Abstract
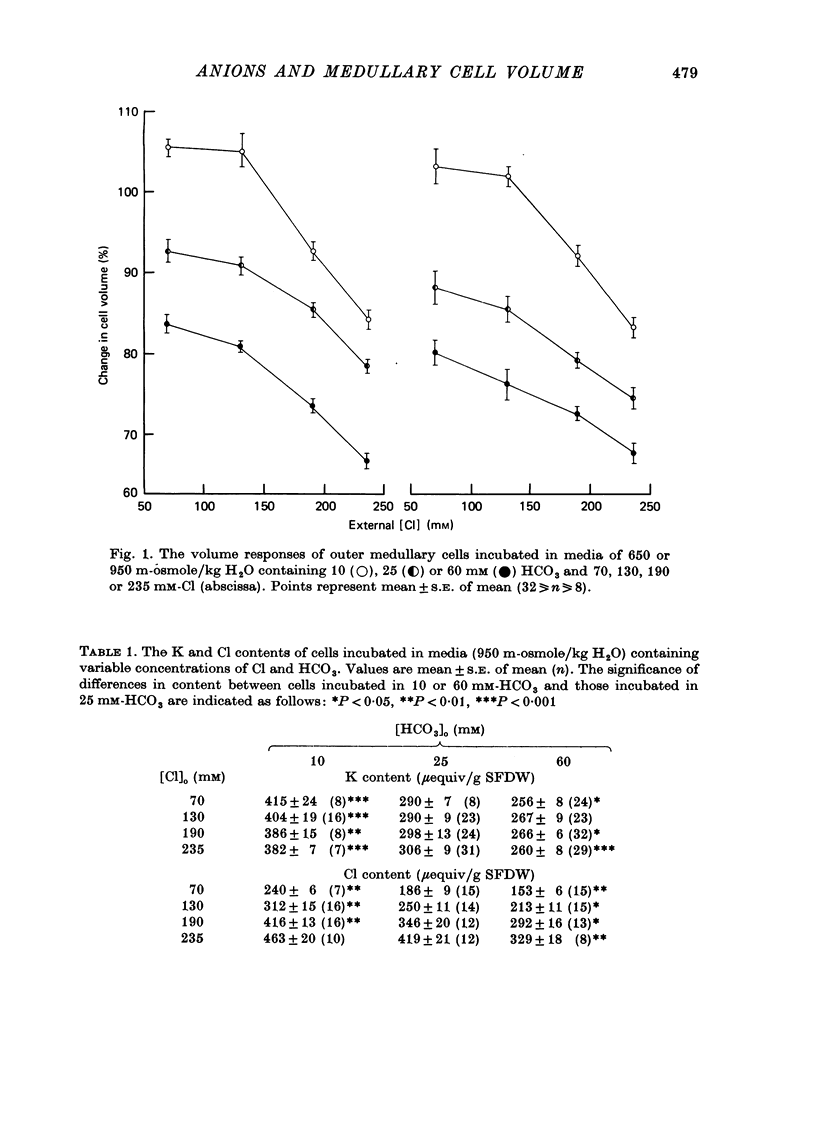
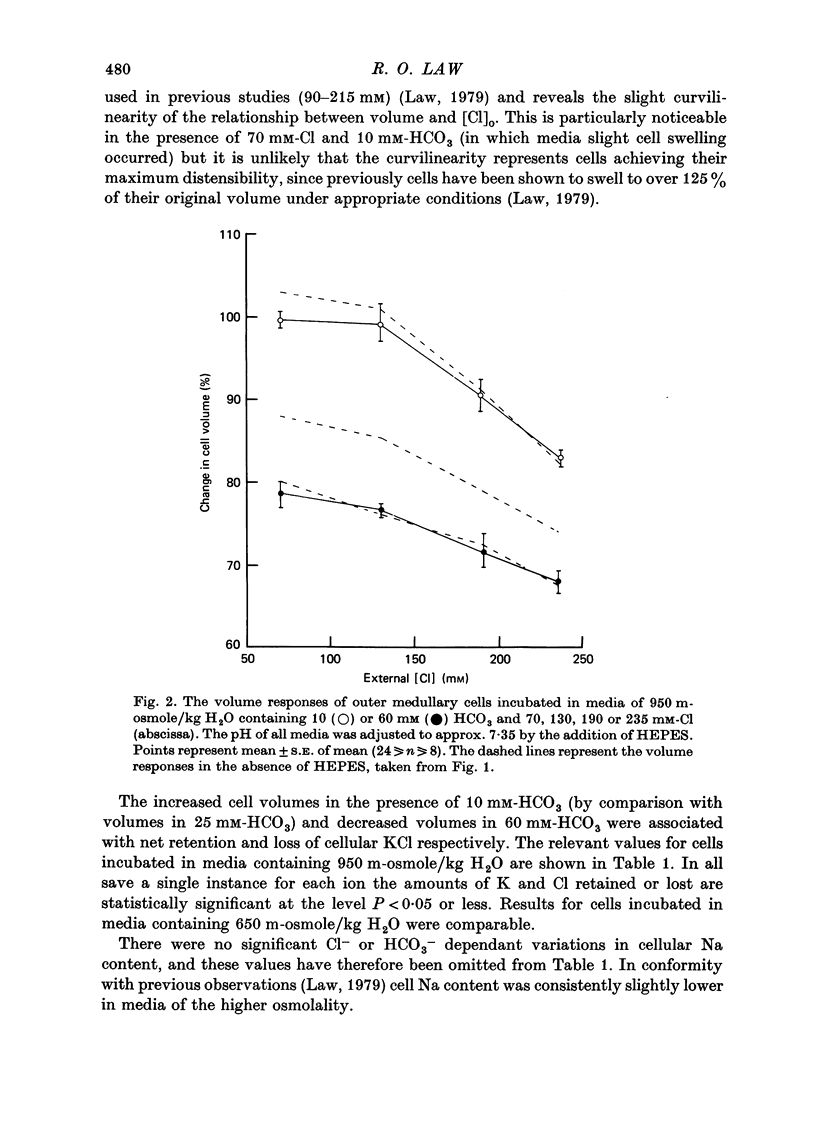
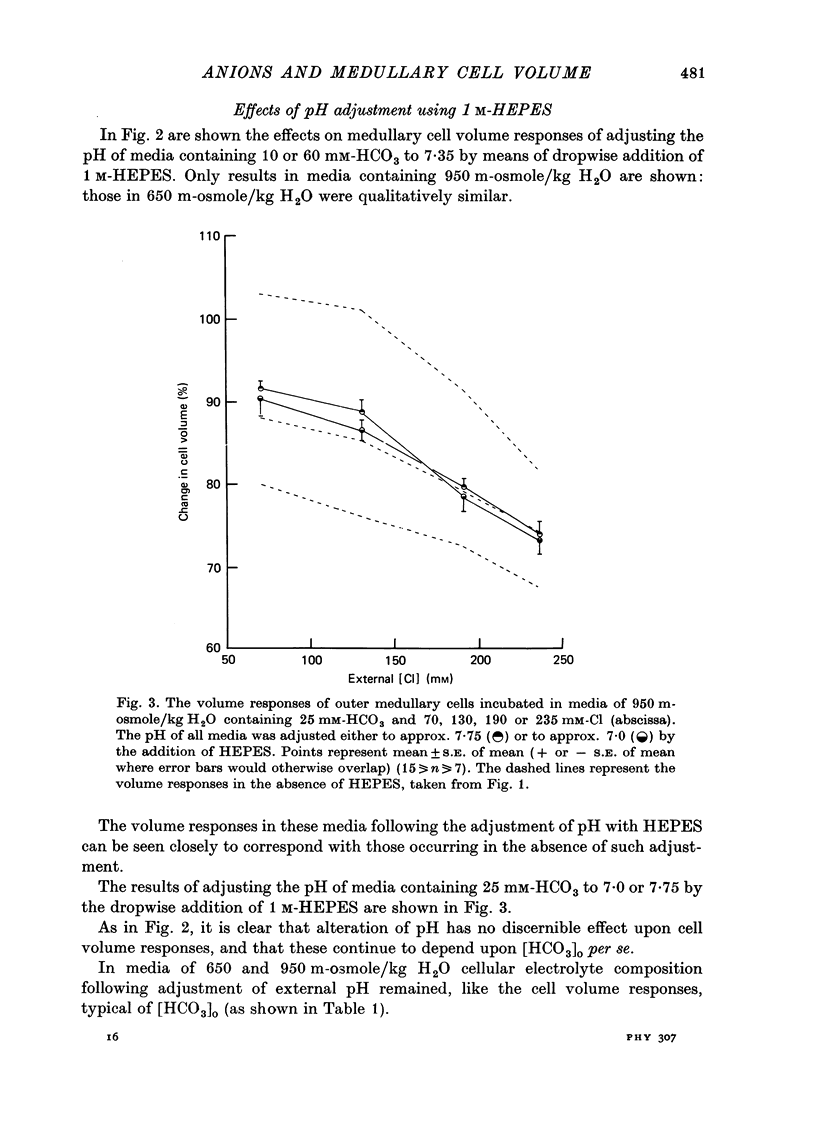
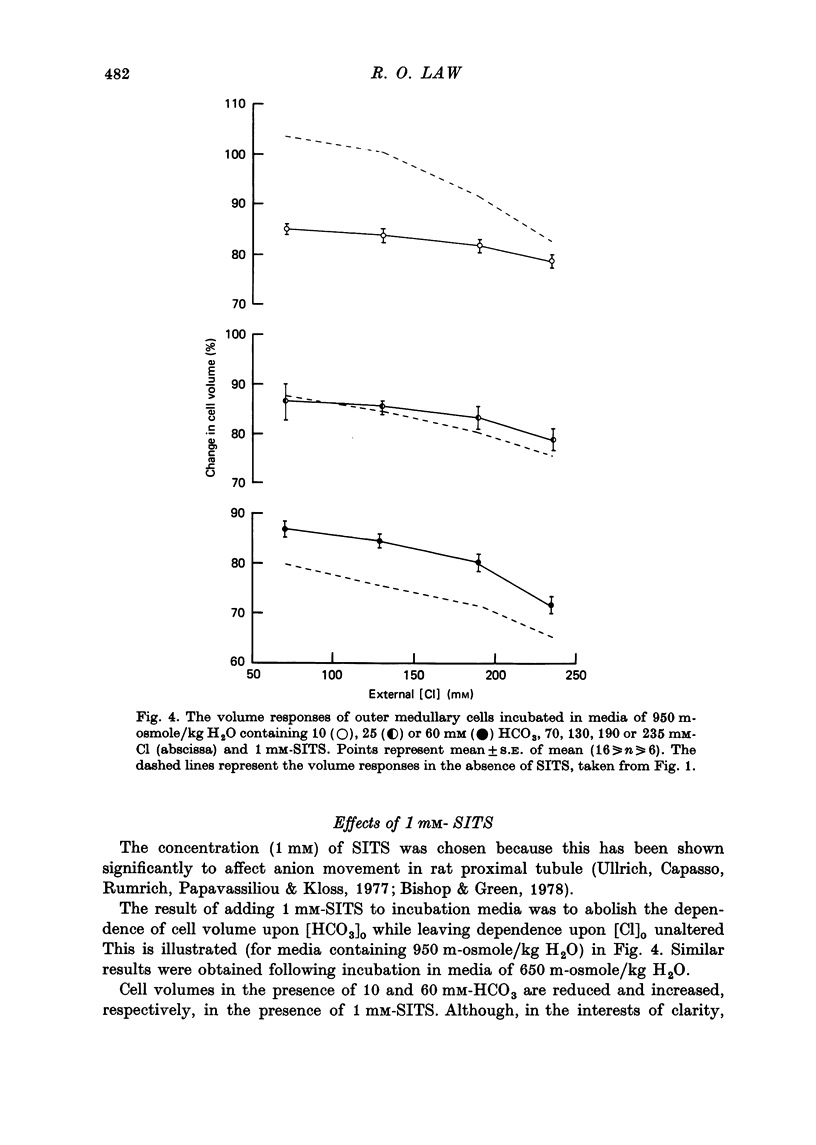
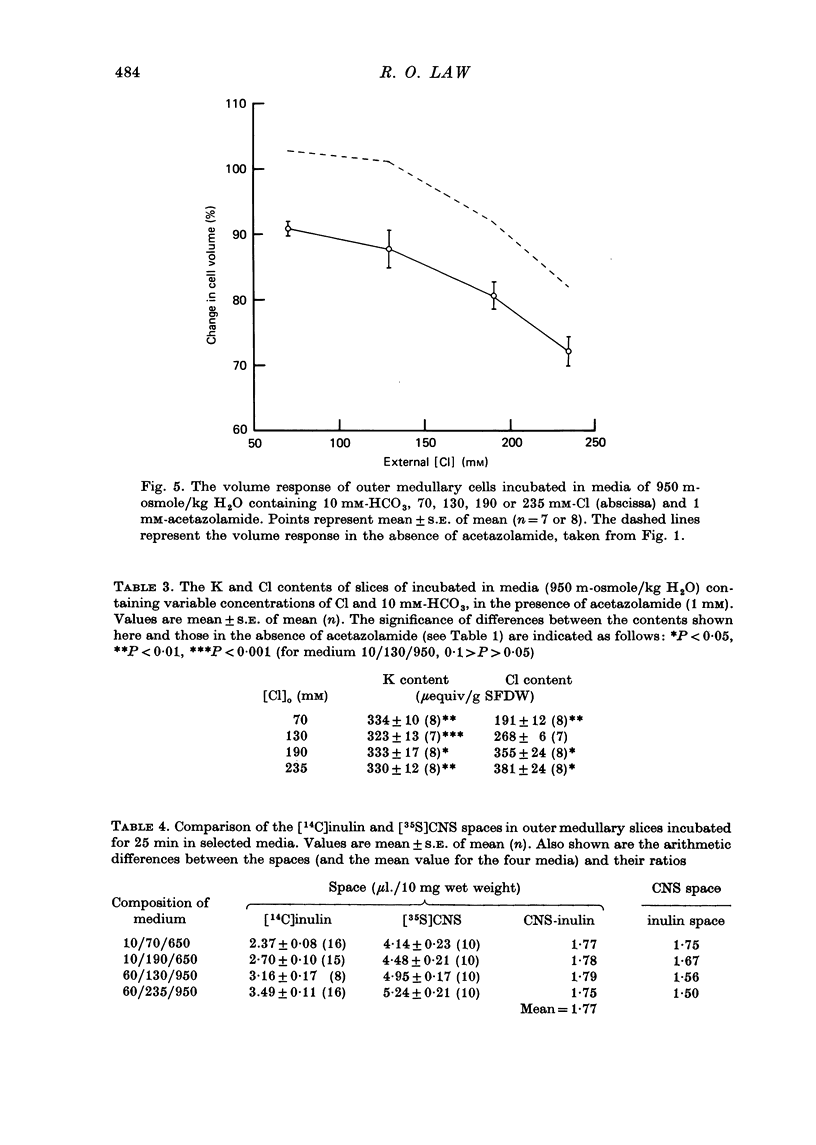
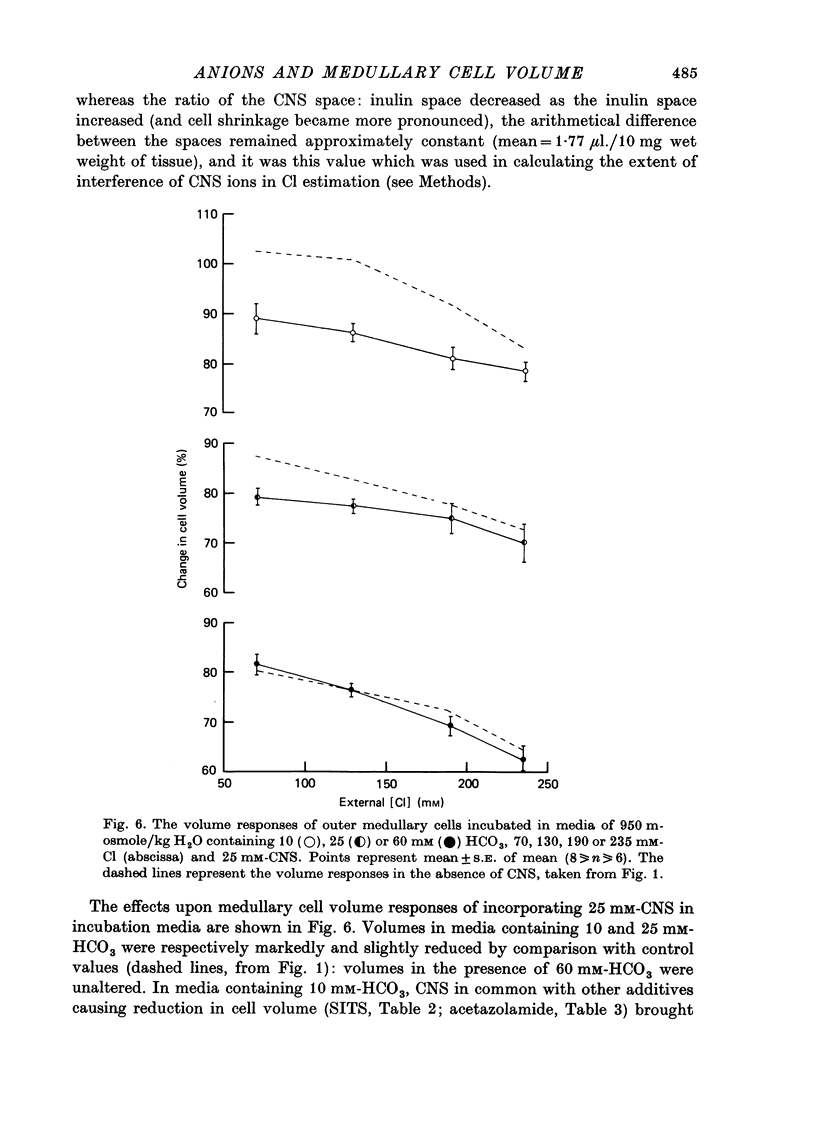
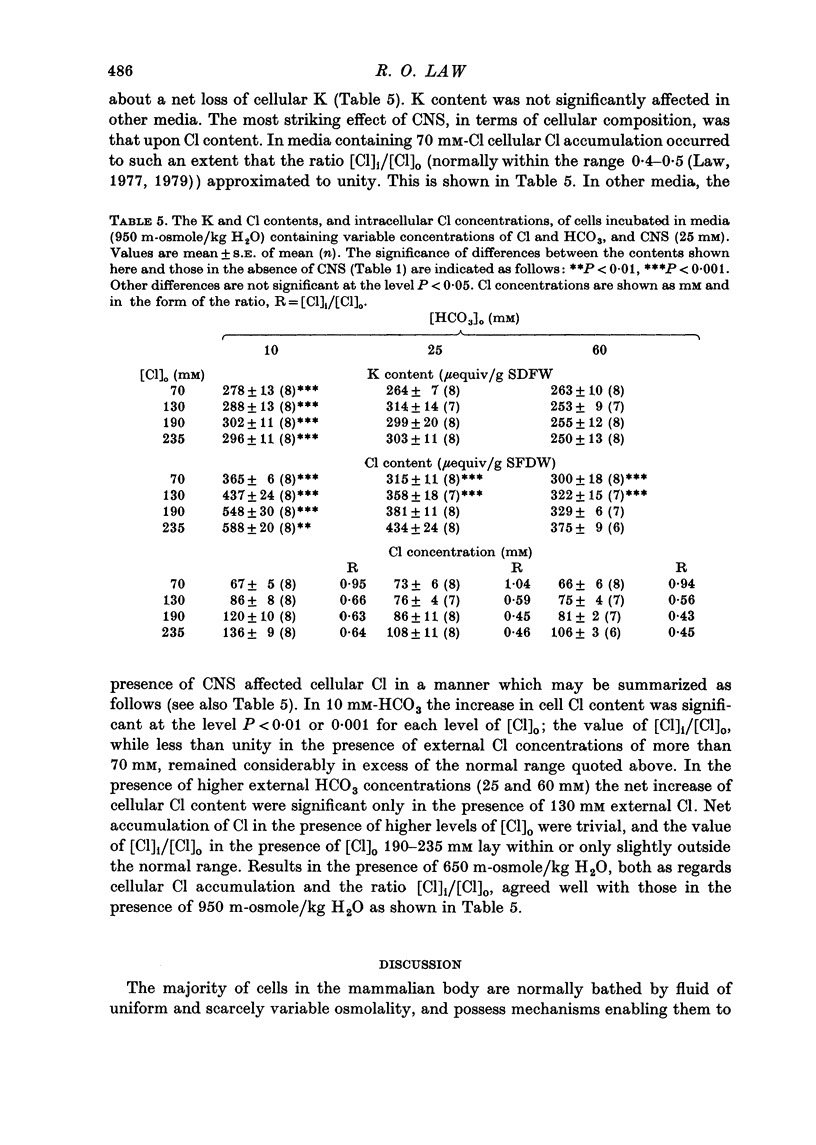
1. The volumes of cells in slices of rat renal outer medulla have been examined following incubation or 25 min in hyperosmolal media (650 and 950 m-osmole/kg H2O) containing independently variable concentrations of Cl (70-325 mM) and HCO3 (10-60 mM) (gas phase 95% O2/5% CO2). 2. For any given level of external Cl concentration cell volumes were reduced by increasing the external HCO3 concentration. These reductions were accompanied by net loss of cellular K and Cl. In confirmation of earlier findings, cell volumes were also reduced by increasing external Cl concentration. 3. Experiments in which the HCO3 concentration and pH of the incubation media were independently varied by the use of N-2-hydroxyethylpiperazine-N'-2-ethanesulphonic acid (HEPES)/100% O2 showed that it is the HCO3 anion per se which influences cell volume. 