Abstract
Mice immunized with heat-inactivated, whole yeast-form cells (Y cells) of Candida albicans developed intense, specific humoral and cell-mediated immune responses. However, they were modestly protected against a lethal challenge by the fungus, and their sera did not confer passive protection upon nonimmunized animals. Surprisingly, this immune serum conferred an elevated degree of passive protection to normal and SCID mice when preadsorbed on whole C. albicans cells. After adsorption, no antibodies specific to mannoprotein (MP)-rich extracts or secretions were detected by indirect enzyme-linked immunosorbent assay and no serum reaction with the fungal cell surface was seen in immunofluorescence assays. However, this serum had totally preserved the level of other antibodies, in particular those reacting with β-1,3 and β-1,6 glucan (GG). The hypothesis that anti-GG antibodies contributed to the passive protection was suggested by the following circumstantial evidence: (i) mice immunized with C. albicans cells treated with dithiothreitol and protease (YDP cells), which exposed GG on their surfaces and generated anti-GG but not anti-MP antibodies, were substantially protected against a lethal fungus challenge; (ii) the sera, and their immunoglobulin fractions, of mice immunized with YDP cells transferred protection to nonimmune animals; and (iii) this passive protection was substantially abolished by preadsorption on GG but not on intact cells. Overall, our findings demonstrate that some anti-Candida antibodies can block the protective potential of immune serum, a potential to which anti-GG antibodies appear to contribute. Our observations may also help explain why subjects with elevated anti-Candida antibody titers, inclusive of anti-MP and anti-GG antibodies, remain nonetheless susceptible to invasive candidiasis.
Various forms of candidiasis have become increasingly prevalent in several clinical settings (22, 27, 37, 52), and a therapeutic or even an immuno-prophylactic vaccine would represent an important new tool in the fight against this disease (16, 19). This notion has gained some wider acceptance since the emergence of resistance to antimycotics, in particular to the azoles (1, 25, 53, 58), coupled with several advances in the knowledge of the immune response to Candida albicans (49, 50), which is by far the most prevalent etiological agent of candidiasis in humans (22, 53). Of primary relevance in this issue is the understanding of the nature of the protective anti-Candida response, the identification of antigenic and nonantigenic constituents involved, and their interaction. It is clear indeed that immune responses to this fungus are complex and, probably, redundant, in parallel with the multifaceted diseases it causes. In particular, the fungus has several mechanisms to evade potentially eradicating immunity, thereby persisting as a commensal or succeeding as a pathogen (13, 15, 16, 17, 20, 21, 38, 49, 50, 54).
In experimental animal models of candidiasis, optimal antifungal protection has been achieved by vaccination with an attenuated low-virulence strain or after spontaneous recovery from the initial infection (2, 10, 24, 48). Since candidiasis is especially prevalent among immunocompromised subjects, however, the use of inactivated whole-cell or subunit vaccines should be, in principle, a safer and more convenient approach. Various preparations of inactivated whole cells of the fungus as well as secretory, cell surface-located molecules and major cytoplasmic or cell wall enzymes have indeed been studied for the above purpose (reviewed in reference 16). A variable degree of protection has been demonstrated in animals immunized with some of these preparations, although the protective levels usually achievable with their use are generally perceived to be lower in magnitude and/or persistence than those obtained with the use of virulence-attenuated strains (6, 16, 18, 28, 30, 31, 40, 43).
With the possible exception of some secretory constituents (4, 14, 26, 55), whole cells of the fungus contain all the advocated protective antigens, and it is therefore somewhat surprising that the protection achieved with inactivated whole-cell preparations has been so variable and inconsistent. While inactivation can obviously decrease the immunogenic potential of one or more antigens, other reasons for the low vaccinating potential of these preparations may reside in the induction of immune responses which block or decrease the efficiency of protective responses. In other fungi, antibodies directed against cell surface structures appear to be involved in this negative interaction (8).
Having this in mind, we have investigated here the reasons why a vaccine consisting of heat-inactivated whole cells of C. albicans was poorly protective, even though it consistently elicited high-level humoral and cell-mediated immune responses directed against secretory and structural cell wall and cytoplasmic antigens of the fungus. We show here that the low level of protection was not due to the absence of immune responses to particular antigens but rather to the presence in the animal serum of blocking factors that are adsorbable on the intact cell surface and probably consist of or include anti-mannoprotein (MP) antibodies. We also show here the elevated efficacy of a vaccine preparation consisting of C. albicans cells deprived of mannoproteic surface constituents.
MATERIALS AND METHODS
Microorganism, culture conditions, and preparation of Y or YDP cells.
C. albicans strain BP, serotype A, from the type collection of the Istituto Superiore di Sanità (Rome, Italy), was routinely maintained on Sabouraud agar slants. For experimental purposes, the fungus was cultured in the yeast form in liquid Winge medium (4) at 28°C, washed twice in saline, counted in a hemocytometer, and resuspended to the desired concentration in sterile saline. For the preparation of the inactivated whole-cell vaccine (Y cells), yeast cell suspensions (108 cells/ml) were inactivated at 80°C for 30 min, washed, and stored at 4°C for no more than a week. For the preparation of the YDP cells, heat-inactivated cells as described above (108/ml) were treated with 50 mM dithiothreitol (DTT) in 5 mM sodium EDTA (1 h, 37°C). Proteinase K (500 μg/ml) (Sigma Chemical Co., St. Louis, Mo.) was then added to the digestion mixture and the cells were treated for one additional hour at 37°C. After thorough washing with saline to remove the enzyme, the cells were resuspended in saline and immediately used. Germ-tube forms of C. albicans were obtained by culturing yeast cells in Lee's medium at 37°C, as previously described (4).
Animals and immunization schedule.
Female 4-week-old CD2F1 and SCID mice were from Charles River Laboratory (Calco, Italy). For immunization with Y or YDP cells, mice were injected twice, at weekly intervals, by the subcutaneous route with each respective cell preparation (106 cells/100 μl/mouse) in incomplete Freund's adjuvant (Sigma) and five times by the intraperitoneal (i.p.) route with the same number of immunizing cells without adjuvant. Control animals were injected with Freund's adjuvant and saline only.
Systemic infection with C. albicans and assessment of protection.
Immunized or control mice were infected by the intravenous (i.v.) route with a lethal dose of C. albicans cells (106 or 2 × 106 in 0.1 ml, as specified in single experiments). Passively immunized mice (see below) received a sublethal challenge with 5 × 105 fungal cells or, in one experiment, a lethal challenge with 106 fungal cells. Protection was evaluated by monitoring animal survival for 60 days and/or by quantifying the extent of Candida outgrowth in the kidneys of the infected animals. For the latter purpose, the left kidneys of sacrificed mice were aseptically removed on day 2 or 7 postchallenge, as specified in single experiments, and homogenized in sterile saline containing 0.1% Triton X-100 (Sigma). The number of CFU per organ was determined by a plate dilution method on Sabouraud dextrose agar. Each kidney was examined separately and at least three distinct dilutions from each sample were assayed in triplicate.
Serological assays.
