Abstract
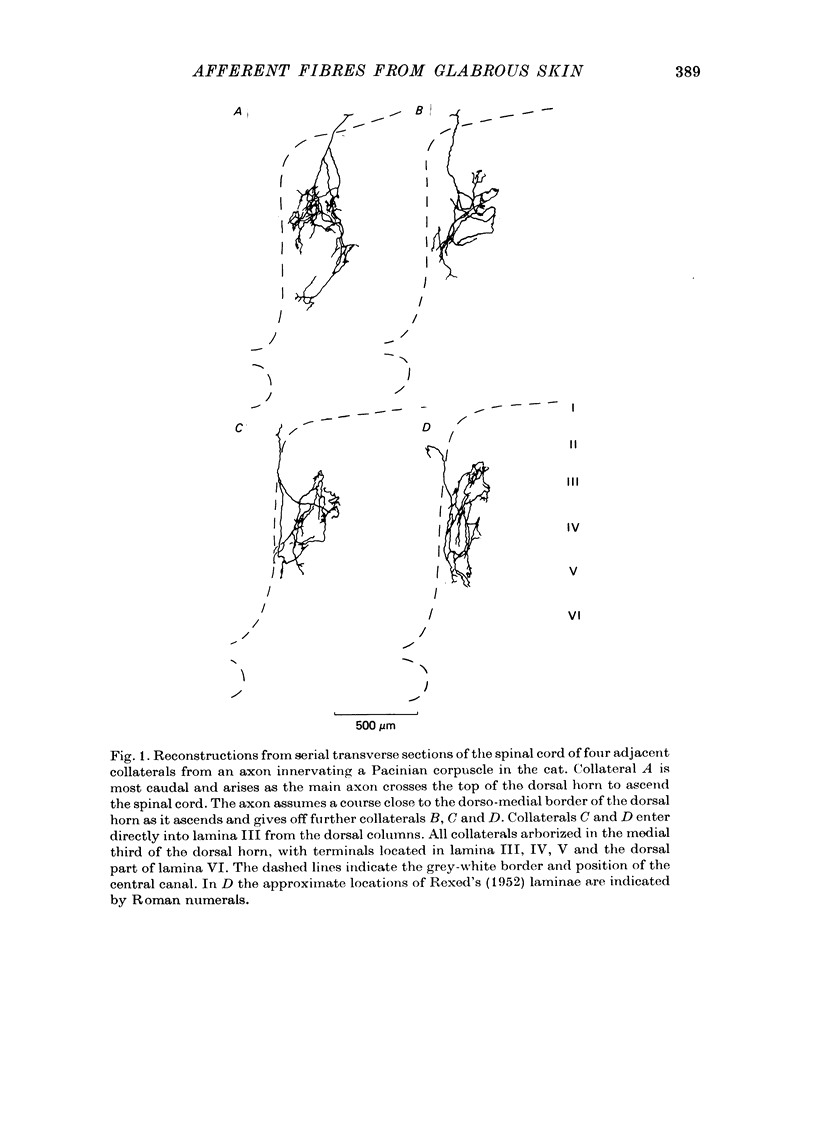
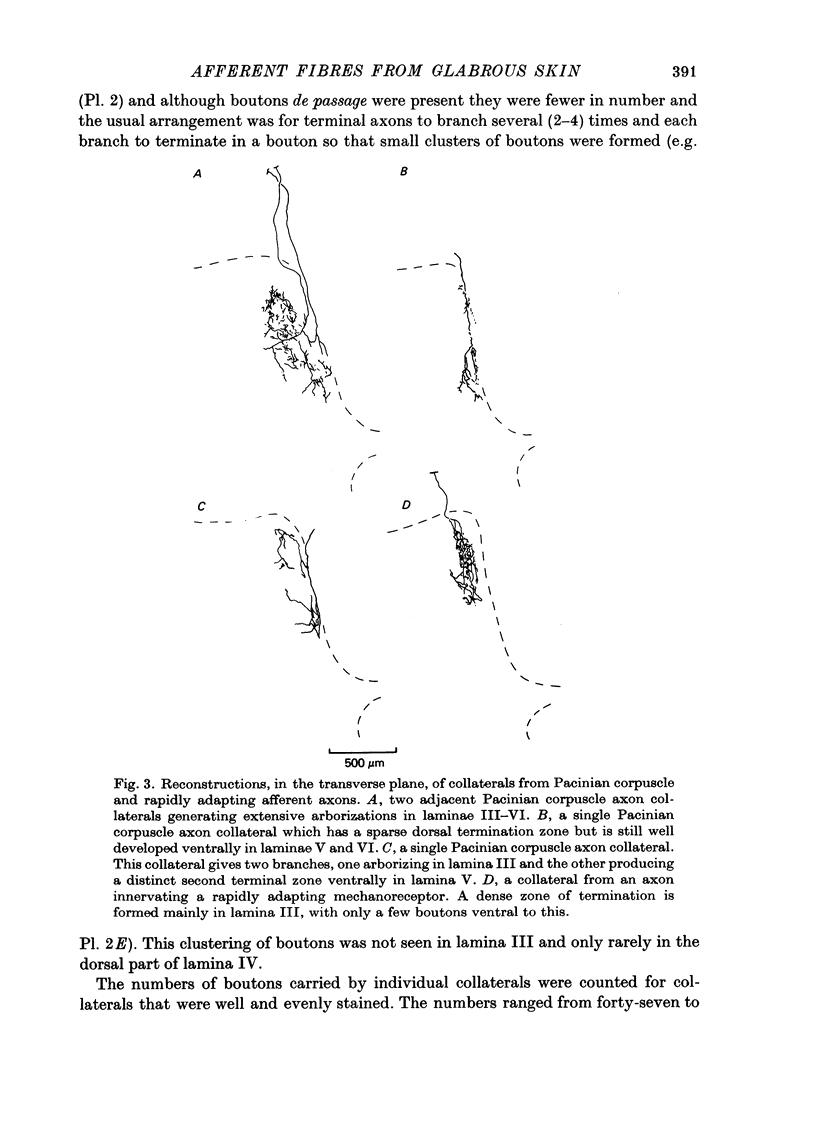
1. Single axons innervating Pacinian corpuscles and rapidly adapting mechanoreceptors of the foot and toe pads were injected with horseradish peroxidase near their entrance to the lumbosacral spinal cord in cats anaesthetized with chloralose and paralysed with gallamine triethiodide. Subsequent histochemistry revealed the morphology of the intra-spinal parts of the axons. 2. All Pacinian corpuscle axons that could be traced into the dorsal root bifurcated upon entering the cord into ascending and descending branches. All Pacinian corpuscle axons gave rise to collaterals that entered the dorsal horn. 3. The collaterals of Pacinian corpuscle afferent fibres had a distinctive morphology. They provided two regions of termination, a larger dorsal region in laminae III and IV and a smaller ventral region in laminae V and VI. Within the dorsal region the terminal axons ran mainly in the longitudinal axis of the cord and carried many boutons en passant. Within the ventral region the axons ran dorso-ventrally in the transverse plane of the cord and although carrying some boutons en passant also gave rise to clusters of boutons. 4. The collaterals of rapidly adapting afferent fibres had a distinctive morphology different from that of the Pacinian corpuscle afferent fibre collaterals. The termination region of rapidly adapting afferents was limited almost exclusively to lamina III, with only slight extension into lamina IV. Boutons were mainly of the en passant type and terminal axons were generally orientated within the longitudinal axis of the cord. 5. The morphology of the afferent fibre collaterals is discussed in relation to the physiology of the dorsal horn.