4. The anion exchange inhibitor 4-acetamido-4'-isothiocyanatostilbene-2,2'-disulphonic acid (SITS, disodium salt, 1 mM) abolished the dependence of cell volume upon HCO3 but not upon Cl. 5. Acetazolamide (1 mM) influenced (reduced) cell volumes only in the presence of low (10 mM) HCO3. 6. CNS (25 mM) also markedly reduced cell volumes in media containing 10mM-HCO3 and, to a lesser extent, 25 mM-HCO3. It was without effect on cell volume when external HCO3 was 60 mM. 7. The presence of CNS was associated with the significant cellular net accumulation of Cl in media in which either Cl or HCO3 concentration (or both) was low (70 or 130 mM and 19 mM respectively). 8. The outer medullary [35S]CNS space at 25 min, determined for slices incubated in a representative selection of the various media employed in this study, exceeded the [14C]inulin space by 1.77 microliters/10 mg wet weight.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aickin C. C., Thomas R. C. An investigation of the ionic mechanism of intracellular pH regulation in mouse soleus muscle fibres. J Physiol. 1977 Dec;273(1):295–316. doi: 10.1113/jphysiol.1977.sp012095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert L., Motais R. Molecular features of organic anion permeablity in ox red blood cell. J Physiol. 1975 Mar;246(1):159–179. doi: 10.1113/jphysiol.1975.sp010884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. H., Green R. Effects of a disulphonic stilbene, SITS, on anion reabsorption from the proximal tubule of the rat [proceedings]. J Physiol. 1978 Dec;285:42P–42P. [PubMed] [Google Scholar]
- Cabantchik Z. I., Knauf P. A., Rothstein A. The anion transport system of the red blood cell. The role of membrane protein evaluated by the use of 'probes'. Biochim Biophys Acta. 1978 Sep 29;515(3):239–302. doi: 10.1016/0304-4157(78)90016-3. [DOI] [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. The nature of the membrane sites controlling anion permeability of human red blood cells as determined by studies with disulfonic stilbene derivatives. J Membr Biol. 1972 Dec 29;10(3):311–330. doi: 10.1007/BF01867863. [DOI] [PubMed] [Google Scholar]
- Cole C. H. Bicarbonate-activated ATPase activity in renal cortex of chronically acidotic rats. Can J Physiol Pharmacol. 1979 Mar;57(3):271–276. doi: 10.1139/y79-040. [DOI] [PubMed] [Google Scholar]
