Abstract
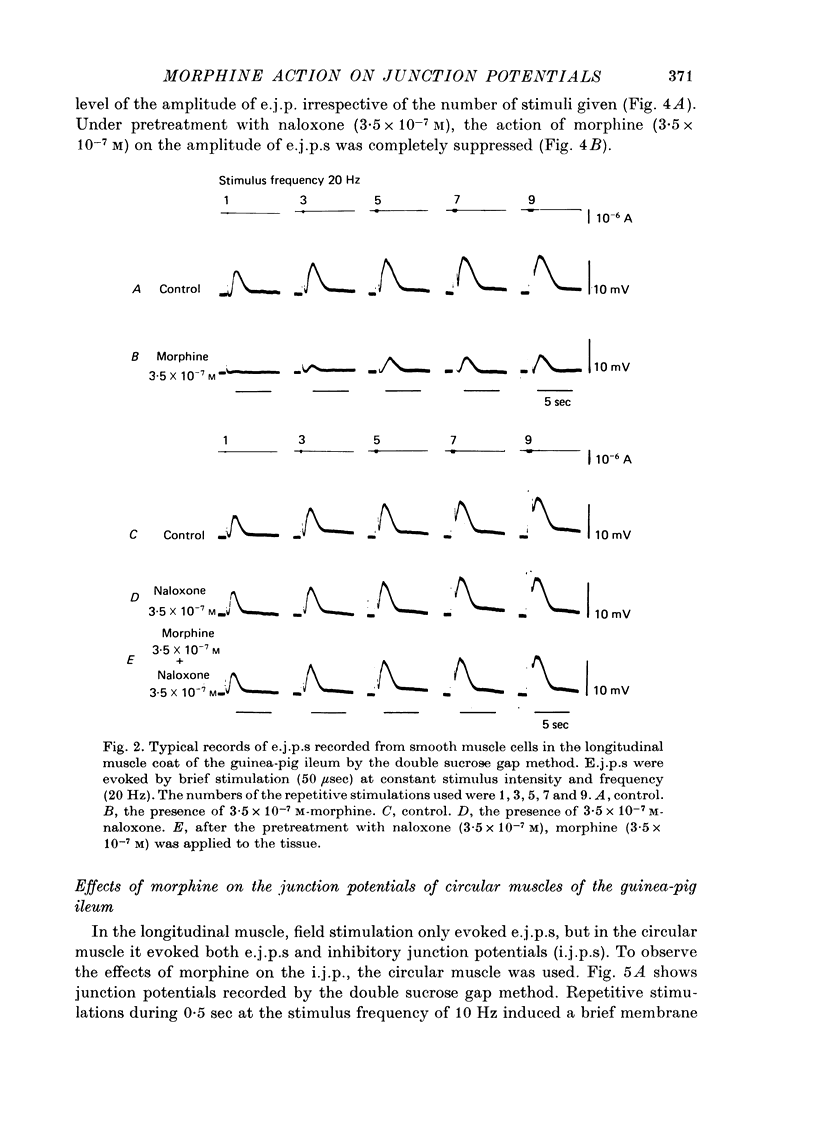
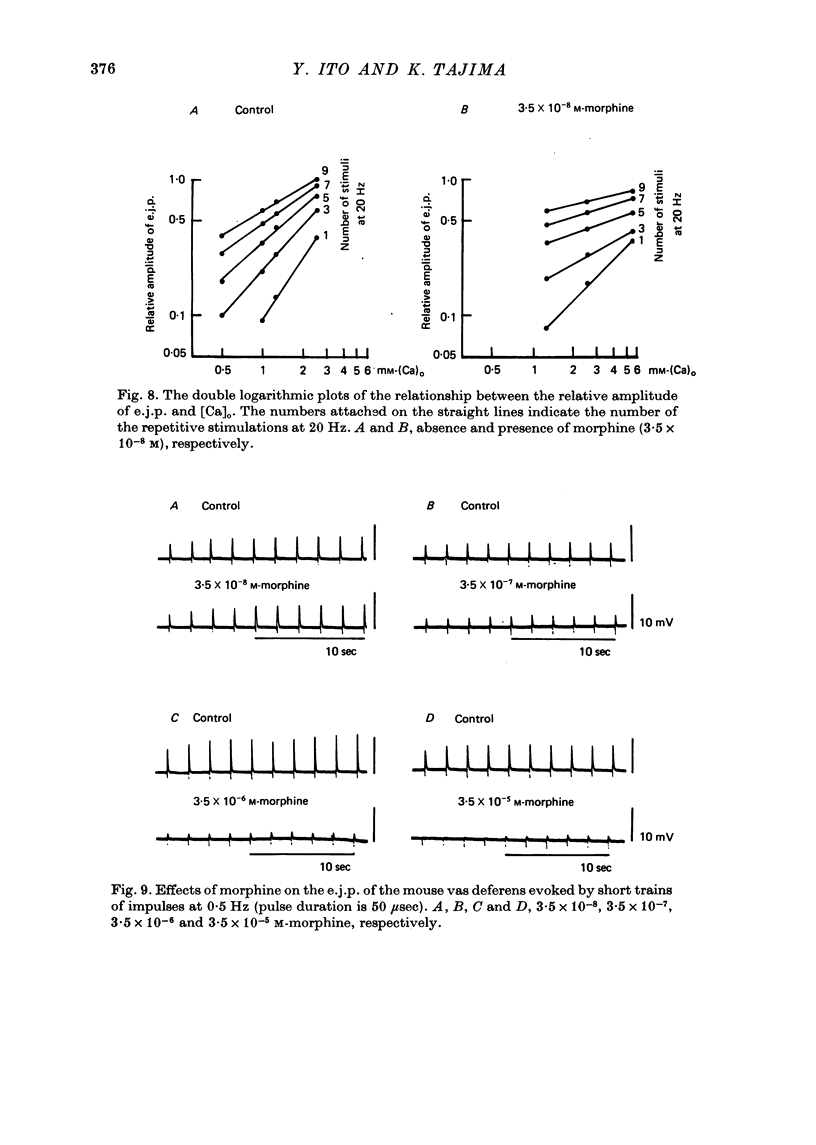
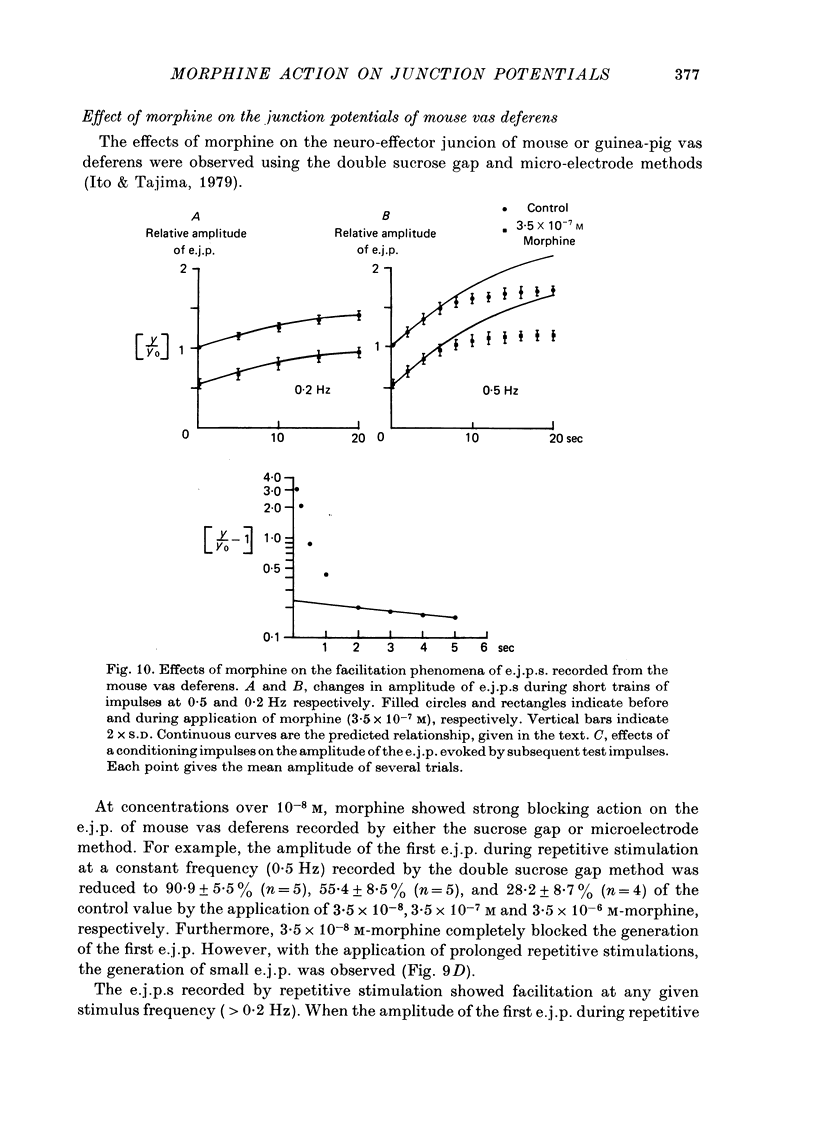
Effects of morphine on the neuro-effector junction of the guinea-pig ileum or mouse vas deferens were investigated by the micro-electrode and double sucrose gap methods. 1. Morphine (10(-8)-10(-5) M) did not change the membrane potential, membrane resistance and electrical threshold required to produce the action potential of smooth muscle cells of guinea-pig ileum or mouse vas deferens. 2. Morphine (10(-8)-10(-7) M) markedly suppressed the amplitude of excitatory junction potential (e.j.p.) of ileum or that of vas deferens. However the same concentration of morphine did not suppress the inhibitory junction potential recorded from the guinea-pig ileum or the facilitation phenomena observed with repetitive stimulation in the mouse vas deferens. In addition, this opiate (3.5 x 10(-5) M) did not alter the amplitude or the frequency of miniature excitatory junction potential recorded from the mouse vas deferens. 3. Naloxone (3.5 x 10(-7) M), itself, exerted no effect on the membrane potential and amplitude of the e.j.p. After pretreatment with naloxone, however, the inhibitory action of morphine on the e.j.p. was suppressed. 4. The extracellularly recorded action potential from the small nerve bundle innervating the mouse vas deferens was not affected by morphine (3.5 x 10(-5) M). 5. The amplitude of the e.j.p. of the guinea-pig ileum was dependent on the concentration of [Ca]o. When [Ca]o and the relative amplitude of e.j.p. were plotted on a double logarithmic scale, the above relation yielded a straight line with a slope of 1.7. Application of morphine resulted in a reduction in the slope of the straight line to 1.0. 6. These results indicate that morphine probably suppresses the influx of Ca during spike electrogenesis in the nerve terminal, however, there is no modification of the action of Ca in nerve terminals.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cox B. M., Goldstein A., Hi C. H. Opioid activity of a peptide, beta-lipotropin-(61-91), derived from beta-lipotropin. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1821–1823. doi: 10.1073/pnas.73.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R., Goldstein A. Effect of synaptic transmission blockade on morphine action in the guinea-pig myenteric plexus. J Pharmacol Exp Ther. 1976 Jan;196(1):97–106. [PubMed] [Google Scholar]
