-5 days post-natal age). Experiments were also conducted on adult cats to permit precise comparisons between neonatal and adult fibres.
2. Neonatal fibres with receptive fields on the glabrous skin of the foot pads were classified into two broad groups, a slowly adapting class (40%) which responded throughout a 1 sec period of steady indentation and a rapidly adapting or dynamically sensitive class comprising 60% of units. Fibres in these two groups had overlapping conduction velocities in the range 4·3 to 7·5 m/sec and were believed to be the developing Group II afferents of the adult.
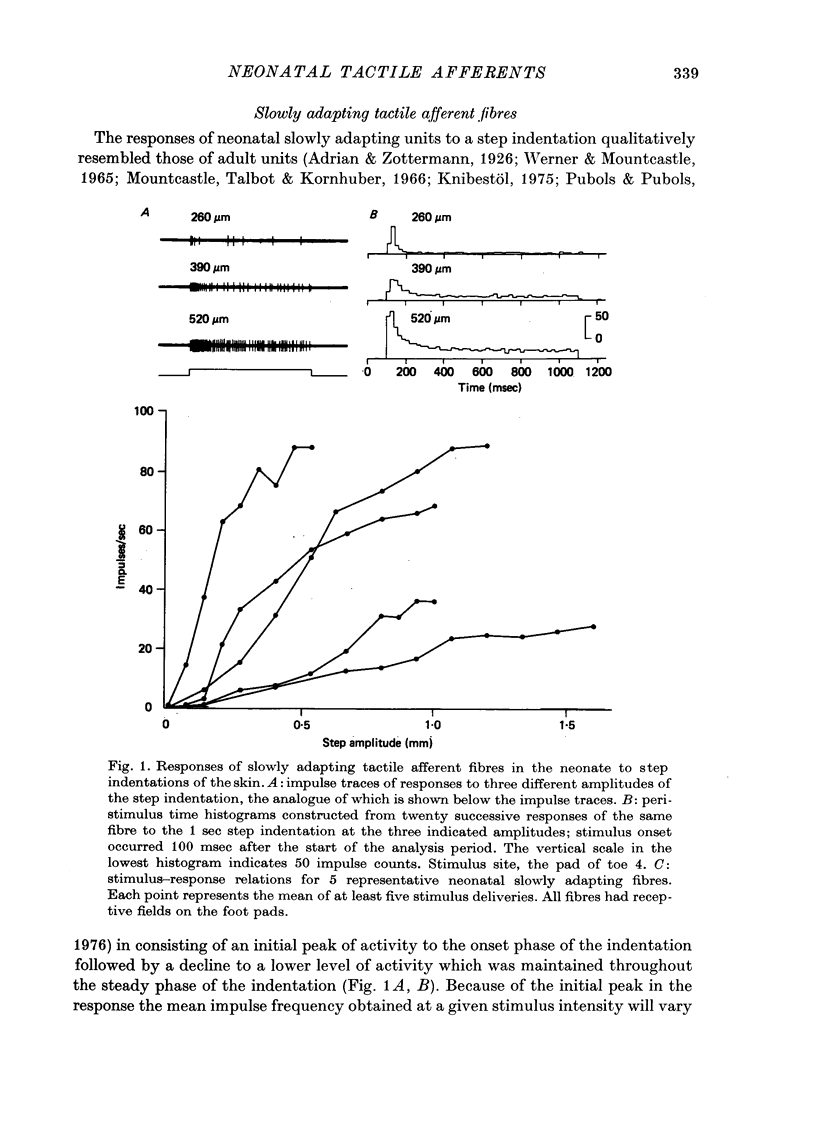
3. Neonatal slowly adapting fibres qualitatively resembled their adult counter-parts. They displayed graded stimulus-response relations which, over the steepest segment of the curves, had mean slopes of 15·7 impulses/100 μm of indentation. Plateau levels of response were often reached at amplitudes of skin indentation of < 0·5-0·7 mm.