- Cousin J. L., Motais R., Sola F. Transmembrane exchange of chloride with bicarbonate ion in mammalian red blood cells: evidence for a sulphonamide-sensitive "carrier". J Physiol. 1975 Dec;253(2):385–399. doi: 10.1113/jphysiol.1975.sp011195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin J. L., Motais R. The role of carbonic anhydrase inhibitors on anion permeability into ox red blood cells. J Physiol. 1976 Mar;256(1):61–80. doi: 10.1113/jphysiol.1976.sp011311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizzell R. A., Koch M. J., Schultz S. G. Ion transport by rabbit colon. I. Active and passive components. J Membr Biol. 1976;27(3):297–316. doi: 10.1007/BF01869142. [DOI] [PubMed] [Google Scholar]
- Hai M. A., Thomas S. The time-course of changes in renal tissue composition during lysine vasopressin infusion in the rat. Pflugers Arch. 1969;310(4):297–317. doi: 10.1007/BF00587241. [DOI] [PubMed] [Google Scholar]
- Heintze K., Petersen K. U., Olles P., Saverymuttu S. H., Wood J. R. Effects of bicarbonate on fluid and electrolyte transport by the guinea pig gallbladder: a bicarbonate-chloride exchange. J Membr Biol. 1979 Mar 28;45(1-2):43–59. doi: 10.1007/BF01869294. [DOI] [PubMed] [Google Scholar]