Full text
PDF




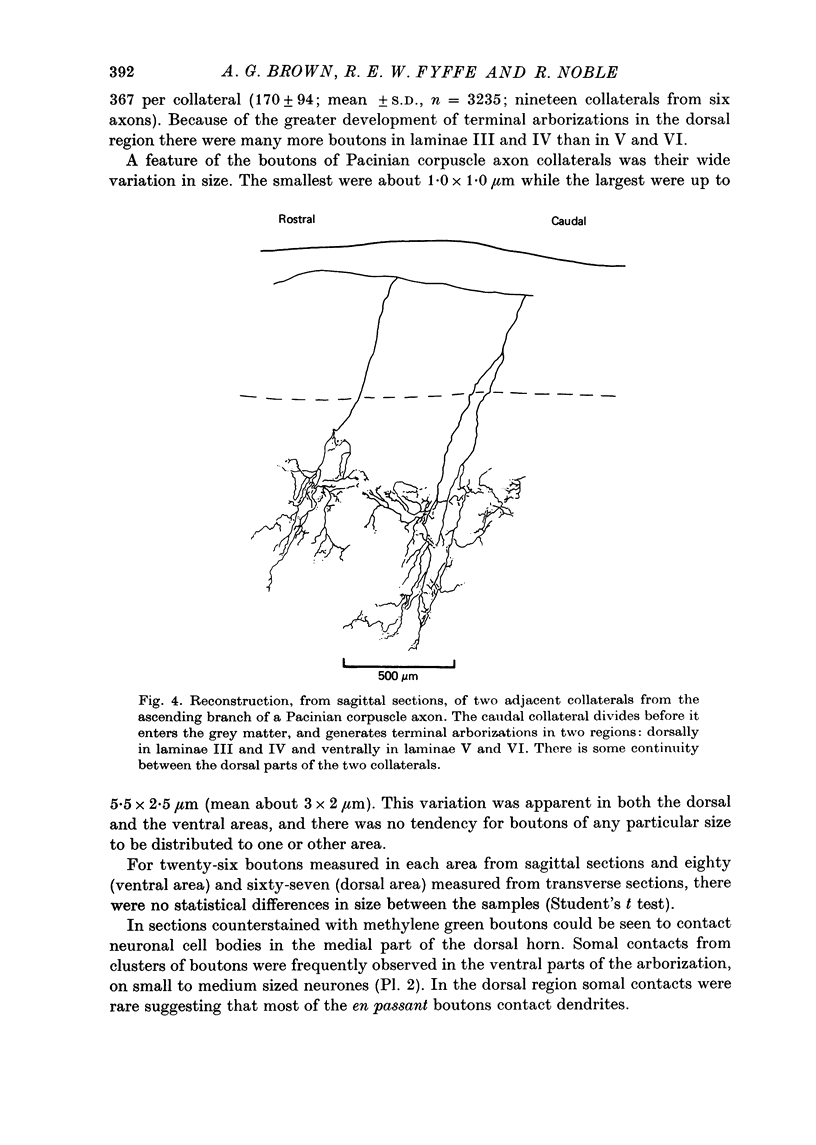
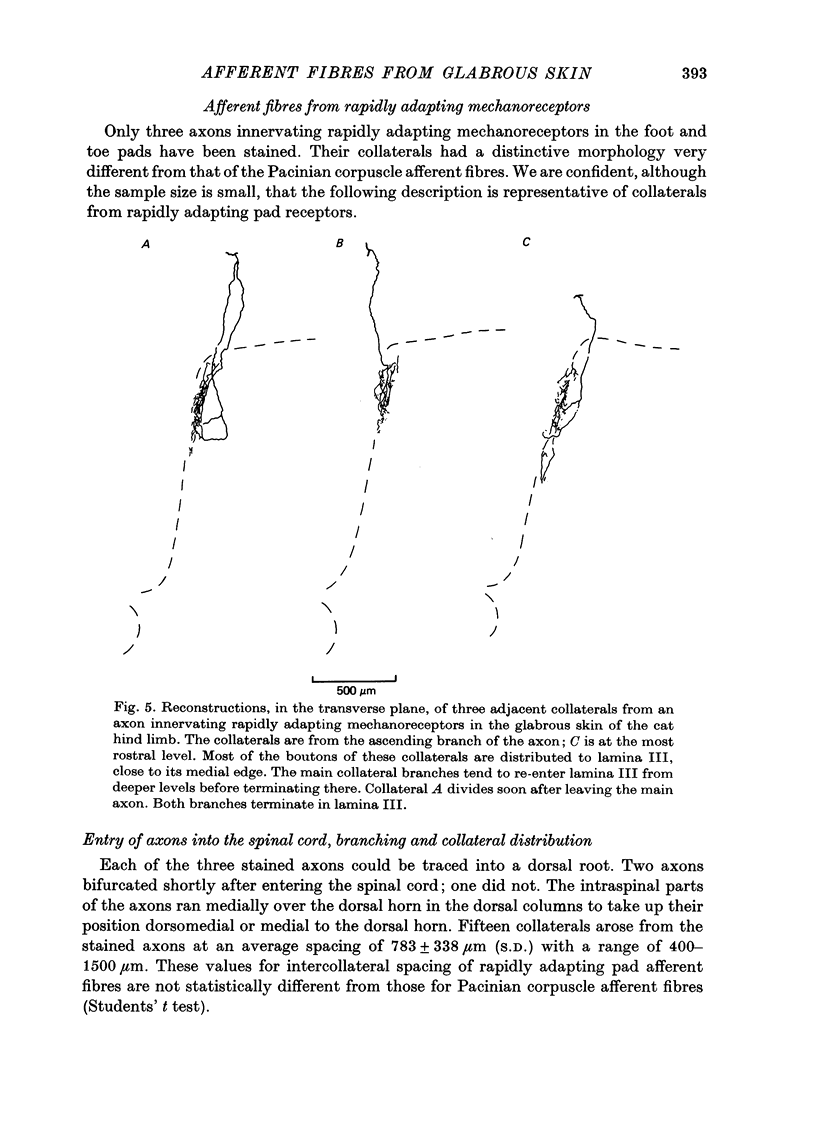
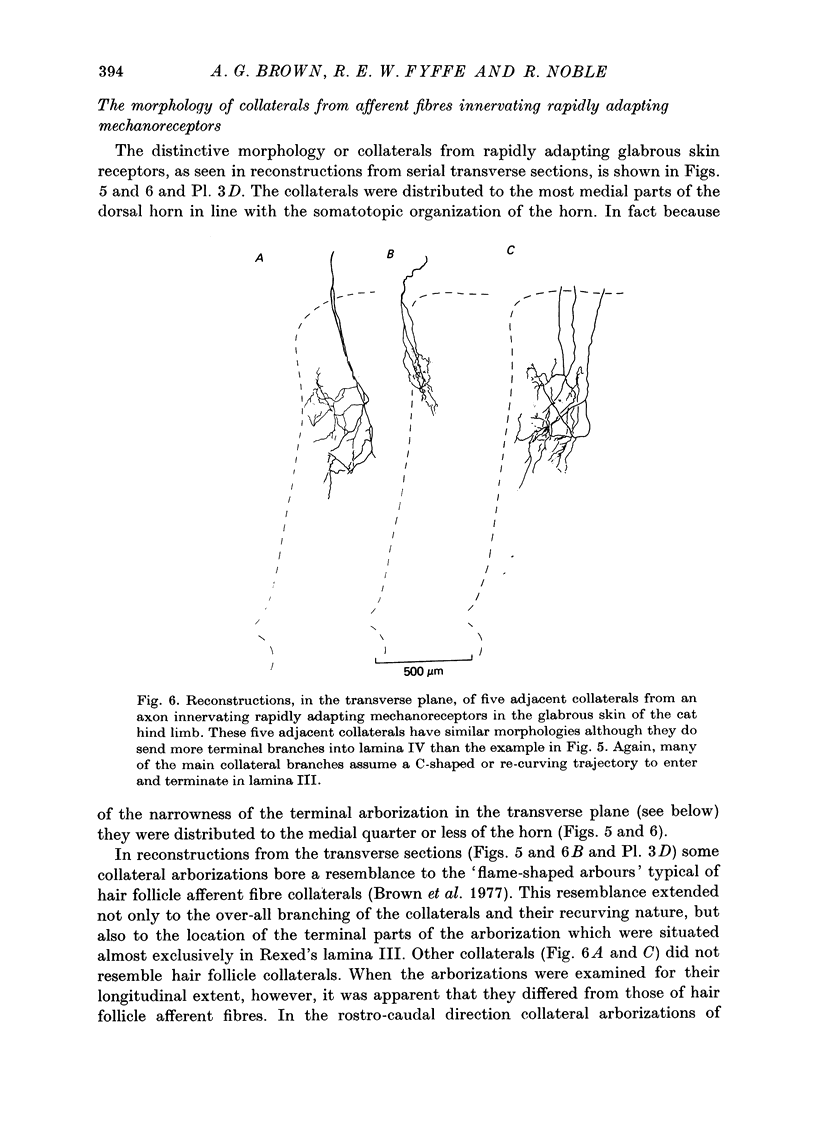

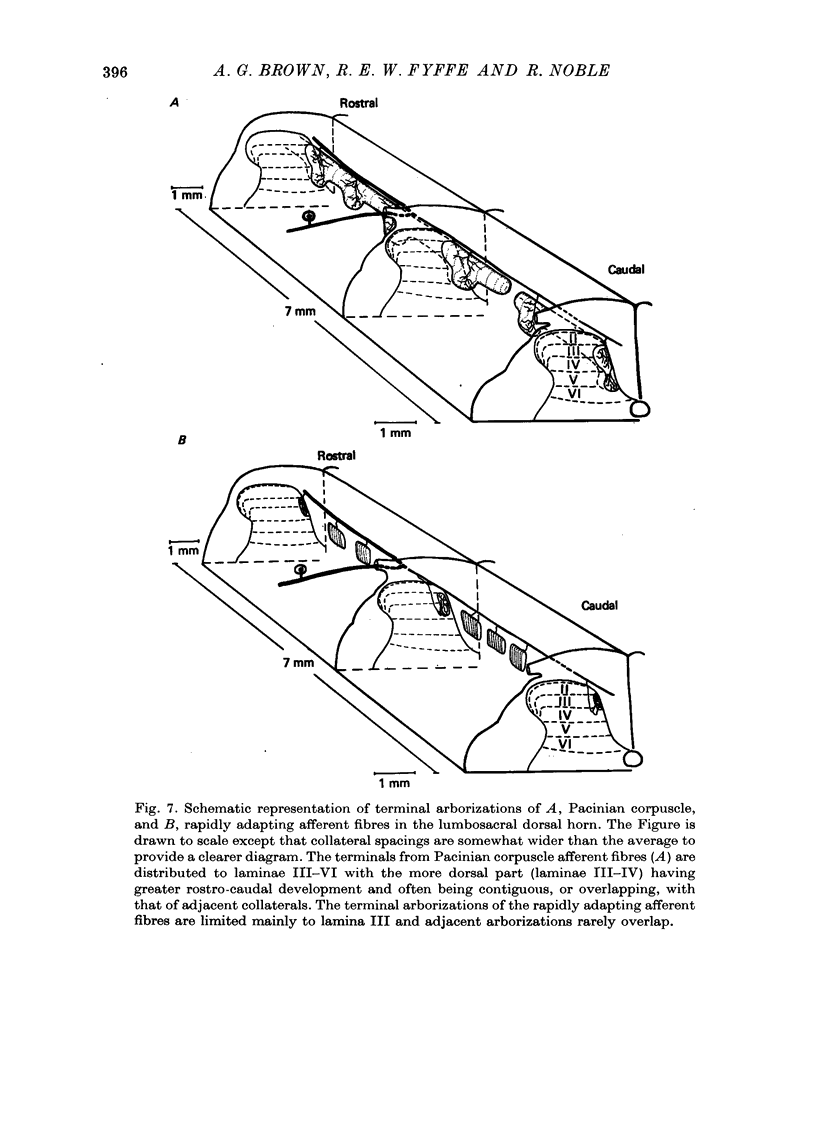





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMETT C. J., GRAY J. A., PALMER J. F. A group of neurones in the dorsal horn associated with cutaneous mechanoreceptors. J Physiol. 1961 May;156:611–622. doi: 10.1113/jphysiol.1961.sp006697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARMETT C. J., HUNSPERGER R. W. Excitation of receptors in the pad of the cat by single and double mechanical pulses. J Physiol. 1961 Sep;158:15–38. doi: 10.1113/jphysiol.1961.sp006751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian E. D., Umrath K. The impulse discharge from the pacinian corpuscle. J Physiol. 1929 Oct 23;68(2):139–154. doi: 10.1113/jphysiol.1929.sp002601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian E. D., Zotterman Y. The impulses produced by sensory nerve endings: Part 3. Impulses set up by Touch and Pressure. J Physiol. 1926 Aug 6;61(4):465–483. doi: 10.1113/jphysiol.1926.sp002308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angaut-Petit D. The dorsal column system: II. Functional properties and bulbar relay of the postsynaptic fibres of the cat's fasciculus gracilis. Exp Brain Res. 1975 May 22;22(5):471–493. doi: 10.1007/BF00237349. [DOI] [PubMed] [Google Scholar]