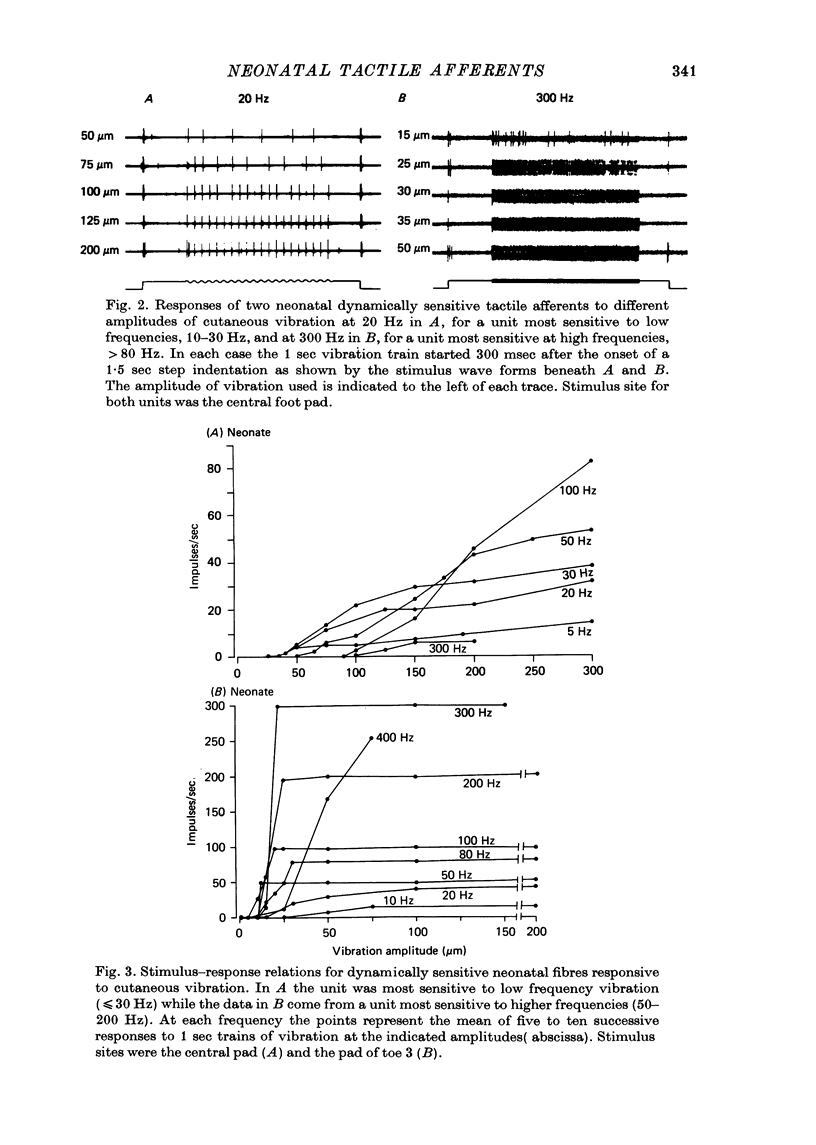
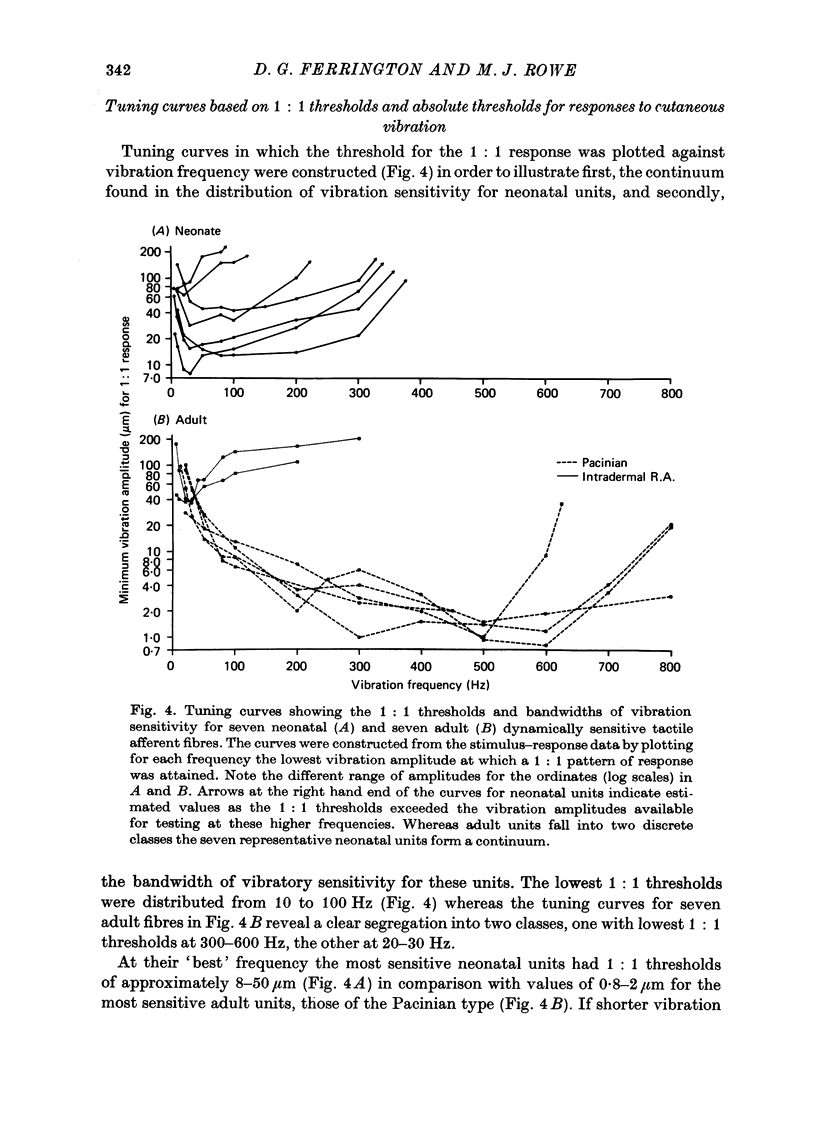
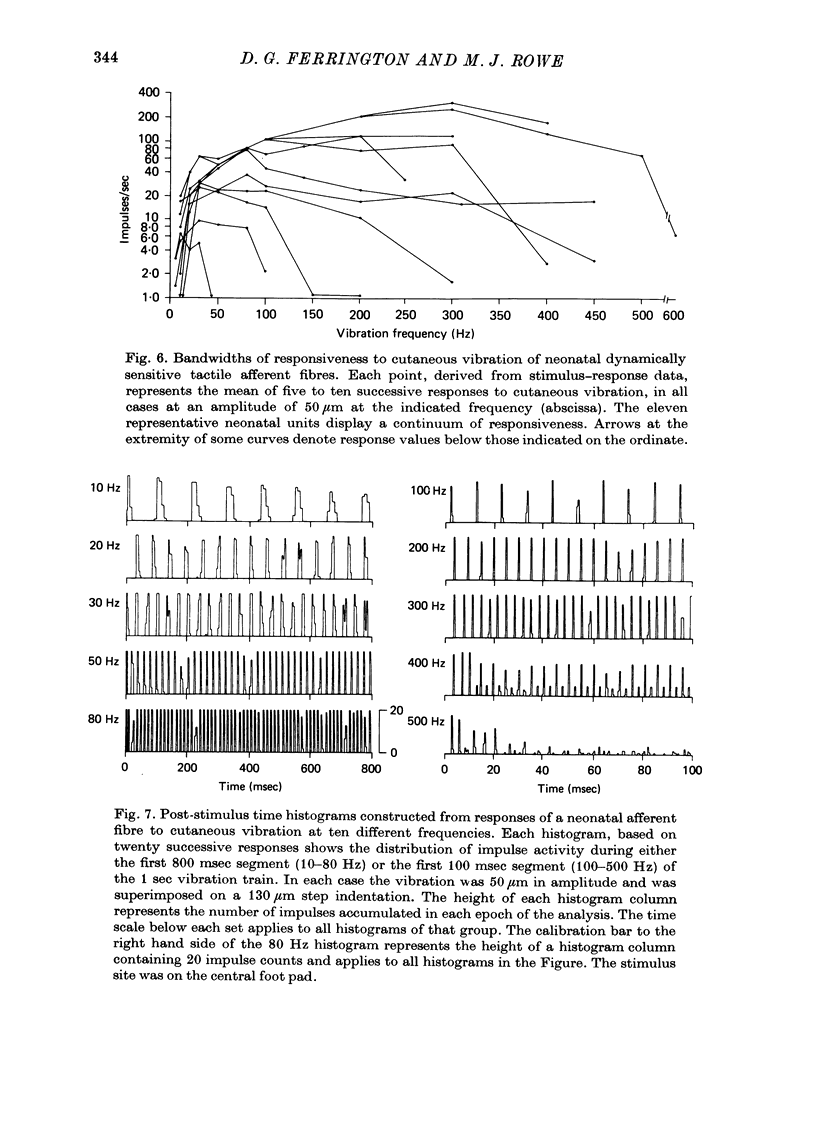
4. Dynamically sensitive fibres with receptive fields on the glabrous skin were studied using sinusoidal cutaneous vibration which in the adult enables them to be divided into two distinct classes. However, in the neonate, they formed a continuum whether criteria of sensitivity or responsiveness were used.