- Jacobson H. R., Gross J. B., Kawamura S., Waters J. D., Kokko J. P. Electrophysiological study of isolated perfused human collecting ducts: Ion dependency of the transepithelial potential difference. J Clin Invest. 1976 Nov;58(5):1233–1239. doi: 10.1172/JCI108577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinne-Saffran E., Kinne R. Presence of bicarbonate stimulated ATPase in the brush border microvillus membranes of the proximal tubule. Proc Soc Exp Biol Med. 1974 Jul;146(3):751–753. doi: 10.3181/00379727-146-38186. [DOI] [PubMed] [Google Scholar]
- Lambert A., Lowe A. G. Chloride/bicarbonate exchange in human erythrocytes. J Physiol. 1978 Feb;275:51–63. doi: 10.1113/jphysiol.1978.sp012177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law R. O. A pH-indpendent effect of external bicarbonate ions on the osmotic volume response of renal medullary cells [proceedings]. J Physiol. 1980 Jan;298:27P–28P. [PubMed] [Google Scholar]
- Law R. O. Factors affecting the distribution of chloride ions in rat renal outer medulla. J Physiol. 1977 Mar;266(1):173–189. doi: 10.1113/jphysiol.1977.sp011762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law R. O. The influence of chloride ions on renal outer medullary cell volume. J Physiol. 