- Armett C. J., Gray J. A., Hunsperger R. W., Lal S. The transmission of information in primary receptor neurones and second-order neurones of a phasic system. J Physiol. 1962 Dec;164(3):395–421. doi: 10.1113/jphysiol.1962.sp007028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G. Cutaneous afferent fibre collaterals in the dorsal columns of the cat. Exp Brain Res. 1968;5(4):293–305. doi: 10.1007/BF00235904. [DOI] [PubMed] [Google Scholar]
- Brown A. G. Cutaneous axons and sensory neurones in the spinal cord. Br Med Bull. 1977 May;33(2):109–112. doi: 10.1093/oxfordjournals.bmb.a071409. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Franz D. N. Responses of spinocervical tract neurones to natural stimulation of identified cutaneous receptors. Exp Brain Res. 1969;7(3):231–249. doi: 10.1007/BF00239031. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E., Heavner J. E., Noble R. The morphology of collaterals from axons innervating Pacinian corpuscles [proceedings]. J Physiol. 1979 Jul;292:24P–25P. [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E. The morphology of group Ia afferent fibre collaterals in the spinal cord of the cat. J Physiol. 1978 Jan;274:111–127. doi: 10.1113/jphysiol.1978.sp012137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E. The morphology of group Ib afferent fibre collaterals in the spinal cord of the cat. J Physiol. 1979 Nov;296:215–226. doi: 10.1113/jphysiol.1979.sp013001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Rose P. K., Snow P. J. Morphology and organization of axon collaterals from afferent fibres of slowly adapting type I units in cat spinal cord. J Physiol. 