Immunized animals were bled by retroorbital puncture and the sera pooled from each immunization group were examined for their antibody content by enzyme-linked immunosorbent assay (ELISA) or by immunofluorescence assays (IFA). For the ELISA tests, polystyrene microtiter plates (MaxiSorp; NUNC, Roskilde, Denmark) were coated with Y or germ-tube cells (106/well) of C. albicans, with commercial standard glucan compounds (laminarin and pustulan), or with C. albicans MP or soluble glucan antigens (see below) at a concentration of 50 μg/ml in 0.05 M carbonate buffer, pH 9.6. Plates were blocked with 3% skim milk (Difco, Detroit, Mich.) in phosphate-buffered saline (PBS), reacted with twofold dilutions of mouse sera in PBS-0.05% Tween 20 (Sigma), and developed with alkaline phosphatase-conjugated rabbit anti-mouse immunoglobulin G or M (IgG or IgM) (Sigma) as the secondary antibody and p-nitrophenyl phosphate disodium (Sigma) as the enzyme substrate. Pooled sera from adjuvant-immunized mice were used as the negative control. Plates were read at 405 nm. Antibody titers were defined as the highest dilution of mouse sera that gave an optical density reading which was at least twice that of the negative control.
For IFA, Y or YDP cells were spotted onto microscope slides and reacted with various dilutions of murine anti-Y or anti-YDP sera or with the MP-recognizing monoclonal antibody AF1 (9, 17) (see below) in 0.01 M PBS. After extensive washings, slides were treated with fluorescein isothiocyanate-conjugated anti-mouse IgM antibody (Sigma) and observed with a Leitz Diaplan fluorescence microscope.
The reactivity of fungal cytoplasmic protein with anti-Y- or anti YDP-cell serum was assessed by the Western blotting technique, as previously described (4). Reactive protein bands were detected by using the murine serum at a dilution of 1:50 and alkaline phosphatase-conjugated goat anti-mouse polyvalent immunoglobulins as the revealing antibody (Sigma).
Adsorption of immune sera.
Anti-Y- or anti-YDP-cell sera were selectively adsorbed to remove glucan-specific or anti-cell-surface antibodies. To this end, sera (2 ml) were treated (1 h, 0°C) with 10 mg of particulate glucan (glucan ghosts) (51) or with 2 × 108 live yeast cells of C. albicans. The adsorbents were removed by centrifugation, and the procedure was repeated three times. The efficacy of the adsorption procedure was evaluated by ELISA, using yeast cells or glucan as the coating antigens. Typically, this procedure lowered the anti-glucan or anti-Y-cell antibody titers of anti-YDP or anti-Y sera 2 or 3 logs, respectively. Antibodies directed against glucan were not removed by adsorption with intact Y cells.
Lymphoproliferation assay.
Proliferation of splenocytes from adjuvant-, Y cell-, or YDP cell-immunized mice was assessed as previously described (3). Briefly, splenocyte suspensions in 3 ml of 0.16 M Tris-buffered NH4Cl, pH 7.2, were added to 9 ml of complete medium (RPMI 1640; Invitrogen, Grand Island, N.Y.) supplemented with 5% fetal calf serum (Invitrogen), 100 U of penicillin per ml, 100 mg of streptomycin per ml, 1 mM sodium pyruvate, 2 mM l-glutamine, minimal essential medium (MEM)-nonessential amino acids, and 10−5 M 2-mercaptoethanol (Invitrogen). The splenocytes were washed by centrifugation, plated in multiwell plates (106/ml, 200 μl/well), and stimulated with Y or YDP cells (105/well) or with concanavalin A (ConA; 2 μg/ml) (Sigma). Each condition was assayed in triplicate. Splenocyte cultures were incubated at 37°C in a 5% CO2 atmosphere. Proliferation was evaluated by [3H]thymidine (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom) incorporation after 4 days of incubation for the antigenic stimuli and after 2 days of incubation for the polyclonal control stimulant. Stimulation indices were the ratios of mean counts per minute values of stimulated splenocyte cultures to those of unstimulated control cultures.
Passive immunization of mice and assessment of protection.
CD2F1 or SCID mice were passively immunized by a single i.p. injection of 0.5 ml of nonadsorbed or adsorbed anti-Y-cell or anti-YDP-cell serum. Control animals received serum from adjuvant-immunized mice. Each serum was heat treated (56°C, 30 min) before transfer to inactivate heat-labile nonantibody constituents. Mice were challenged i.v. 2 h after the serum transfer and protection was evaluated by the extent of kidney invasion or survival of the infected animals (see above for details).
Candida antigens and other reagents.
The MP-rich secretion from C. albicans yeast cells was prepared from the supernatant of a 24-h fungal culture in Lee's medium at 28°C, as reported elsewhere (4). The MP-rich fraction MP-F2 was purified from the C. albicans cell wall, as already described (56). Purified, particulate glucan (glucan ghosts), exclusively composed of β-1,3 and β-1,6 glucan, was obtained by repeated hot alkali-acid extractions of fungal cell walls (51). The soluble glucan fraction was an enzymatic digest (1 h, 37°C) of insoluble glucan ghosts with purified β-1,3 glucanase (Zymoliase 100T; Seikagaku Co., Tokyo, Japan). Total cytoplasmic proteins were extracted from yeast cells by treatment (100°C, 10 min) with 0.5 M Tris-HCl buffer containing 10% sodium dodecyl sulfate and 3.5 M 2-mercaptoethanol or by hypotonic lysis of C. albicans protoplasts obtained as described by Miragall et al. (42). The monoclonal antibody AF1, recognizing a β-1,2 oligomannoside epitope highly expressed on the surface of C. albicans yeast cells, has already been described (9, 57). Standard β-1,3 glucan (laminarin) and β-1,6 glucan (pustulan) were purchased from Sigma and CalbioChem (La Jolla, Calif.), respectively.
Statistical evaluation.
The data were assessed for statistical significance by Fisher's exact test or a two-tailed Mann-Whitney U test, as appropriate and as stated in the legends to the figures. Multiple comparisons were made by analysis of variance (one-way ANOVA or Kruskal-Wallis ANOVA) followed by Bonferroni's multiple t test.
RESULTS
Immune responses of mice immunized with Y or YDP cells.
Because of the methods used for their preparation, Y cells should be able to immunize the mice against all major antigenic cell wall and cytoplasmic constituents of C. albicans while immunization with YDP cells should not be able to induce consistent immune reactivity against some cell surface constituents of the fungus. As shown in Table 1, the anti-Y-cell serum contained antibodies against all major cell wall constituents, present in both Y and germ-tube forms, including β-1,6 (pustulan) and β-1,3 (laminarin) glucans. Confirming our expectations, the anti-YDP-cell serum had an elevated titer of antibodies against glucan but low titers of antibodies against the whole yeast or hyphal (germ-tube) cells as well as the cell surface-located or secretory MP-rich constituents. Both the anti-Y-cell and the anti-YDP-cell sera contained antibodies against major cytoplasmic constituents, as seen by Western blots, without substantially differing in the reactivity of the different protein bands (data not shown).
TABLE 1.