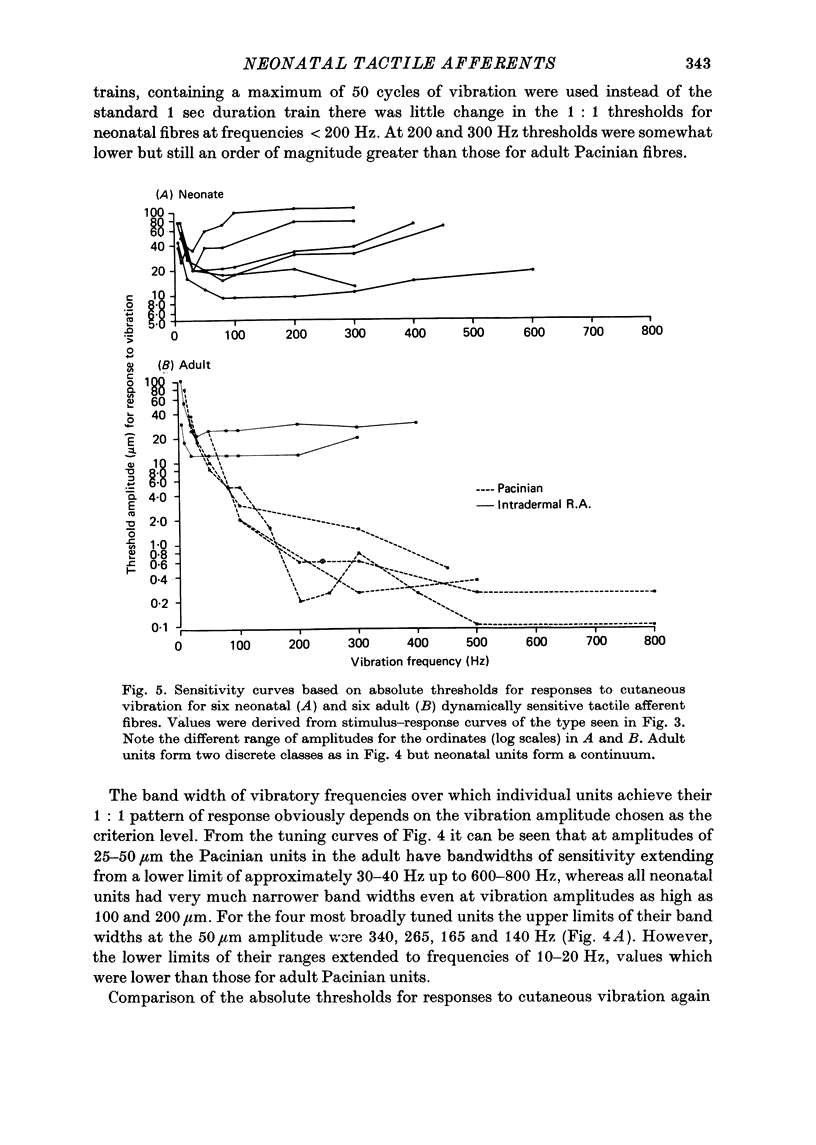
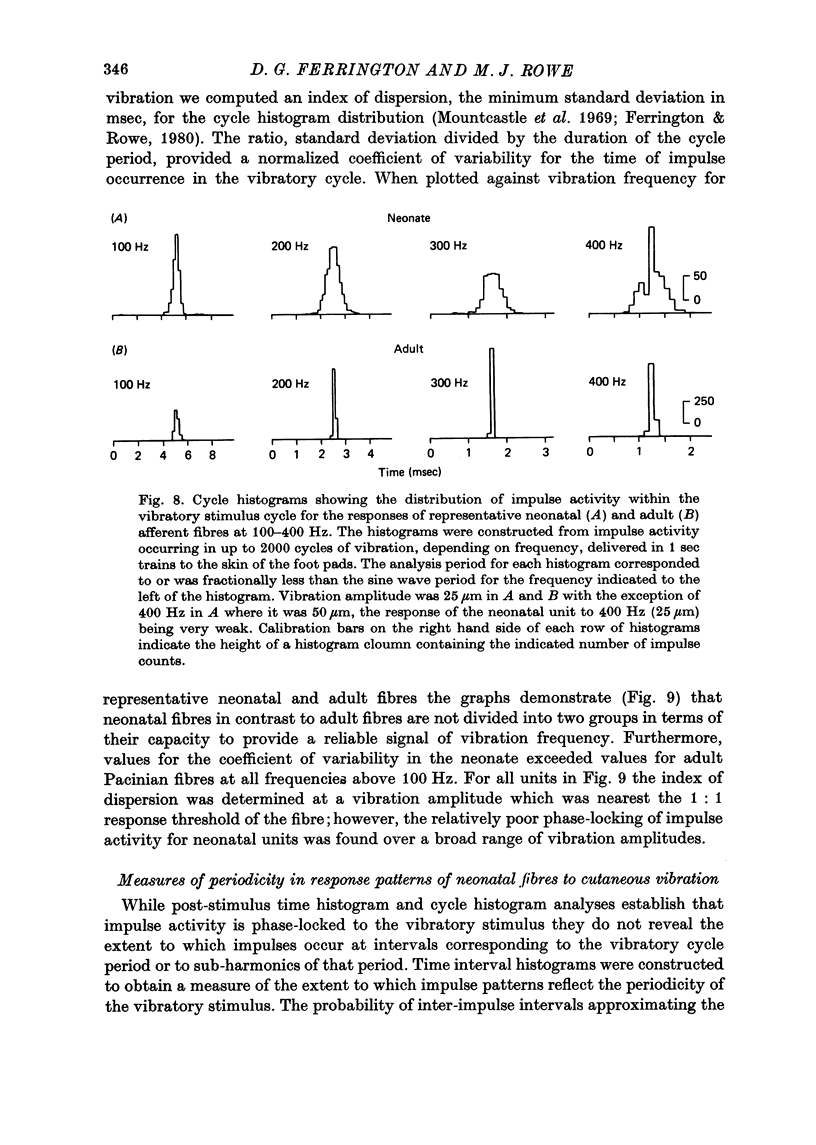
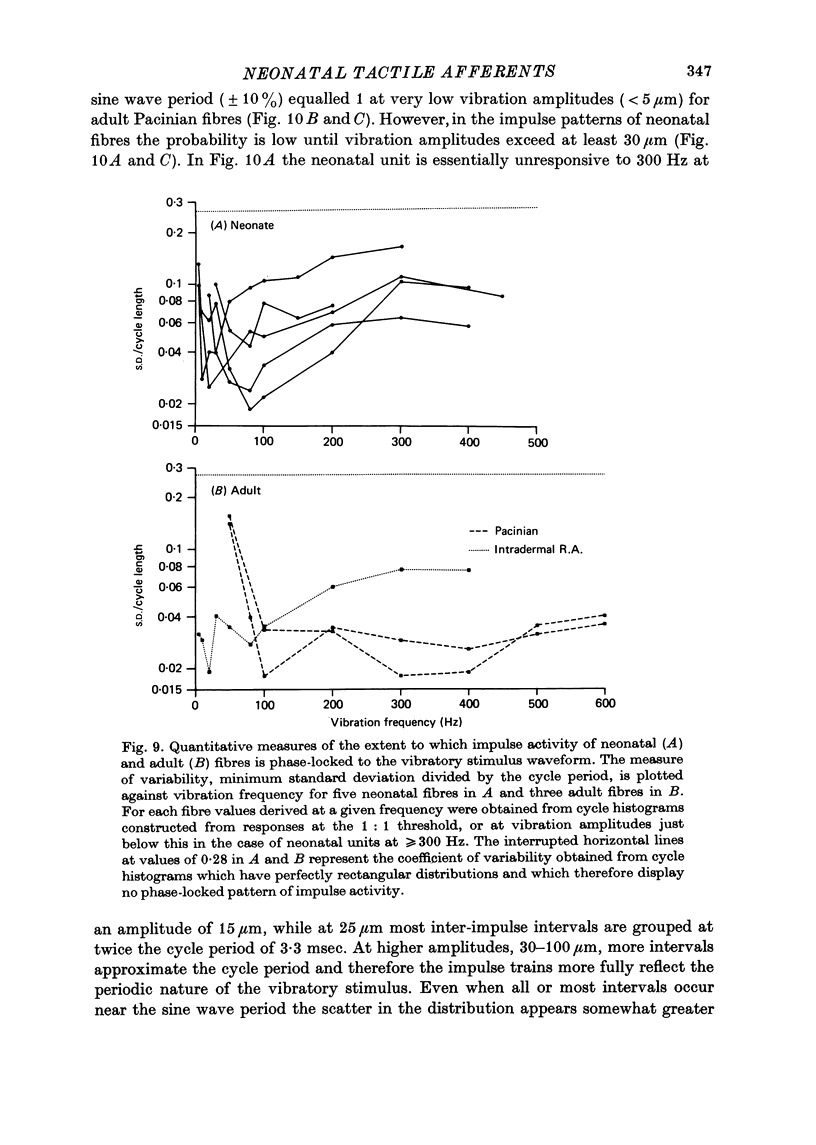
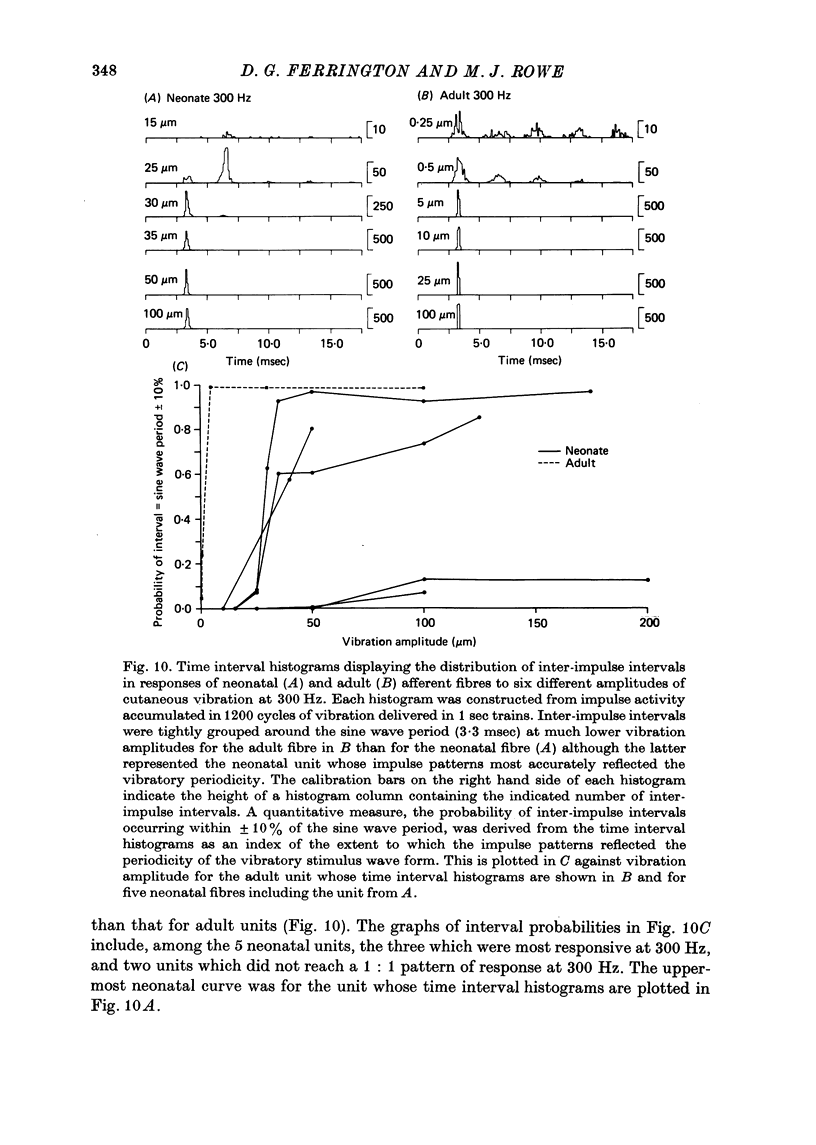
5. In response to vibration neonatal fibres differed from adult ones according to the following quantitative indices: (i) sensitivity as measured by both absolute thresholds and thresholds for a 1: 1 pattern of response, both of which were higher in the neonate than in the adult at all frequencies > 50 Hz and differed by an order of magnitude at frequencies ≥ 200 Hz; (ii) responsiveness based on the mean impulse rate evoked at a fixed amplitude of cutaneous vibration; (iii) band width of vibratory sensitivity which in the neonate was confined to approximately 5-300 Hz whereas in the two classes of adult units it covered the range 5-800 Hz; (iv) capacity for coding information about vibration frequency. Impulse activity of neonatal fibres was less tightly phase-locked to the vibratory stimulus and showed a poorer reflection of the periodic nature of the vibratory stimulus than impulse patterns of adult units.