1979 Aug;293:485–500. doi: 10.1113/jphysiol.1979.sp012902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Williams P. G. The effect of vasopressin (Pitressin) administration and dehydration on the concentration of solutes in renal fluids of rats with and without hereditary hypothalamic diabetes insipidus. J Physiol. 1972 Feb;220(3):729–743. doi: 10.1113/jphysiol.1972.sp009732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie B. R., Schwartz J. H., Steinmetz P. R. Coupling between Cl- absorption and HCO3- secretion in turtle urinary bladder. Am J Physiol. 1973 Sep;225(3):610–617. doi: 10.1152/ajplegacy.1973.225.3.610. [DOI] [PubMed] [Google Scholar]
- Levine D. Z., Byers M. K., McLeod R. A., Luisello J. A., Raman S. Loop of Henle bicarbonate accumulation in vivo in the rat. J Clin Invest. 1979 Jan;63(1):59–66. doi: 10.1172/JCI109278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macknight A. D., Leaf A. Regulation of cellular volume. Physiol Rev. 1977 Jul;57(3):510–573. doi: 10.1152/physrev.1977.57.3.510. [DOI] [PubMed] [Google Scholar]
- Malnic G., Steinmetz P. R. Transport processes in urinary acidification. Kidney Int. 1976 Feb;9(2):172–188. doi: 10.1038/ki.1976.19. [DOI] [PubMed] [Google Scholar]