Reactivity of the antibodies raised in mice by Y- or YDP-cell immunizationa
| Antigenb | Antibody titerc
|
|
|---|---|---|
| Anti-Y-cell serum | Anti-YDP-cell serum | |
| Y cells | 1,280 | 40 |
| Germ tubes | 1,280 | 40 |
| Secr-MP | 2,560 | 80 |
| MP-F2 | >2,560 | 320 |
| GGZym | 2,560 | 2,560 |
| Lam | 2,560 | 2,560 |
| Pust | 2,560 | 1,280 |
Reactivity of sera pooled from groups of 10 mice immunized with Y or YDP cells was assessed by indirect ELISA, as described in Materials and Methods. Values are from one representative experiment out of three performed with similar results.
Secr-MP, MP-rich secretion; MP-F2, purified mannoproteic extract from the fungal cell wall; GGZym, soluble-β-1,3 glucanase digest from a purified cell wall glucan preparation; Lam, laminarin (β-glucan); Pust, pustulan (β-1,6-glucan).
Titers are the reciprocals of the highest dilution giving a positive reading (see Materials and Methods).
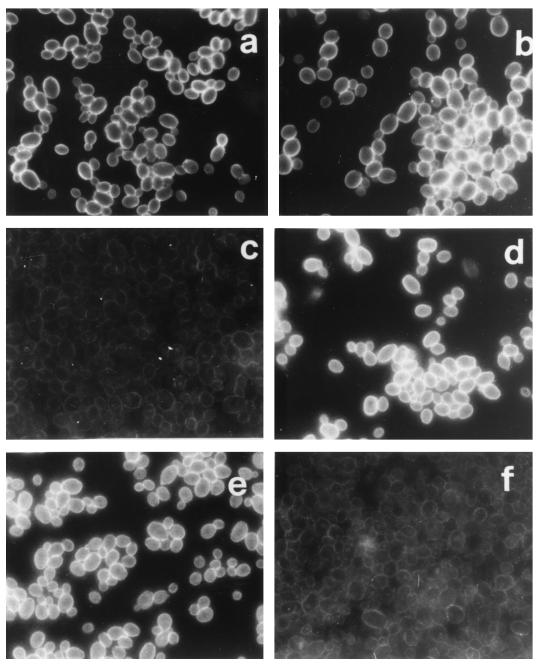
The anti-Y-cell serum was strongly reactive in immunofluorescence with both Y and YDP cells (Fig. 1a and b), while the anti-YDP-cell serum almost exclusively recognized the YDP cells (Fig. 1c and d). As a further control, YDP cells of C. albicans were seen to lose reactivity with a monoclonal antibody (AF1) which detects a β-oligomannoside epitope largely shared by cell surface MP and therefore strongly reactive with the fungal cell surface (Fig. 1e and f).
FIG. 1.
IFA reactivity of anti-Y-cell or anti-YDP-cell serum with C. albicans and effect of treatment with DTT and proteinase K on the antigenic array of the fungal cell surface. Untreated Y cells (a, c, and e) or DTT-proteinase-treated YDP cells (b, d, and f) were immunostained as described in Materials and Methods with the anti-Y-cell (a and b) or anti-YDP-cell (c and d) serum or with a monoclonal antibody (AF1) (e and f). Anti-Y-cell and anti-YDP-cell sera were used at a dilution of 1:200; the monoclonal antibody AF1 was used at a dilution of 1:5,000. Magnification, ×1,000. The figure represents the results of five repeated experiments.
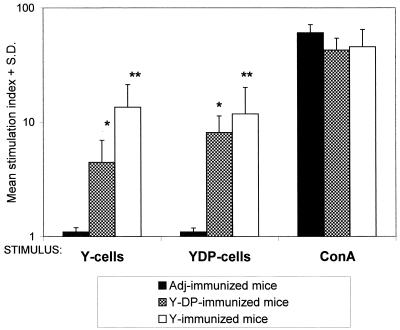
To assay for the induction of cell-mediated immunity (CMI) following Y- or YDP-cell immunization, spleen cells of control or immunized mice were induced to proliferate in vitro in the presence of Y or YDP cells. ConA-stimulated splenocytes served as the positive control. As shown in Fig. 2, immunization with Y or YDP cells was largely cross-reactive in stimulating a consistent degree of splenocyte proliferation, although a more intense response (though statistically nonsignificant) was apparent with splenocytes stimulated in vitro with the specific immunizing antigenic preparation. The splenocytes of all animals, including the nonimmunized controls, responded to the polyclonal stimulation with ConA.
FIG. 2.
In vitro proliferative response of splenocytes of mice immunized with Y cells or YDP cells. Individual splenocyte cultures from four mice for each immunization group were stimulated in vitro with Y or YDP cells or with ConA, as described in Materials and Methods. Proliferative responses were evaluated after 3 days by measuring [3H]thymidine incorporation. Values are mean stimulation indexes ± standard deviations measured for each experimental group compared to unstimulated control cultures. The asterisks indicate a significant difference (∗, P < 0.05; ∗∗, P < 0.001; ANOVA and Bonferroni's multiple t test) with respect to values measured in adjuvant (Adj)-treated animals. All other differences in the proliferative response were not significant. The data are from a representative experiment of three with similar results.
Overall, immunization with Y or YDP cells induced largely cross-reactive humoral and CMI responses to the plethora of antigens present on both cellular preparations. However, anti-MP-rich material and anti-Y-cell surface-directed antibodies were consistently present only in mice immunized with whole Y cells.
Anti-Candida protection.
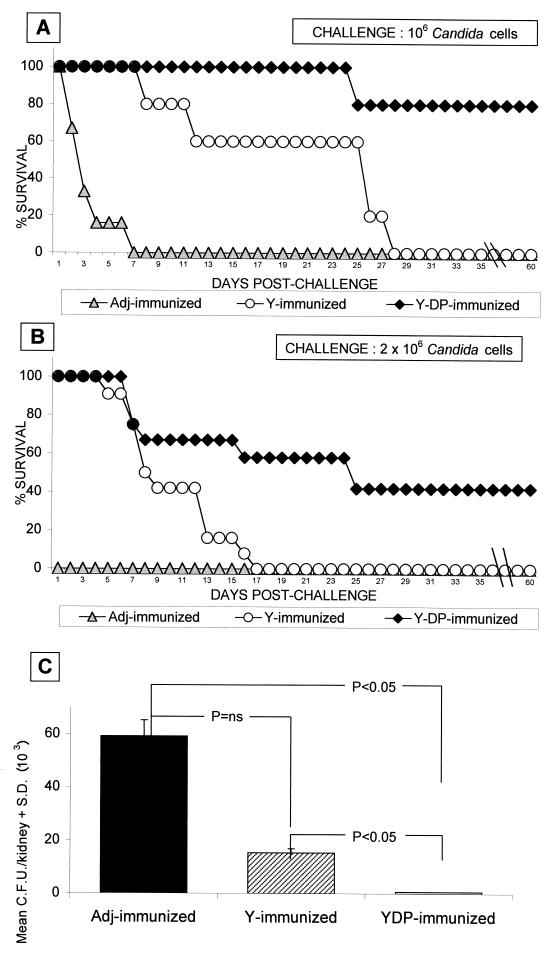
Having demonstrated that immunization with Y cells induced consistent humoral and CMI responses against major antigenic constituents of the fungus, we assessed the protective capacity of the Y-cell vaccine in an acutely lethal mouse candidiasis model. As shown in Fig. 3 (which reports data from two independent experiments with CD2 F1 mice challenged with different lethal doses of the fungus), adjuvant only-treated mice had a median survival time (MST) of 1 to 3 days following the virulent i.v. challenges and a kidney burden of 105 to 106 fungal cells close to the lethal event. The animals immunized with Y cells somewhat resisted, in terms of increased MST, a fungal challenge which rapidly killed all nonimmunized animals (MST = 7 and 25 days for C. albicans challenge with 2 × 106 and 106 cells, respectively; P < 0.01 with respect to adjuvant-treated mice; Mann-Whitney U test). Nonetheless, they all died by days 17 to 35, in proportion to the size of the challenging dose (Fig. 3A and B). In contrast, 50 to 80% of the animals immunized with the YDP cells survived the lethal challenge with 2 × 106 or 106 Candida cells, respectively, with an MST of >60 days and a mean fungus burden in the kidney significantly lower than that measured in Y cell-immunized mice (<103 compared to 15.4 × 103 ± 0.6 × 103 on day 7; P < 0.05) (Fig. 3).
