Abstract
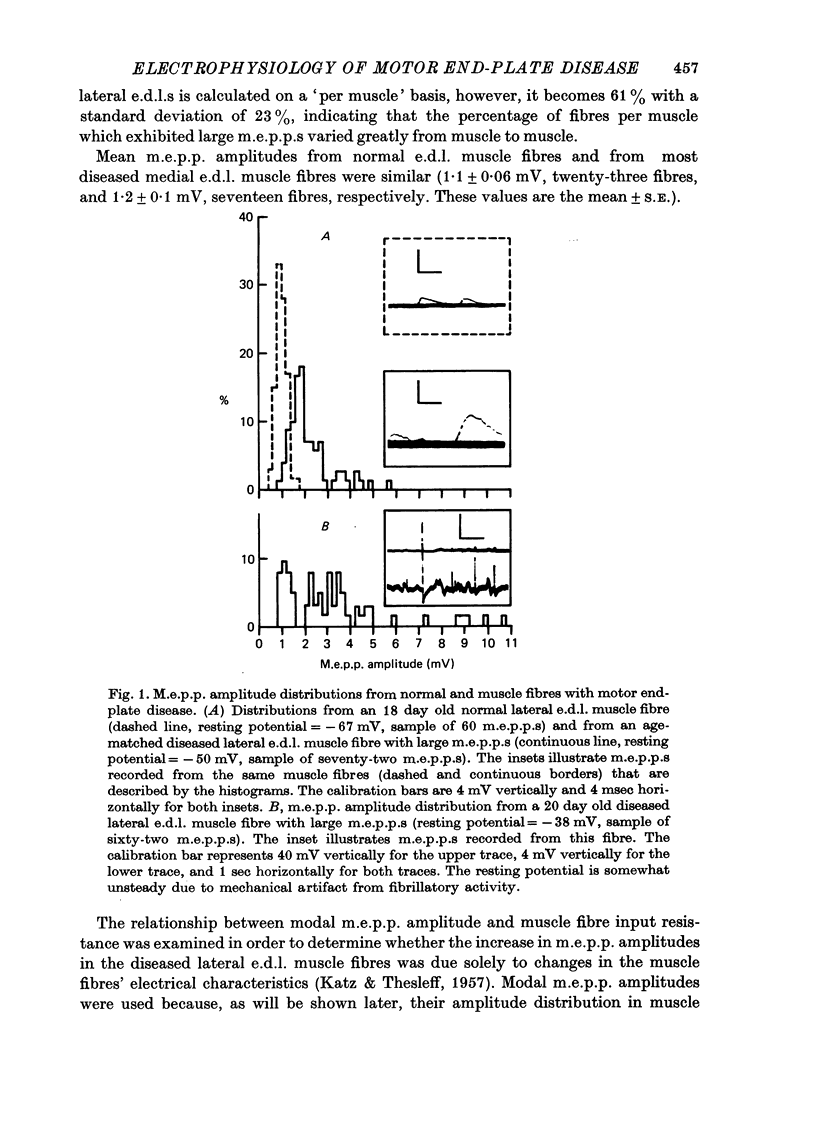
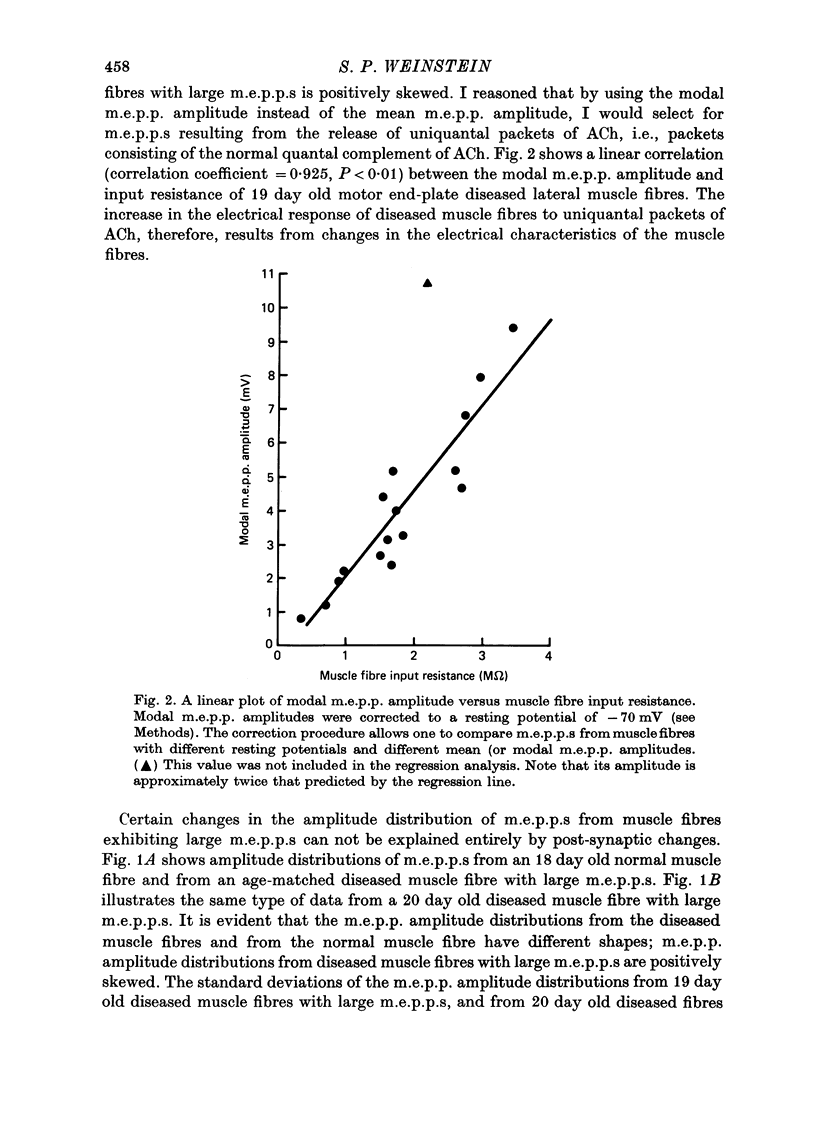
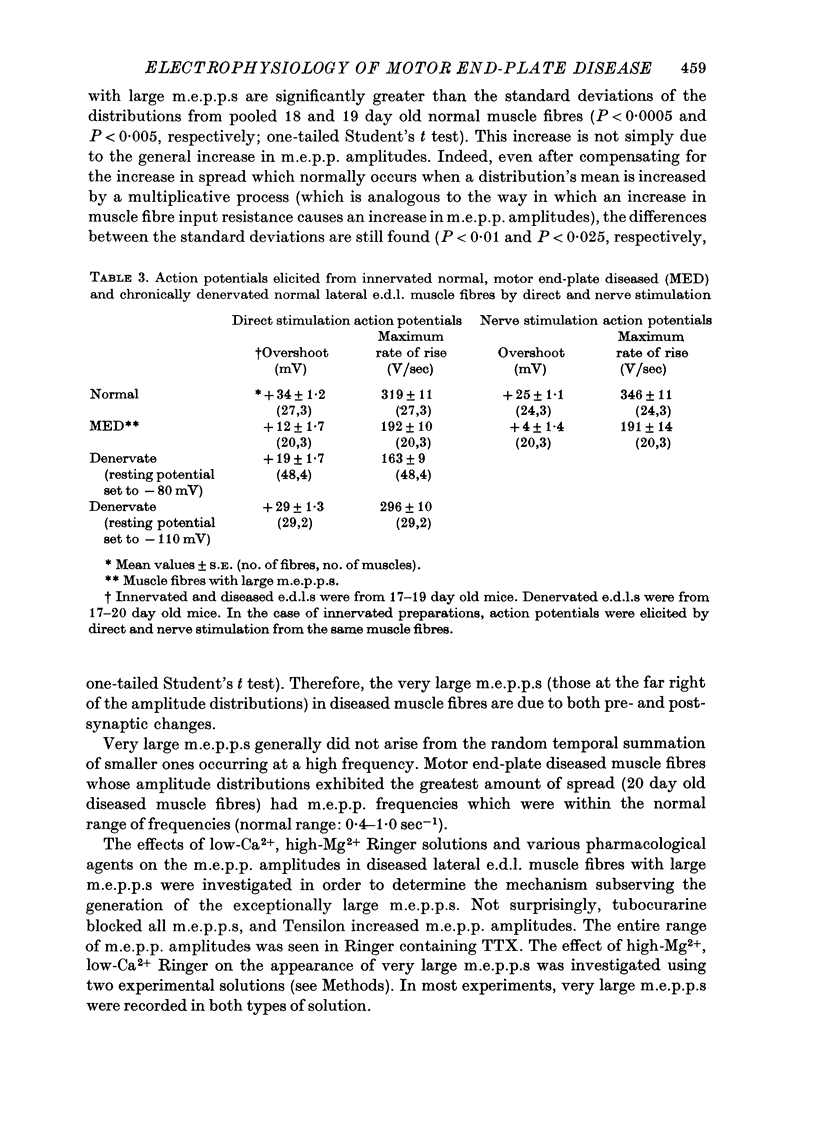
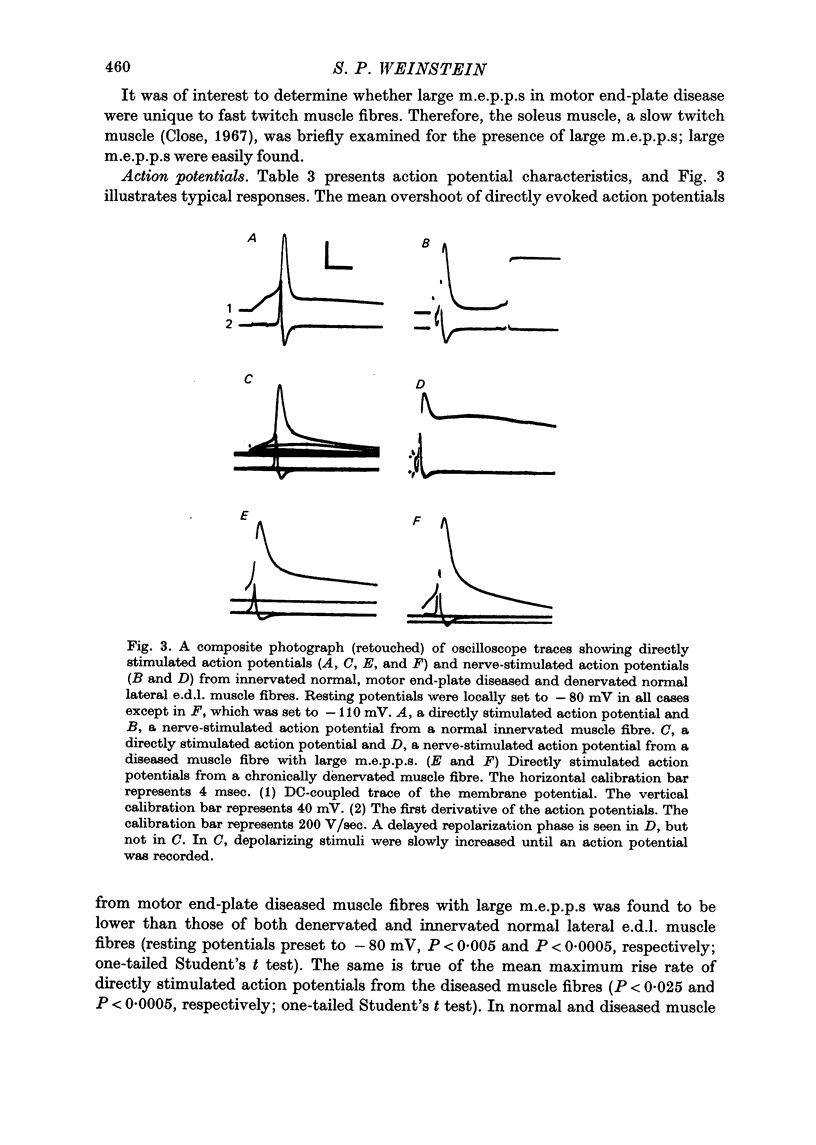
1. Experiments using intracellular recording, stimulation, and microionophoretic techniques were performed on extensor digitorum longus nerve-muscle preparations excised from mice having hereditary 'motor end-plate disease'. Control experiments were performed on normal innervated and chronically denervated nerve-muscle preparations. 2. Two physiologically distinct groups of muscle fibres were found in the diseased muscles. Group I is similar to normal innervated muscle with respect to resting potentials, cable properties, neuromuscular transmission, miniature end-plate potentials, and extrajunctional acetylcholine sensitivity. Group II is similar to denervated muscle in the above respects except that (i) neuromuscular transmission, though abnormal, was present, and (ii) miniature end-plate potentials (m.e.p.p.s), often having large amplitudes, were found in these muscle fibres. 3. Large m.e.p.p.s appear to be due to an increase in muscle fibre input resistance and to the quantal release of abnormally large amounts of acetylcholine from motor nerve terminals. 4. Nerve stimulation of group II muscle fibres evoked action potentials with a delayed repolarization phase, suggesting that a prolonged acetylcholine-induced conductance change occurs at motor end-plates. 