1978 Apr;277:15–27. doi: 10.1113/jphysiol.1978.sp012257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Rose P. K., Snow P. J. The morphology of hair follicle afferent fibre collaterals in the spinal cord of the cat. J Physiol. 1977 Nov;272(3):779–797. doi: 10.1113/jphysiol.1977.sp012073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G. Subcortical mechanisms concerned in somatic sensation. Br Med Bull. 1977 May;33(2):121–128. doi: 10.1093/oxfordjournals.bmb.a071411. [DOI] [PubMed] [Google Scholar]
- GRAY J. A. B., MATTHEWS P. B. C. Response of Pacinian corpuscles in the cat's toe. J Physiol. 1951 May;113(4):475–482. doi: 10.1113/jphysiol.1951.sp004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horch K. W., Burgess P. R., Whitehorn D. Ascending collaterals of cutaneous neurons in the fasciculus gracilis of the cat. Brain Res. 1976 Nov 19;117(1):1–17. doi: 10.1016/0006-8993(76)90552-7. [DOI] [PubMed] [Google Scholar]
- Iggo A., Ogawa H. Correlative physiological and morphological studies of rapidly adapting mechanoreceptors in cat's glabrous skin. J Physiol. 1977 Apr;266(2):275–296. doi: 10.1113/jphysiol.1977.sp011768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänig W., Schmidt R. F., Zimmermann M. Single unit responses and the total afferent outflow from the cat's foot pad upon mechanical stimulation. Exp Brain Res. 1968;6(2):100–115. doi: 10.1007/BF00239165. [DOI] [PubMed] [Google Scholar]