FIG. 3.
Different protective effect of Y- or YDP-cell immunization against murine disseminated candidiasis. (A and B) Survival rates of Y or YDP cell-vaccinated mice compared to those of control, nonimmunized mice. Mice (6 for panel A and 15 for panel B) were immunized with Y or YDP cells or with adjuvant (Adj) only and challenged i.v. with 106 (A) or 2 × 106 (B) Candida cells. Data represent percent survival, recorded daily for 60 days postchallenge. Differences in survival rates (on day 60) between YDP cell- and adjuvant- or Y cell-immunized animals were found to be statistically significant (P < 0.05) as assessed by Fisher's exact test. For the statistical significance of the MST (in days), see the text. The data in panel A refer to a single experiment, while the data in panel B represent the results of two separate experiments. (C) Kidney invasion in Y or YDP cell-vaccinated mice following an i.v. challenge with C. albicans. Groups of mice immunized with Y or YDP cells were challenged i.v. with 106 Candida cells. On day 7 postchallenge, three mice per group were sacrificed and fungal invasion in the left kidney was evaluated by individual CFU counts. Values are weighted means of CFU count measured in each group of animals ± standard deviations. Probability, as indicated in the graph, was evaluated by Kruskal-Wallis ANOVA and Bonferroni-type nonparametric multiple comparison. The data are from a representative experiment of two with similar results (independent of the data in panels A and B).
Two experiments were also performed with SCID mice immunized as the immunocompetent animals. No protection was observed (data not shown), demonstrating that adaptive immune responses were essential to achieve protection.
Passive immunization and role of anti-β-glucan antibodies in anti-Candida protection.
Since there is growing evidence of the protective role of anti-candidal antibodies (11, 16) and since a major difference in the immune response to Y or YDP cells was in the antibody specificity to cell wall constituents (see above), we wondered whether and which immune serum could transfer some protection to nonimmune animals. In these experiments, we also evaluated the potential contribution of the immune system of the recipient mice to the protection conferred by the passively administered serum. Thus, CD2F1 or SCID mice were given a single injection (0.5 ml) of serum from vaccinated animals, followed 2 h later by challenge with C. albicans. Other animals were given serum from mice administered adjuvant only as a control. Two days after challenge, the animals were sacrificed and fungus burden in the kidney was assessed as a measure of protection. These experiments were performed with various batches of serum from animals independently immunized with the YDP- or Y-cell vaccine.
As shown in Table 2, the animals receiving the anti-Y-cell serum had the same elevated fungus burden in their kidneys as those receiving the control nonimmune serum. In contrast, those receiving the anti-YDP-cell serum had significantly fewer fungal cells in their kidneys than the animals receiving adjuvant serum. This was observed with different batches of the immune sera and in both the immunocompetent and SCID mice (Table 2).
TABLE 2.
Outcome of experimental disseminated candidiasis in mice passively immunized with serum from Y or YDP cell-immunized micea
| Recipient mouse strain | Prechallenge treatment | Fungal burden in kidney (CFU [103] ± SD) | Pb (compared to control) |
|---|---|---|---|
| CD2F1 | Control (adjuvant only) serum | 361.7 ± 17.6 | |
| Anti-Y-cell serum 1 | 393.8 ± 7.7 | NS | |
| Anti-YDP-cell serum 1 | 8.7 ± 0.4 | <0.05 | |
| SCID | Control (adjuvant only) serum | 214.4 ± 1.8 | |
| Anti-Y-cell serum 2 | 392.2 ± 7.7 | NS | |
| Anti-YDP-cell serum 2 | 44.2 ± 0.8 | <0.05 |
Groups of four CD2F1 or SCID mice received a single dose (0.5 ml, i.p.) of the indicated immune serum. Two hours later mice were challenged i.v. with a sublethal dose of C. albicans (5 × 105 cells). Fungal burden in the left kidneys of the challenged mice was evaluated 48 h postchallenge by CFU counts. Data represent the weighted means (weighted averages) of the individual CFU counts enumerated from each group of mice. Data are from one experiment out of two with similar results.
P, probability statistical analysis of data was performed by Kruskal-Wallis ANOVA followed by a nonparametric Bonferroni-type multiple comparison test. NS, not significant. Differences between anti-Y-cell serum (1 or 2) and anti-YDP-cell serum (1 or 2) were also statistically significant (P < 0.05; not shown).
Since these data suggested that antibodies could indeed play a role in the protection, we partially purified the IgM fraction of serum from mice immunized with YDP cells and used this fraction for passive immunization. The same fraction purified from the serum of animals given adjuvant only was used as a control. In a single experiment, the fungus kidney burden on day 2 postchallenge of four mice intravenously injected with 106 cells of C. albicans was 290 × 103 ± 8 × 103 CFU compared to 1,359 × 103 ± 18 × 103 CFU in the kidneys of four mice given the IgM fraction from adjuvant serum (P < 0.01). The IgM fraction of YDP-cell serum was highly reactive against the glucan extract of C. albicans (data not shown).
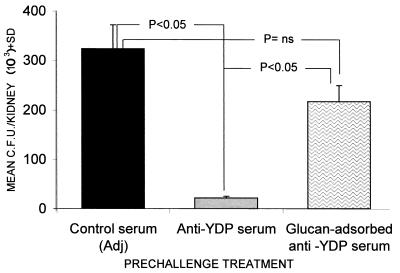
Finally, considering that antibodies in serum generated by immunization with the YDP cells mostly recognized β-glucan among the cell wall constituents (Table 1; Fig. 1), we also attempted passive transfer of immune sera preadsorbed on pure glucan particles to remove specific antibodies. As shown in Fig. 4, when using the serum preadsorbed on glucan particles, thus removing most of the anti-β-glucan antibodies (see Materials and Methods), the fungus burden in the kidney was significantly higher than that found in animals which received the nonadsorbed anti-YDP-cell serum. A nonsignificant difference in Candida kidney burden was found between mice given the glucan-adsorbed serum and those receiving the serum from adjuvant-treated animals.
FIG. 4.