- ELLIOTT H. W., KOKKA N., WAY E. L. INFLUENCE OF CALCIUM-DEFICIT ON MORPHINE INHIBITION OF QO2 OF RAT CEREBRAL CORTEX SLICES. Proc Soc Exp Biol Med. 1963 Aug-Sep;113:1049–1052. doi: 10.3181/00379727-113-28569. [DOI] [PubMed] [Google Scholar]
- GYANG E. A., KOSTERLITZ H. W., LEES G. M. THE INHIBITION OF AUTONOMIC NEURO-EFFECTOR TRANSMISSION BY MORPHINE-LIKE DRUGS AND ITS USE AS A SCREENING TEST FOR NARCOTIC ANALGESIC DRUGS . Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1964 May 25;248:231–246. doi: 10.1007/BF00348594. [DOI] [PubMed] [Google Scholar]
- Gyand E. A., Kosterlitz H. W. Agonist and antagonist actions of morphine-like drugs on the guinea-pig isolated ileum. Br J Pharmacol Chemother. 1966 Sep;27(3):514–527. doi: 10.1111/j.1476-5381.1966.tb01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R. A., Loh H. H., Way E. L. Effects of divalent cations, cation chelators and an ionophore on morphine analgesia and tolerance. J Pharmacol Exp Ther. 1975 Dec;195(3):488–498. [PubMed] [Google Scholar]
- Henderson G., Hughes J., Kosterlitz H. W. A new example of a morphine-sensitive neuro-effector junction: adrenergic transmission in the mouse vas deferens. Br J Pharmacol. 1972 Dec;46(4):764–766. doi: 10.1111/j.1476-5381.1972.tb06901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J. Isolation of an endogenous compound from the brain with pharmacological properties similar to morphine. Brain Res. 1975 May 2;88(2):295–308. doi: 10.1016/0006-8993(75)90391-1. [DOI] [PubMed] [Google Scholar]
- Hughes J., Kosterlitz H. W., Leslie F. M. Effect of morphine on adrenergic transmission in the mouse vas deferens. Assessment of agonist and antogonist potencies of narcotic analgesics. Br J Pharmacol. 1975 Mar;53(3):371–381. doi: 10.1111/j.1476-5381.1975.tb07373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J., Kosterlitz H. W., Leslie F. M. Proceedings: Assessment of the agonist and antagonist activities of narcotic analgesic drugs by means of the mouse vas deferens. Br J Pharmacol. 1974 May;51(1):139P–140P. [PMC free article] [PubMed] [Google Scholar]