6. The results reveal that tactile receptors and afferent fibres in the neonate are functionally immature. Their restricted coding capacities suggest that peripheral tactile sensory mechanisms impose limits on the ability of the new-born animal to derive information about its tactile environment.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian E. D., Zotterman Y. The impulses produced by sensory nerve endings: Part 3. Impulses set up by Touch and Pressure. J Physiol. 1926 Aug 6;61(4):465–483. doi: 10.1113/jphysiol.1926.sp002308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitel R. E., Gibson J. M., Welker W. I. Functional development of mechanoreceptive neurons innervating the glabrous skin in postnatal kittens. Brain Res. 1977 Jul 1;129(2):213–226. doi: 10.1016/0006-8993(77)90002-6. [DOI] [PubMed] [Google Scholar]
- Bennett R. E., Ferrington D. G., Rowe M. Tactile neuron classes within second somatosensory area (SII) of cat cerebral cortex. J Neurophysiol. 1980 Feb;43(2):292–309. doi: 10.1152/jn.1980.43.2.292. [DOI] [PubMed] [Google Scholar]
- Bystrzycka E., NAil B. S., Rowe M. Inhibition of cuneate neurones: its afferent source and influence on dynamically sensitive "tactile" neurones. J Physiol. 1977 Jun;268(1):251–270. doi: 10.1113/jphysiol.1977.sp011856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmody J., Rowe M. Inhibition within the trigeminal nucleus induced by afferent inputs and its influence on stimulus coding by mechanosensitive neurones. J Physiol. 1974 Nov;243(1):195–210. doi: 10.1113/jphysiol.1974.sp010749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darian-Smith I., Rowe M. J., Sessle B. J. "Tactile" stimulus intensity: information transmission by relay neurons in different trigeminal nuclei. Science. 1968 May 17;160(3829):791–794. doi: 10.1126/science.160.3829.791. [DOI] [PubMed] [Google Scholar]
- Douglas P. R., Ferrington D. G., Rowe M. Coding of information about tactile stimuli by neurones of the cuneate nucleus. J Physiol. 1978 Dec;285:493–513. doi: 10.1113/jphysiol.1978.sp012585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekholm J. Postnatal changes in cutaneous reflexes and in the discharge pattern of cutaneous and articular sense organs. A morphological and physiological study in the cat. Acta Physiol Scand Suppl. 1967;297:1–130. [PubMed] [Google Scholar]
- Ferrington D. G., Nail B. S., Rowe M. Human tactile detection thresholds: modification by inputs from specific tactile receptor classes. J Physiol. 1977 Nov;272(2):415–433. doi: 10.1113/jphysiol.1977.sp012052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrington D. G., Rowe M. Differential contributions to coding of cutaneous vibratory information by cortical somatosensory areas I and II. J Neurophysiol. 1980 Feb;43(2):310–331. doi: 10.1152/jn.1980.43.2.310. [DOI] [PubMed] [Google Scholar]
- Gibson J. M., Beitel R. E., Welker W. Diversity of coding profiles of mechanoreceptors in glabrous skin of kittens. Brain Res. 1975 Mar 21;86(2):181–203. doi: 10.1016/0006-8993(75)90696-4. [DOI] [PubMed] [Google Scholar]
- HUNT C. C., McINTYRE A. K. Characteristics of responses from receptors from the flexor longus digitorum muscle and the adjoining interosseous region of the cat. J Physiol. 1960 Aug;153:74–87. doi: 10.1113/jphysiol.1960.sp006519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C. On the nature of vibration receptors in the hind limb of the cat. J Physiol. 1961 Jan;155:175–186. doi: 10.1113/jphysiol.1961.sp006621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A., Ogawa H. Correlative physiological and morphological studies of rapidly adapting mechanoreceptors in cat's glabrous skin. J Physiol. 1977 Apr;266(2):275–296. doi: 10.1113/jphysiol.1977.sp011768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänig W. Morphology of rapidly and slowly adapting mechanoreceptors in the hairless skin of the cat's hind foot. Brain Res. 1971 May 7;28(2):217–231. doi: 10.1016/0006-8993(71)90656-1. [DOI] [PubMed] [Google Scholar]