Effect of preadsorption with particulate glucan on the protective action of anti-YDP-cell serum against a systemic fungal challenge. Control unadsorbed or preadsorbed serum was given i.p. (0.5 ml/mouse) to three mice per group 2 h before an i.v., sublethal challenge with C. albicans (5 × 105 cells/mouse). Kidney invasion was assessed 48 h postchallenge by individual CFU counts of individual kidneys. Data are weighted means of CFU counts measured in mice from each experimental group. Statistical assessment was performed by Kruskal-Wallis ANOVA and Bonferroni-type nonparametric multiple comparison. The data are the results of one representative experiment out of three with similar results. Adj, adjuvant.
Protective and nonprotective antagonistic antibodies.
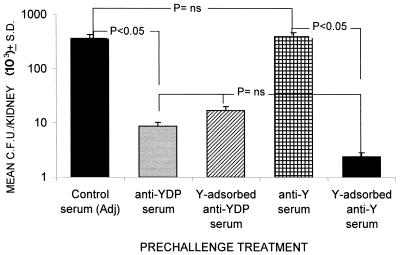
The previous data suggested that anti-β-glucan antibodies played a role in the protection conferred by the YDP-cell vaccine. Thus, we wondered why the serum of animals immunized with the Y-cell vaccine, which also contained high titers of anti-β-glucan antibodies (Table 1), did not transfer protection. We hypothesized that these sera could contain antagonistic or blocking antibodies, probably directed against cell surface constituents of the fungus, since these antibodies were absent in the protective serum from YDP cell-immunized mice (Table 1; Fig. 1). Thus, we evaluated the fungus burden in the kidneys of SCID mice challenged with C. albicans and injected a serum obtained from Y cell-immunized animals and preadsorbed on Y cells. The adsorption was effective, as demonstrated by the substantial reduction of anti-MP antibodies and maintenance of the elevated anti-β-glucan antibody level. As shown in Fig. 5, the animals receiving the preadsorbed Y-cell serum had a kidney burden that was about 2 logs lower than that of the animals given nonadsorbed Y-cell serum and comparable to that of the animals given the protective unadsorbed YDP-cell serum. As a control, adsorption of anti-YDP-cell serum with Y cells did not substantially affect its ability to induce protection. Thus, the anti-Y-cell serum contains antibodies to the yeast cell surface which are inhibitory for protection conferred by antibodies against underlying cell wall antigens (β-glucan).
FIG. 5.
Effect of preadsorption with intact Y cells on the protective anti-Candida activity of anti-Y-cell or anti-YDP-cell serum. Mice (three per group) were injected i.p. with 0.5 ml of the indicated serum. Two hours later, the animals were challenged i.v. with 5 × 105 cells of C. albicans. Kidney invasion was evaluated 48 h postchallenge by individual CFU counts of individual kidneys. Data represent weighted means of CFU counts for each experimental group. Statistical comparisons were made by Kruskal-Wallis ANOVA and Bonferrroni-type nonparametric multiple comparison. The data are the results of one representative experiment out of two with similar results. Adj, adjuvant.
DISCUSSION
In this paper, we report three main interrelated and novel findings addressing the issue of a protective vaccine against C. albicans, one of the principal agents of widespread opportunistic infections in humans. First, we show that the animals immunized with whole, intact Y cells of C. albicans contain cell surface-reactive antagonistic or blocking antibodies. Second, we demonstrate the immunogenicity and efficacy of a preparation of fungal cells substantially deprived of these cell surface constituents (YDP cells), thus exposing inner, β-glucan-rich constituents. Notably, cell surface MPs are usually considered the dominant antigenic components of the fungus while β-glucans are not (12-14). Third, we present some circumstantial evidence that the anti-Candida protection induced by the YDP cells is in part mediated by antibodies, with anti-β-glucan IgM likely playing a relevant role. Overall, our findings constitute the first evidence that serum-transferable, antibody-mediated protection against C. albicans can be negated by immune (antibody) responses to cell surface-located immunodominant antigens of the fungus.
Mouse models of systemic, lethal infections by C. albicans have been largely used to investigate the Candida-host relationship, and in particular a wealth of information has been generated on anti-Candida protective immune mechanisms. Thus, a T-helper type 1 response producing, and induced by, gamma interferon and interleukin-12 (49) is widely considered to be critical for protection induced by attenuated strains (2, 48) or inactivated fungal cells (6). Gamma interferon itself or other cytokines profusely released during an expanded Th1 response have been demonstrated to be strong phagocyte activators, in keeping with clinical evidence that phagocytic cells, in particular polymorphonuclear phagocytes, are primarily involved in eradicating the systemic infection. While it is therefore clear that CMI responses are critically involved in the protection, other immune mechanisms can also operate for anti-Candida defense, as recently discussed by Casadevall et al. (7), Han et al. (28, 30), Cutler et al. (16), and ourselves (11, 18, 46). Specifically, it has been shown that antibodies may be protective against both mucosal and systemic infection, provided that they are of the right specificity and isotype (11, 18, 29, 30, 39, 47). Few antigens have, however, been characterized for their ability to elicit protective anti-Candida antibodies and, to our knowledge, the poorly immunogenic β-glucan constituents of the inner Candida cell wall (36) were never considered among the putatively protective antigens.
We obtained here suggestive evidence that some anti-Candida antibodies may be deleterious for protection, as they appear to antagonize the action of protective antibodies. Although we have not exactly identified these antagonistic antibodies, they are clearly directed against some of the cell surface constituents of the fungus, for instance, the MP-rich cell extract or cell secretion (here collectively defined as MP). The idea that some antibodies are enhancing rather than combating infection is not new. It has been particularly promoted by the studies by Casadevall and collaborators through the use of monoclonal antibodies against Cryptococcus neoformans (8, 45, 59). We have previously shown that animals with high antibody titers against the 70-kDa heat shock protein are more susceptible to lethal Candida disease (3). Others have shown that antibodies may inhibit critical phagocyte functions in vitro (8). However, this is, to our knowledge, the first demonstration that a nonprotective anti-Candida serum becomes protective against a lethal systemic challenge when deprived of some antibodies. Our observation may explain why anti-Candida sera have been so inconsistent in transferring protection and why immunization with whole inactivated cells of C. albicans has been so variably protective though always stimulating a delayed-type hypersensitivity reaction, other CMI responses, and abundant anti-Candida antibodies (23, 33, 44). Our data strongly suggest that antibody-mediated protection against C. albicans not only requires the presence of the right antibody but also requires the absence of other antibodies. Considering that antibodies against abundantly expressed cell surface constituents are so prevalent in healthy people colonized by C. albicans, the generation of antagonistic or blocking antibodies may be seen as a manner by which the fungus defends itself from the eradicating capacity of other antibodies and CMI responses, also clearly detectable and intense in almost all healthy subjects (also see below).
On the other hand, the idea that antibodies were at least in part responsible for the high-level protection induced by the YDP-cell vaccine and possibly also mediated the low degree of protection achieved with Y-cell immunization is suggested by the facts that an appreciable level of protection was transferred to naive animals by the serum of YDP-cell recipient animals, that the protective serum factor was heat stable, and that the immunoglobulin fraction was also protective. The protective serum was rich in anti-β-glucan and poor in anti-MP antibodies. When adsorbed on pure β-glucan, this serum loses much of its protective capacity. Moreover, the anti-Y-cell serum was protective when deprived of the anti-MP but not the anti-β-glucan antibodies. Overall, direct and indirect evidence suggest that at least part of the protective IgM antibodies are those recognizing β-glucan.