5. Neuromuscular physiology in motor end-plate disease is similar to that reported for frog nerve-muscle preparations which have been incubated in high Ca2+ Ringer.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albuquerque E. X., McIsaac R. J. Fast and slow mammalian muscles after denervation. Exp Neurol. 1970 Jan;26(1):183–202. doi: 10.1016/0014-4886(70)90099-3. [DOI] [PubMed] [Google Scholar]
- Close R. Properties of motor units in fast and slow skeletal muscles of the rat. J Physiol. 1967 Nov;193(1):45–55. doi: 10.1113/jphysiol.1967.sp008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. On the localization of acetylcholine receptors. J Physiol. 1955 Apr 28;128(1):157–181. doi: 10.1113/jphysiol.1955.sp005297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. The membrane change produced by the neuromuscular transmitter. J Physiol. 1954 Sep 28;125(3):546–565. doi: 10.1113/jphysiol.1954.sp005180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen L. W. Hereditary motor end-plate disease in the mouse: light and electron microscopic studies. J Neurol Neurosurg Psychiatry. 1970 Apr;33(2):238–250. doi: 10.1136/jnnp.33.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen L. W., Stefani E. Electrophysiological studies of neuromuscular transmission in hereditary 'motor end-plate disease' of the mouse. J Physiol. 1971 Jan;212(2):535–548. doi: 10.1113/jphysiol.1971.sp009340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis K. O., Bryant S. H. Excitation-contraction uncoupling in skeletal muscle by dantrolene sodium. Naunyn Schmiedebergs Arch Pharmacol. 