- Jänig W., Schmidt R. F., Zimmermann M. Single unit responses and the total afferent outflow from the cat's foot pad upon mechanical stimulation. Exp Brain Res. 1968;6(2):100–115. doi: 10.1007/BF00239165. [DOI] [PubMed] [Google Scholar]
- Kasprzak H., Tapper D. N., Craig P. H. Functional development of the tactile pad receptor system. Exp Neurol. 1970 Mar;26(3):439–446. doi: 10.1016/0014-4886(70)90140-8. [DOI] [PubMed] [Google Scholar]
- Kruger L., Kenton B. Quantitative neural and psychophysical data for cutaneous mechanoreceptor function. Brain Res. 1973 Jan 15;49(1):1–24. doi: 10.1016/0006-8993(73)90398-3. [DOI] [PubMed] [Google Scholar]
- LaMotte R. H., Mountcastle V. B. Capacities of humans and monkeys to discriminate vibratory stimuli of different frequency and amplitude: a correlation between neural events and psychological measurements. J Neurophysiol. 1975 May;38(3):539–559. doi: 10.1152/jn.1975.38.3.539. [DOI] [PubMed] [Google Scholar]
- Lindblom U., Lund L. The discharge from vibration-sensitive receptors in the monkey foot. Exp Neurol. 1966 Aug;15(4):401–417. doi: 10.1016/0014-4886(66)90138-5. [DOI] [PubMed] [Google Scholar]
- Lynn B. The nature and location of certain phasic mechanoreceptors in the cat's foot. J Physiol. 1969 May;201(3):765–773. doi: 10.1113/jphysiol.1969.sp008786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinovský L. Ein Beitrag zur Entwicklung einfacher sensibler Körperchen bei der Hauskatze (Felis silvestris f. catus L.) Acta Anat (Basel) 1970;76(2):220–235. [PubMed] [Google Scholar]
- Malinovský L., Sommerová J. Die postnatale Entwicklung der Vater-Pacinischen Körperchen in den Fussballen der Hauskatze (Felis silvestris f. catus L. Acta Anat (Basel) 1972;81(2):183–201. [PubMed] [Google Scholar]
- Mountcastle V. B., Talbot W. H., Sakata H., Hyvärinen J. Cortical neuronal mechanisms in flutter-vibration studied in unanesthetized monkeys. Neuronal periodicity and frequency discrimination. J Neurophysiol. 1969 May;32(3):452–484. doi: 10.1152/jn.1969.32.3.452. [DOI] [PubMed] [Google Scholar]
- Munger B. L., Pubols L. M., Pubols B. H. The Merkel rete papilla--a slowly adapting sensory receptor in mammalian glabrous skin. Brain Res. 1971 Jun 4;29(1):47–61. doi: 10.1016/0006-8993(71)90416-1. [DOI] [PubMed] [Google Scholar]
- Munger B. L., Pubols L. M. The sensorineural organization of the digital skin of the raccoon. Brain Behav Evol. 1972;5(4):367–393. doi: 10.1159/000123757. [DOI] [PubMed] [Google Scholar]
- Pubols B. H., Pubols L. M. Coding of mechanical stimulus velocity and indentation depth by squirrel monkey and raccoon glabrous skin mechanoreceptors. J Neurophysiol. 1976 Jul;39(4):773–787. doi: 10.1152/jn.1976.39.4.773. [DOI] [PubMed] [Google Scholar]
- Pubols L. M., Pubols B. H., Jr, Munger B. L. Functional properties of mechanoreceptors in glabrous skin of the raccoon's forepaw. Exp Neurol. 1971 May;31(2):165–182. doi: 10.1016/0014-4886(71)90185-3. [DOI] [PubMed] [Google Scholar]
- Rose J. E., Brugge J. F., Anderson D. J., Hind J. E. Phase-locked response to low-frequency tones in single auditory nerve fibers of the squirrel monkey. J Neurophysiol. 1967 Jul;30(4):769–793. doi: 10.1152/jn.1967.30.4.769. [DOI] [PubMed] [Google Scholar]
- SATO M. Response of Pacinian corpuscles to sinusoidal vibration. J Physiol. 1961 Dec;159:391–409. doi: 10.1113/jphysiol.1961.sp006817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHERRICK C. E., Jr Variables affecting sensitivity of the human skin to mechanical vibration. J Exp Psychol. 1953 May;45(5):273–282. doi: 10.1037/h0059511. [DOI] [PubMed] [Google Scholar]
- Talbot W. H., Darian-Smith I., Kornhuber H. H., Mountcastle V. B. The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J Neurophysiol. 1968 Mar;31(2):301–334. doi: 10.1152/jn.1968.31.2.301. [DOI] [PubMed] [Google Scholar]
- WERNER G., MOUNTCASTLE V. B. NEURAL ACTIVITY IN MECHANORECEPTIVE CUTANEOUS AFFERENTS: STIMULUS-RESPONSE RELATIONS, WEBER FUNCTIONS, AND INFORMATION TRANSMISSION. J Neurophysiol. 1965 Mar;28:359–397. doi: 10.1152/jn.1965.28.2.359. [DOI] [PubMed] [Google Scholar]
- Zelená J. The development of Pacinian corpuscles. J Neurocytol. 1978 Feb;7(1):71–91. doi: 10.1007/BF01213461. [DOI] [PubMed] [Google Scholar]