Note that antibodies to β-glucan are present in normal human sera (34). Since they do not react (as confirmed here) with cell surface components and do not obviously opsonize the fungal cell, they are not usually considered in the mechanism of protection. The anti-β-glucan IgG2 antibodies described by Keller et al. (34) and by Merkel and Scofield (41) were indeed seen to be dispensable for opsonic activity of nonencapsulated, β-glucan-exposing C. neoformans cells. Our data, however, invite us to reconsider this aspect, as anti-β-glucan antibodies might well play a role, mostly when other blocking antibodies are absent, as discussed above. Anti-β-glucan antibodies have been involved in rapid opsonizing complement activation by Blastomyces dermatitidis yeast cells (61), which expose β-glucan on their surfaces (35). However, no evidence has been provided that these anti-β-glucan antibodies are indeed protective. Also note that one of the pioneering evidences of protective anti-Candida antibodies (44) was achieved by the use of sonically disrupted cells. Although the protection observed has been attributed by Cutler et al. (16) to a nonspecific agglutinating antibody, it might have been caused by antibodies to β-glucan or other antigens present in inner cell wall layers and exposed in a suitable form on disrupted cells.
The potential of protection exerted by the passive transfer of anti-β-glucan antibody-rich serum can be better appreciated if we consider the isotype (IgM) of the protective antibody. IgM antibodies are very avid but they also have a very short half-life compared to IgG antibodies. The fact that a single i.p. injection of the serum was protective is also notable. Nonetheless, other antibodies could be involved in the protection. While there is clear evidence that YDP cells had lost most of their surface-located antigenic material following the treatment with DTT and protease, it can be safely assumed that many other strongly immunogenic cellular constituents are antigenically expressed by these cells. β-Mannan, for instance, has been shown to contain protective antigenic epitopes in systemic infection models (29), supposedly owing to a rapid binding of complement factor 3, thus causing efficient opsonization and killing of C. albicans by phagocytes (32). Importantly, the protective antibodies elicited by the YDP-cell vaccine were adsorbed by β-glucan particles which do not contain mannose (12, 51). They did not appreciably bind, thus could not opsonize, the whole Y cells of the fungus or facilitate rapid deposition of complement, phagocytosis, and killing (32). Moreover, the YDP cells did not bind a monoclonal antibody (AF1) which recognizes a β-oligomannoside epitope (9, 57). Clearly, a distinct possibility still exists that antibodies against a particular β-mannan structure (16) or other unknown, non-β-glucan antigen present in YDP cells are involved in anti-Candida protection. This is currently being investigated in our laboratories.
Although we cannot explain the mechanisms of action of anti-β-glucan antibodies, our findings rule out that anti-YDP antibodies owe their protective effect to indirectly promoting one or more adaptive immune responses against the fungus. In fact, passive transfer of serum was equally protective in normal and SCID mice, suggesting that the anti-Candida activity exerted by the anti-YDP-cell antibody is not supported by and does not require early help or mediation by adaptive responses of the recipient, an event which is likely happening for other mechanisms of antibody-mediated protection (60).
All of the above does not mean that antibodies are uniquely responsible for protection in the normal mouse. We have demonstrated here that a rather potent CMI response is elicited by YDP cells. This response can have a contributory role, as previously reported for immunization with MP-65, a major target of the anti-Candida CMI response in humans (26), which elicited little antibody response but induced a potent, Th1-oriented CMI response (40). However, the degree of protection induced by vaccination with MP-65 was definitely lower than that reported here with the YDP-cell vaccine (40). In the present study, the Y-cell vaccine also induced a potent CMI response that was substantially cross-reactive to YDP cells but was minimally protective. In addition, Y cells have an intrinsic adjuvanticity which can be expressed, for instance, by a degree of macrophage activation or activation of other natural immunoeffectors. There is no reason to believe that cells exposing inner β-glucan constituents lose their adjuvanticity. As a matter of fact, this can be enhanced in the YDP cells since β-glucan is a well-known immunomodulator (12, 51) capable of strongly activating the whole system of natural immunity through several receptors, some of which have recently been identified (5). This activation can indeed collaborate with antibodies and CMI in protecting against C. albicans.
Acknowledgments
We are grateful to J. E. Cutler for reading the manuscript and providing constructive suggestions. Thanks are also due to A. Botzios and A. M. Marella for help in the preparation of the manuscript.
This investigation was supported by the “Programma Nazionale AIDS”—Istituto Superiore di Sanità, Italy, under contract no. 50D.2.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Alexander, B. D., and J. R. Perfect. 1997. Antifungal resistance trends towards the year 2000: implications for therapy and new approaches. Drugs 54:657-678. [DOI] [PubMed] [Google Scholar]
- 2.Bistoni, F., A. Vecchiarelli, E. Cenci, P. Puccetti, P. Marconi, and A. Cassone. 1986. Evidence for macrophage-mediated protection against lethal Candida albicans infection. Infect. Immun. 51:668-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bromuro, C., R. La Valle, S. Sandini, F. Urbani, C. M. Ausiello, L. Morelli, C. Fè d'Ostiani, L. Romani, and A. Cassone. 1998. A 70-kilodalton recombinant heat shock protein of Candida albicans is highly immunogenic and enhances systemic murine candidiasis. Infect. Immun. 66:2154-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bromuro, C., A. Torosantucci, M. J. Gomez, F. Urbani, and A. Cassone. 1994. Differential release of an immunodominant 65 kDa mannoprotein antigen from yeast and mycelial forms of Candida albicans. J. Med. Vet. Mycol. 32:447-459. [DOI] [PubMed] [Google Scholar]
- 5.Brown, G. D., and S. Gordon. 2001. Immune recognition: a new receptor for β-glucans. Nature 413:36-37. [DOI] [PubMed] [Google Scholar]
- 6.Cardenas-Freytag, L., E. Cheng, P. Mayeux, J. E. Doner, and J. D. Clements. 1999. Effectiveness of a vaccine composed of heat-killed Candida albicans and a novel mucosal adjuvant, LT (R192G), against systemic candidiasis. Infect. Immun. 67:826-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casadevall, A., A. Cassone, F. Bistoni, J. E. Cutler, W. Magliani, J. W. Murphy, L. Polonelli, and L. Romani. 1998. Antibody and/or cell-mediated immunity, protective mechanisms in fungal disease: an ongoing dilemma or an unnecessary dispute? Med. Mycol. 36:95-105. [PubMed] [Google Scholar]
- 8.Casadevall, A. 2001. Humoral immunity and Cryptococcus neoformans, p. 293-324. In R. A. Calderone and R. L. Cihlar (ed.), Fungal pathogenesis, principles and clinical applications. Marcel Dekker, New York, N.Y.