- Maren T. H. Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol Rev. 1967 Oct;47(4):595–781. doi: 10.1152/physrev.1967.47.4.595. [DOI] [PubMed] [Google Scholar]
- Mattenheimer H., Pollak V. E., Muehrcke R. C. Quantitative enzyme patterns in the nephron of the healthy human kidney. Nephron. 1970;7(2):144–154. doi: 10.1159/000179816. [DOI] [PubMed] [Google Scholar]
- Rocha A. S., Kokko J. P. Sodium chloride and water transport in the medullary thick ascending limb of Henle. Evidence for active chloride transport. J Clin Invest. 1973 Mar;52(3):612–623. doi: 10.1172/JCI107223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAIKIA T. C. COMPOSITION OF THE RENAL CORTEX AND MEDULLA OF RATS DURING WATER DIURESIS AND ANTIDIURESIS. Q J Exp Physiol Cogn Med Sci. 1965 Apr;50:146–157. doi: 10.1113/expphysiol.1965.sp001777. [DOI] [PubMed] [Google Scholar]
- Sanders S. S., O'Callaghan J., Butler C. F., Rehm W. S. Conductance of submucosal-facing membrane of frog gastric mucosa. Am J Physiol. 1972 Jun;222(6):1348–1354. doi: 10.1152/ajplegacy.1972.222.6.1348. [DOI] [PubMed] [Google Scholar]