- Jänig W., Schmidt R. F., Zimmermann M. Two specific feedback pathways to the central afferent terminals of phasic and tonic mechanoreceptors. Exp Brain Res. 1968;6(2):116–129. doi: 10.1007/BF00239166. [DOI] [PubMed] [Google Scholar]
- Jänig W. The afferent innervation of the central pad of the cat's hind foot. Brain Res. 1971 May 7;28(2):203–216. doi: 10.1016/0006-8993(71)90655-x. [DOI] [PubMed] [Google Scholar]
- Lynn B. The form and distribution of the receptive fields of Pacinian corpuscles found in and around the cat's large foot pad. J Physiol. 1971 Sep;217(3):755–771. doi: 10.1113/jphysiol.1971.sp009598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn B. The nature and location of certain phasic mechanoreceptors in the cat's foot. J Physiol. 1969 May;201(3):765–773. doi: 10.1113/jphysiol.1969.sp008786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARUHASHI J., MIZUGUCHI K., TASAKI I. Action currents in single afferent nerve fibres elicited by stimulation of the skin of the toad and the cat. J Physiol. 1952 Jun;117(2):129–151. doi: 10.1113/jphysiol.1952.sp004736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit D., Burgess P. R. Dorsal column projection of receptors in cat hairy skin supplied by myelinated fibers. J Neurophysiol. 1968 Nov;31(6):849–855. doi: 10.1152/jn.1968.31.6.849. [DOI] [PubMed] [Google Scholar]