- 9.Cassone, A., A. Torosantucci, M. Boccanera, G. Pellegrini, C. Palma, and F. Malavasi. 1988. Production and characterization of a monoclonal antibody to a cell-surface, glucomannoprotein constituent of Candida albicans and other pathogenic Candida species. J. Med. Microbiol. 27:233-238. [DOI] [PubMed] [Google Scholar]
- 10.Cassone, A., M. Boccanera, D. Adriani, G. Santoni, and F. De Bernardis. 1995. Rats clearing a vaginal infection by Candida albicans acquire specific, antibody-mediated resistance to vaginal reinfection. Infect. Immun. 63:2619-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cassone, A., S. Conti, F. De Bernardis, and L. Polonelli. 1997. Antibodies, killer toxin and antifungal immunoprotection: a lesson from nature? Immunol. Today 18:164-169. [DOI] [PubMed] [Google Scholar]
- 12.Cassone, A., and A. Torosantucci. 1991. Immunological moieties of the cell wall, p. 89-107. In R. Prasad (ed.), Candida albicans. Springer-Verlag, Berlin, Germany.
- 13.Cassone, A., F. De Bernardis, C. M. Ausiello, M. J. Gomez, M. Boccanera, R. La Valle, and A. Torosantucci. 1998. Immunogenic and protective Candida albicans constituents. Res. Immunol. 149:289-298. [DOI] [PubMed] [Google Scholar]
- 14.Cutler, J. E. N. Glycosylation of yeast, with emphasis on Candida albicans. Med. Mycol. 39(Suppl. 1):75-86. [PubMed]
- 15.Cutler, J. E., and T. Kanbe. 1993. Antigenic variability of Candida albicans cell surface. Curr. Top. Med. Mycol. 5:27-47. [PubMed] [Google Scholar]
- 16.Cutler, J. E., B. L. Granger, and Y. Han. 2001. Vaccines, antibodies and passive immunity in candidiasis, p. 325-352. In R. A. Calderone and R. L. Cihlar (ed.), Fungal pathogenesis, principles and clinical applications. Marcel Dekker, New York, N.Y.
- 17.De Bernardis, F., A. Molinari, M. Boccanera, A. Stringaro, R. Robert, J. M. Senet, G. Arancia, and A. Cassone. 1994. Modulation of cell surface-associated mannoprotein antigen expression in experimental candidal vaginitis. Infect. Immun. 62:509-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Bernardis, F., M. Boccanera, D. A. Adriani, E. Spreghini, G. Santoni, and A. Cassone. 1997. Protective role of antimannan and anti-aspartyl proteinase antibodies in an experimental model of Candida albicans vaginitis in rats. Infect. Immun. 65:3399-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deepe, G. S., Jr. 1997. Prospects for the development of fungal vaccines. Clin. Microbiol. Rev. 10:585-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deepe, G. S., Jr., L. Romani, V. L. Calich, G. Huffnagle, C. Arruda, E. E. Molinari-Madlun, and J. R. Perfect. 2000. Knockout mice as experimental models of virulence. Med. Mycol. 38(Suppl. 1):87-98. [PubMed] [Google Scholar]
- 21.d'Ostiani, C. F., G. Del Sero, A. Bacci, C. Montagnoli, A. Spreca, A. Mencacci, P. Ricciardi-Castagnoli, and L. Romani. 2000. Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo. J. Exp. Med. 191:1661-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edmond, M. B., S. E. Wallace, D. F. Mc Clish, M. A. Pfaller, R. N. Jones, and R. P. Wenzel. 1999. Nosocomial bloodstream infections in United States hospitals: a three year analysis. Clin. Infect. Dis. 29:239-244. [DOI] [PubMed] [Google Scholar]
- 23.Elahi, S., R. Clancy, and G. Pong. 2001. A therapeutic vaccine for mucosal candidiasis. Vaccine 19:2516-2521. [DOI] [PubMed] [Google Scholar]
- 24.Fidel, P. L., Jr., and J. D. Sobel. 1998. Protective immunity in experimental Candida vaginitis. Res. Immunol. 149:361-373. [DOI] [PubMed] [Google Scholar]
- 25.Goldman, M., G. A. Cloud, M. Smedema, A. La Monte, P. Connolly, D. S. McKinsey, C. A. Kauffman, B. Moskowitz, L. J. Wheat, et al. 2000. Does long-term itraconazole prophylaxis result in in vitro azole resistance in mucosal Candida albicans isolates from persons with advanced human immunodeficiency virus infection? Antimicrob. Ag. Chemother. 44:1585-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomez, M. J., A. Torosantucci, S. Arancia, B. Maras, L. Parisi, and A. Cassone. 1996. Purification and biochemical characterization of a 65-kilodalton mannoprotein (MP65), a main target of anti-Candida cell-mediated immune responses in humans. Infect. Immun. 64:2577-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grossi, P., C. Farina, R. Fiocchi, and D. Dalla Gasperina. 2000. Prevalence and outcome of invasive fungal infection in 1,963 thoracic organ transplant recipients: a multicenter retrospective study. Transplantation 70:112-116. [PubMed] [Google Scholar]
- 28.Han, Y., and J. E. Cutler. 1995. Antibody response that protects against disseminated candidiasis. Infect. Immun. 63:2714-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han, Y., T. Kanbe, R. Cherniak, and J. E. Cutler. 1997. Biochemical characterization of Candida albicans epitopes that can elicit protective and nonprotective antibodies. Infect. Immun. 65:4100-4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han, Y., R. P. Morrison, and J. E. Cutler. 1998. A vaccine and monoclonal antibodies that enhance mouse resistance to Candida albicans vaginal infection. Infect. Immun. 66:5771-5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han, Y., M. A. Ulrich, and J. E. Cutler. 1999. Candida albicans mannan extract-protein conjugates induce a protective immune response against experimental candidiasis. J. Infect. Dis. 179:1169-1175. [DOI] [PubMed] [Google Scholar]
- 32.Han, Y., T. R. Kozel, M. X. Zhang, R. S. McGill, M. C. Carrol, and J. E. Cutler. 2001. Complement is essential for protection by IgM and an IgG3 monoclonal antibody against experimental, hematogeneously disseminated candidiasis. J. Immunol. 167:1550-1557. [DOI] [PubMed] [Google Scholar]
- 33.Hurd, R. C., and C. H. Drake. 1953. Candida albicans infections in actively and passively immunized animals. Mycopathol. Mycol. Appl. 6:290-297. [DOI] [PubMed] [Google Scholar]
- 34.Keller, R. G., G. S. Pfrommer, and T. R. Kozel. 1994. Occurrences, specificities, and functions of ubiquitous antibodies in human serum that are reactive with the Cryptococcus neoformans cell wall. Infect. Immun. 62:215-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klein, B. S., and S. L. Newman. 1996. Role of cell-surface molecules of Blastomyces dermatiditis in host-pathogen interactions. Trends Microbiol. 4:246-251. [DOI] [PubMed] [Google Scholar]
- 36.Klis, F. M., P. De Groot, and K. Hellingwerf. 2001. Molecular organization of the cell wall of Candida albicans. Med. Mycol. 39(Suppl. 1):1-8. [PubMed] [Google Scholar]