1972;274(1):107–109. doi: 10.1007/BF00501011. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach G. D., Robbins N. Effect of chronic disuse of rat soleus neuromuscular junctions on postsynaptic membrane. J Neurophysiol. 1971 Jul;34(4):562–569. doi: 10.1152/jn.1971.34.4.562. [DOI] [PubMed] [Google Scholar]
- Harris J. B., Ward M. R. A comparative study of "denervation" in muscles from mice with inherited progressive neuromuscular disorders. Exp Neurol. 1974 Jan;42(1):169–180. doi: 10.1016/0014-4886(74)90015-6. [DOI] [PubMed] [Google Scholar]
- Heuser J., Katz B., Miledi R. Structural and functional changes of frog neuromuscular junctions in high calcium solutions. Proc R Soc Lond B Biol Sci. 1971 Sep 28;178(1053):407–415. doi: 10.1098/rspb.1971.0072. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. On the factors which determine the amplitude of the miniature end-plate potential. J Physiol. 1957 Jul 11;137(2):267–278. doi: 10.1113/jphysiol.1957.sp005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Spontaneous and evoked activity of motor nerve endings in calcium Ringer. J Physiol. 1969 Aug;203(3):689–706. doi: 10.1113/jphysiol.1969.sp008887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie P. A., Collier B., Tenehouse A. Comparison of alpha-bungarotoxin binding to skeletal muscles after inactivity or denervation. Nature. 1976 Mar 25;260(5549):349–350. doi: 10.1038/260349a0. [DOI] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. The acetylcholine sensitivity of frog muscle fibres after complete or partial devervation. J Physiol. 1960 Apr;151:1–23. [PMC free article] [PubMed] [Google Scholar]