- Proshansky E., Egger M. D. Dendritic spread of dorsal horn neurons in cats. Exp Brain Res. 1977 May 23;28(1-2):153–166. doi: 10.1007/BF00237093. [DOI] [PubMed] [Google Scholar]
- REXED B. The cytoarchitectonic organization of the spinal cord in the cat. J Comp Neurol. 1952 Jun;96(3):414–495. doi: 10.1002/cne.900960303. [DOI] [PubMed] [Google Scholar]
- Rustioni A. Dorsal column nuclei afferents in the lateral funiculus of the cat: distribution pattern and absence of sprouting after chronic deafferentation. Exp Brain Res. 1975 Jul 11;23(1):1–12. doi: 10.1007/BF00238725. [DOI] [PubMed] [Google Scholar]
- Rustioni A., Kaufman A. B. Identification of cells or origin of non-primary afferents to the dorsal column nuclei of the cat. Exp Brain Res. 1977 Jan 18;27(1):1–14. doi: 10.1007/BF00234821. [DOI] [PubMed] [Google Scholar]
- Scheibel M. E., Scheibel A. B. Terminal axonal patterns in cat spinal cord. II. The dorsal horn. Brain Res. 1968 Jun;9(1):32–58. doi: 10.1016/0006-8993(68)90256-4. [DOI] [PubMed] [Google Scholar]
- Snow P. J., Rose P. K., Brown A. G. Tracing axons and axon collaterals of spinal neurons using intracellular injection of horseradish peroxidase. Science. 1976 Jan 23;191(4224):312–313. doi: 10.1126/science.54936. [DOI] [PubMed] [Google Scholar]
- Willis W. D., Trevino D. L., Coulter J. D., Maunz R. A. Responses of primate spinothalamic tract neurons to natural stimulation of hindlimb. J Neurophysiol. 1974 Mar;37(2):358–372. doi: 10.1152/jn.1974.37.2.358. [DOI] [PubMed] [Google Scholar]