- Stoner L. C., Burg M. B., Orloff J. Ion transport in cortical collecting tubule; effect of amiloride. Am J Physiol. 1974 Aug;227(2):453–459. doi: 10.1152/ajplegacy.1974.227.2.453. [DOI] [PubMed] [Google Scholar]
- Swanson C. H., Solomon A. K. Micropuncture analysis of the cellular mechanisms of electrolyte secretion by the in vitro rabbit pancreas. J Gen Physiol. 1975 Jan;65(1):22–45. doi: 10.1085/jgp.65.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. The role of bicarbonate, chloride and sodium ions in the regulation of intracellular pH in snail neurones. J Physiol. 1977 Dec;273(1):317–338. doi: 10.1113/jphysiol.1977.sp012096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich K. J., Capasso G., Rumrich G., Papavassiliou F., Klöss S. Coupling between proximal tubular transport processes. Studies with ouabain, SITS and HCO3-free solutions. Pflugers Arch. 1977 Apr 25;368(3):245–252. doi: 10.1007/BF00585203. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D. Regulation of chloride in quiescent sheep-heart Purkinje fibres studied using intracellular chloride and pH-sensitive micro-electrodes. J Physiol. 1979 Oct;295:111–137. doi: 10.1113/jphysiol.1979.sp012957. [DOI] [PMC free article] [PubMed] [Google Scholar]