- Pestronk A., Drachman D. B., Griffin J. W. Effect of muscle disuse on acetylcholine receptors. Nature. 1976 Mar 25;260(5549):352–353. doi: 10.1038/260352a0. [DOI] [PubMed] [Google Scholar]
- Pestronk A., Drachman D. B. Motor nerve sprouting and acetylcholine receptors. Science. 1978 Mar 17;199(4334):1223–1225. doi: 10.1126/science.204007. [DOI] [PubMed] [Google Scholar]
- Purves D., Sakmann B. Membrane properties underlying spontaneous activity of denervated muscle fibres. J Physiol. 1974 May;239(1):125–153. doi: 10.1113/jphysiol.1974.sp010559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern P., Thesleff S. Action potential generation in denervated rat skeletal muscle. I. Quantitative aspects. Acta Physiol Scand. 1971 Apr;81(4):557–564. doi: 10.1111/j.1748-1716.1971.tb04932.x. [DOI] [PubMed] [Google Scholar]
- Redfern P., Thesleff S. Action potential generation in denervated rat skeletal muscle. II. The action of tetrodotoxin. Acta Physiol Scand. 1971 May;82(1):70–78. doi: 10.1111/j.1748-1716.1971.tb04943.x. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. On the permeability of end-plate membrane during the action of transmitter. J Physiol. 1960 Nov;154:52–67. doi: 10.1113/jphysiol.1960.sp006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacks S. I., Sheff M. F., Rhodes M., Saito A. MED myopathy. A new hereditary myopathy. Lab Invest. 1969 Aug;21(2):143–153. [PubMed] [Google Scholar]