- Hughes J., Smith T. W., Kosterlitz H. W., Fothergill L. A., Morgan B. A., Morris H. R. Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature. 1975 Dec 18;258(5536):577–580. doi: 10.1038/258577a0. [DOI] [PubMed] [Google Scholar]
- Ito Y., Suzuki H., Kuriyama H. On the roles of calcium ion during potassium induced contracture in the smooth muscle cells of the rabbit main pulmonary artery. Jpn J Physiol. 1977;27(6):755–770. doi: 10.2170/jjphysiol.27.755. [DOI] [PubMed] [Google Scholar]
- Ito Y., Tajima K. An electrophysiological analysis of the actions of prostaglandin on neuromuscular transmission in the guinea-pig vas deferens. J Physiol. 1979 Dec;297(0):521–537. doi: 10.1113/jphysiol.1979.sp013054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. PROPAGATION OF ELECTRIC ACTIVITY IN MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:453–482. doi: 10.1098/rspb.1965.0015. [DOI] [PubMed] [Google Scholar]
- Kosterlitz H. W., Waterfield A. A. In vitro models in the study of structure-activity relationships of narcotic analgesics. Annu Rev Pharmacol. 1975;15:29–47. doi: 10.1146/annurev.pa.15.040175.000333. [DOI] [PubMed] [Google Scholar]
- Kosterlitz H. W., Watt A. J. Kinetic parameters of narcotic agonists and antagonists, with particular reference to N-allylnoroxymorphone (naloxone). Br J Pharmacol Chemother. 1968 Jun;33(2):266–276. doi: 10.1111/j.1476-5381.1968.tb00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus L. H., Ling N., Guillemin R. beta-Lipotropin as a prohormone for the morphinomimetic peptides endorphins and enkephalins. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2156–2159. doi: 10.1073/pnas.73.6.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. H., Chung D. Isolation and structure of an untriakontapeptide with opiate activity from camel pituitary glands. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1145–1148. doi: 10.1073/pnas.73.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Martin A. R. An analysis of facilitation of transmitter release at the neuromuscular junction of the frog. J Physiol. 1967 Dec;193(3):679–694. doi: 10.1113/jphysiol.1967.sp008388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Thies R. Tetanic and post-tetanic rise in frequency of miniature end-plate potentials in low-calcium solutions. J Physiol. 1971 Jan;212(1):245–257. doi: 10.1113/jphysiol.1971.sp009320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A., Katayama Y., Williams J. T. On the mechanism and site of action of enkephalin on single myenteric neurons. Brain Res. 1979 Apr 6;165(1):67–77. doi: 10.1016/0006-8993(79)90045-3. [DOI] [PubMed] [Google Scholar]