- 37.Lamagni, T. L., B. G. Evans, M. Shigematsu, and E. M. Johnson. 2001. Emerging trends in the epidemiology of invasive mycoses in England and Wales (1990-9). Epidemiol. Infect. 126:397-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lo, H., J. R. Kohler, B. Di Domenico, D. Loenbenberg, A. Cacciapuoti, and G. R. Fink. 1997. Nonfilamentous C. albicans are avirulent. Cell 90:939-949. [DOI] [PubMed] [Google Scholar]
- 39.Matthews, R., S. Hodgetts, and J. Burnie. 1995. Preliminary assessment of a humoral recombinant antibody fragment to hsp90 in murine invasive candidiasis. J. Infect. Dis. 171:1668-1671. [DOI] [PubMed] [Google Scholar]
- 40.Mencacci, A., A. Torosantucci, R. Spaccapelo, L. Romani, F. Bistoni, and A. Cassone. 1994. A mannoprotein constituent of Candida albicans that elicits different levels of delayed-type hypersensitivity, cytokine production, and anticandidal protection in mice. Infect. Immun. 62:5353-5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merkel, G. J., and B. A. Scofield. 1999. An opsonizing monoclonal antibody that recognizes a noncapsular epitope expressed on Cryptococcus neoformans. Infect. Immun. 67:4994-5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miragall, F., H. Rico, and R. Sentandren. 1986. Changes in the plasmic membrane of regenerating protoplasts of Candida albicans as revealed by freeze-fracture electron microscopy. J. Gen. Microbiol. 132:2845-2853. [DOI] [PubMed] [Google Scholar]
- 43.Mizutani, S., M. Endo, T. Ino-ve, M. Kurosawa, Y. Uno, H. Saito, K. Onogi, I. Kato, and K. Takesako. 2000. CD4-T-cell-mediated resistance to systemic candidiasis induced by a membrane fraction of Candida albicans. Antimicrob. Ag. Chemother. 44:2653-2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mouraud, S., and L. Friedman. 1961. Active immunization of mice against Candida albicans. Proc. Soc. Exp. Biol. Med. 106:570-572. [DOI] [PubMed] [Google Scholar]
- 45.Pirofski, L., and A. Casadevall. 1996. Antibody immunity to Cryptococcus neoformans: paradigm for antibody immunity to the fungi? Zentbl. Bakteriol. 284:475-495. [DOI] [PubMed] [Google Scholar]
- 46.Polonelli, L., F. De Bernardis, M. Boccanera, M. Gerloni, G. Morace, W. Magliani, C. Chezzi, and A. Cassone. 1994. Idiotypic intravaginal vaccination to protect against candidal vaginitis by secretory, yeast killer toxin-like anti-idiotypic antibodies. J. Immunol. 152:3175-3182. [PubMed] [Google Scholar]
- 47.Polonelli, L., A. Casadevall, Y. Han, F. De Bernardis, T. N. Kirkland, R. C. Matthews, D. Adriani, M. Boccanera, J. P. Burnie, A. Cassone, S. Conti, J. E. Cutler, R. Frazzi, C. Gregory, S. Hodgetts, C. Illidge, W. Magliani, G. Rigg, and G. Santoni. 2000. The efficacy of acquired humoral and cellular immunity in the prevention and therapy of experimental fungal infections. Med. Mycol. 38(Suppl. 1):281-292. [PubMed] [Google Scholar]
- 48.Romani, L., A. Mencacci, E. Cenci, P. Mosci, G. Vitellozzi, U. Grohmann, P. Puccetti, and F. Bistoni. 1992. Course of primary candidiasis in T-cell-depleted mice infected with attenuated variant cells. J. Infect. Dis. 166:1384-1392. [DOI] [PubMed] [Google Scholar]
- 49.Romani, L. 1999. Immunity to Candida albicans: Th1, Th2 cells and beyond. Curr. Opin. Microbiol. 2:363-367. [DOI] [PubMed] [Google Scholar]
- 50.Romani, L. 2000. Innate and adaptive immunity in Candida albicans infections and saprophytism. J. Leukoc. Biol. 68:175-179. [PubMed] [Google Scholar]
- 51.Scaringi, L., P. Marconi, M. Boccanera, L. Tissi, F. Bistoni, and A. Cassone. 1988. Cell wall components of Candida albicans as immunomodulators: induction of natural killer and macrophage-mediated peritoneal cell cytotoxicity in mice by mannoprotein and glucan fraction. J. Gen. Microbiol. 134:1265-1274. [DOI] [PubMed] [Google Scholar]
- 52.Schuman, P., J. D. Sobel, S. E. Ohmit, K. H. Warren, R. S. Klein, et al. 1998. Mucosal candidal colonization and candidiasis in women with or at risk for human immunodeficiency virus infection. Clin. Infect. Dis. 27:1161-1167. [DOI] [PubMed] [Google Scholar]
- 53.Sobel, J. D., S. E. Ohmit, P. Schuman, R. S. Klein, K. Mayer, A. Duerr, J. A. Vasquez, A. Rampalo, and the HIV Epidemiology Research Study (HERS) Group. 2001. The evolution of Candida species and fluconazole susceptibility among oral and vaginal isolates recovered from human immunodeficiency virus (HIV)-seropositive and high-risk HIV-seronegative women. J. Infect. Dis. 183:286-293. [DOI] [PubMed] [Google Scholar]
- 54.Tavares, D., P. Ferriera, M. Vilanova, A. Videira, and M. Arala-Chaves. 1995. Immunoprotection against systemic candidiasis in mice. Int. Immunol. 7:785-796. [DOI] [PubMed] [Google Scholar]
- 55.Torosantucci, A., M. J. Gomez, C. Bromuro, J. Casalinuovo, and A. Cassone. 1991. Biochemical and antigenic characterization of mannoprotein constituents released from yeast and mycelial form of Candida albicans. J. Med. Vet. Mycol. 29:361-372. [DOI] [PubMed] [Google Scholar]
- 56.Torosantucci, A., C. Palma, M. Boccanera, C. M. Ausiello, G. C. Spagnoli, and A. Cassone. 1990. Lymphoproliferative and cytotoxic responses of human peripheral blood mononuclear cells to mannoprotein constituents of Candida albicans. J. Gen. Microbiol. 136:2155-2163. [DOI] [PubMed] [Google Scholar]
- 57.Trinel, P. A., C. Faille, P. M. Jacquinot, J. C. Cailliez, and D. Poulain. 1992. Mapping of Candida albicans oligomannosidic epitopes by using monoclonal antibodies. Infect. Immun. 60:3845-3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vazquez, J. A., G. Peng, J. D. Sobel, H. Steele-Moore, P. Shuman, W. Halloway, and J. D. Neaton. 2001. Evolution of antifungal susceptibility among Candida species isolates recovered from human immunodeficiency virus-infected women receiving fluconazole prophylaxis. Clin. Infect. Dis. 33:1069-1075. [DOI] [PubMed] [Google Scholar]
- 59.Yuan, R., A. Casadevall, G. Spira, and M. D. Scharff. 1995. Isotype switching from IgG3 to IgG1 converts a nonprotective murine antibody to Cryptococcus neoformans into a protective antibody. J. Immunol. 154:1810-1816. [PubMed] [Google Scholar]
- 60.Yuan, R. R., A. Casadevall, J. Oh, and M. D. Scharff. 1997. T cells cooperate with passive antibody to modify Cryptococcus neoformans infection in mice. Proc. Natl. Acad. Sci. USA 94:2483-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang, M. X., T. T. Brandhorst, T. R. Kozel, and B. S. Klein. 2001. Role of glucan and surface protein BAD 1 in complement activation by Blastomyces dermatitidis yeast. Infect. Immun. 69:7559-7564. [DOI] [PMC free article] [PubMed] [Google Scholar]