- North R. A., Tonini M. The mechanism of action of narcotic analgesics in the guinea-pig ileum. Br J Pharmacol. 1977 Dec;61(4):541–549. doi: 10.1111/j.1476-5381.1977.tb07546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATON W. D. The action of morphine and related substances on contraction and on acetylcholine output of coaxially stimulated guinea-pig ileum. Br J Pharmacol Chemother. 1957 Mar;12(1):119–127. doi: 10.1111/j.1476-5381.1957.tb01373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton W. D., Zar M. A. The origin of acetylcholine released from guinea-pig intestine and longitudinal muscle strips. J Physiol. 1968 Jan;194(1):13–33. doi: 10.1113/jphysiol.1968.sp008392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak J. M., Bloom S. R., Sullivan S. N., Facer P., Pearse A. G. Enkephalin-like immunoreactivity in the human gastrointestinal tract. Lancet. 1977 May 7;1(8019):972–974. doi: 10.1016/s0140-6736(77)92277-2. [DOI] [PubMed] [Google Scholar]
- Schultzberg M., Dreyfus C. F., Gershon M. D., Hökfelt T., Elde R. P., Nilsson G., Said S., Goldstein M. VIP-, enkephalin-, substance P- and somatostatin-like immunoreactivity in neurons intrinsic to the intestine: immunohistochemical evidence from organotypic tissue cultures. Brain Res. 1978 Oct 27;155(2):239–248. doi: 10.1016/0006-8993(78)91020-x. [DOI] [PubMed] [Google Scholar]
- Schulz R., Wüster M., Simantov R., Snyder S., Herz A. Electrically stimulated release of opiate-like material from the myenteric plexus of the guinea pig ileum. Eur J Pharmacol. 1977 Feb 7;41(3):347–348. doi: 10.1016/0014-2999(77)90331-4. [DOI] [PubMed] [Google Scholar]
- Waterfield A. A., Kosterlitz H. W. Stereospecific increase by narcotic antagonists of evoked acetylcholine output in guinea-pig ileum. Life Sci. 1975 Jun 15;16(12):1787–1792. doi: 10.1016/0024-3205(75)90275-1. [DOI] [PubMed] [Google Scholar]
- Williams J. T., North R. A. Effects of endorphins on single myenteric neurons. Brain Res. 1979 Apr 6;165(1):57–65. doi: 10.1016/0006-8993(79)90044-1. [DOI] [PubMed] [Google Scholar]


