Abstract
Knowledge of whether Mycobacterium tuberculosis resides within a relatively impermeable membrane-bound vacuole or is free within the cytoplasm within its host cell is central to an understanding of the immunobiology of this intracellular parasite but is a matter of controversy. To explore this issue, we assessed the accessibility of medium-size protein molecules (Fab fragments of 50,000 Da) to M. tuberculosis within human macrophages. We infected the macrophages with wild-type or green fluorescent protein-expressing M. tuberculosis, microinjected Fab fragments directed against a major surface antigen of M. tuberculosis into the host cell, and assayed the accessibility of the bacteria to the Fab fragments by both immunofluorescence microscopy and immunogold electron microscopy. Whereas microinjected intact immunoglobulin G molecules against cytoplasmic early endosomal antigen 1 readily stained this antigen, microinjected Fab fragments against M. tuberculosis did not stain the bacterium within its phagosome. In contrast, microinjected Fab fragments against Listeria monocytogenes, an intracellular bacterium known to permeabilize its phagosomal membrane, strongly stained this bacterium. Our study shows that M. tuberculosis resides in an isolated phagosome that is relatively impermeable to cytoplasmic constituents.
Intracellular pathogens fall into two broad categories: those that reside within membrane-bound phagosomes and those that escape their phagosomes and reside freely in the host cell cytoplasm. Examples of intracellular pathogens widely accepted as residing within membrane-bound phagosomes are Legionella pneumophila (13), Chlamydia psittaci (28), and Coxiella burnetii (3). Examples of intracellular pathogens that have been reported to lyse the phagosomal membrane and to multiply freely within the host cell cytoplasm include Trypanosoma cruzi (19, 23), Listeria monocytogenes (9), Shigella spp. (6, 20), and some species of Rickettsia (27). For Mycobacterium tuberculosis, some investigators have reported an exclusively intraphagosomal location (1, 5, 29), whereas other investigators have reported various degrees of intracytoplasmic localization (16, 18). Recently, Teitelbaum et al. (24) have reported that Mycobacterium bovis BCG resides in a phagosome that is permeable to relatively low-molecular-weight molecules (fluorescent dextrans of 70,000 Da and smaller) and hypothesized that the more virulent organism, M. tuberculosis, may permeabilize its phagosome even more.
Whether M. tuberculosis resides within a membrane-bound phagosome, a highly permeabilized phagosome, or the cytoplasm is of considerable importance to our understanding of its pathogenic mechanisms. For example, intracytoplasmically located pathogens tend to present immunoprotective antigens via the class I antigen-processing pathway whereas intraphagosomally located pathogens tend to present such antigens via the class II antigen-processing pathway. Consequently, class I restricted CD8 cells predominate in host defense against the former pathogens, whereas class II restricted CD4 cells predominate in host defense against the latter.
To explore the permeability of the M. tuberculosis phagosome, we investigated the accessibility of intracellular M. tuberculosis to 50,000-Da probes introduced into the host cell cytoplasm. The probes consisted of fluorescence-labeled or unlabeled Fab fragments directed against an abundant surface antigen of M. tuberculosis, lipoarabinomannan (LAM). The probes were introduced into the mononuclear cell host by electroporation before the cells were infected with M. tuberculosis or by microinjection after the cells were infected with M. tuberculosis. Our study shows that the probes are not able to stain M. tuberculosis within its phagosome in host cells. Hence, intracellular M. tuberculosis is inaccessible to cytoplasmic molecules of ≥50,000 Da. While these experiments do not test directly the capacity of molecules to move from the phagosome into the cytoplasm (the direction that is important for major histocompatibility complex class I [MHC-I]) processing and presentation), these results do indicate that M. tuberculosis does not escape into the cytoplasm or create large bidirectional pores in the phagosomal membrane.
MATERIALS AND METHODS
Reagents and antibodies.
Paraformaldehyde and glutaraldehyde were purchased from Polysciences; PIPES (piperazine-N,N′-bis[2-ethanesulfonic acid]), methylcellulose, and polyvinylpyrrolidone were from Sigma Chemical Company; Dulbecco's phosphate-buffered saline (PBS) was from Gibco Laboratories; RPMI 1640 with l-glutamine, fetal bovine serum (FBS), and human AB serum were from Irvine Scientific Co. Brain heart infusion broth, Middlebrook 7H10 agar, 7H9 medium, and Middlebrook oleic acid-albumin-dextrose-catalase were obtained from Becton Dickinson Microbiology Systems.
A mouse monoclonal antibody (immunoglobulin G1 [IgG1]) to the human early endosomal antigen (EEA1) was purchased from Transduction Laboratories and dialyzed against PBS to remove glycerol. Mouse monoclonal antibody CS35 to mycobacterial LAM was a gift from John Belisle (Colorado State University, Fort Collins, Colo.). A rabbit antibody to mycobacterial LAM was prepared as described previously (5). Polyclonal rabbit anti-L. monocytogenes antiserum was prepared by immunizing rabbits with 109 formalin-killed L. monocytogenes strain 10403S cells three times 3 weeks apart. The first immunization was performed with complete Freund's adjuvant (Sigma Chemical Co.), and subsequent immunizations were performed with incomplete Freund's adjuvant. Anti-LAM IgG and anti-L. monocytogenes IgG were purified from the immune rabbit sera by protein A chromatography and enzymatically digested with papain (11). Fc fragments and intact IgG were removed by protein A chromatography, and the Fab fragments were further purified by Superdex 75 gel filtration (Pharmacia Fine Chemicals, Inc.). Texas Red-X was covalently conjugated to the purified Fab fragments with the Texas Red-X coupling kit (Molecular Probes) according to the manufacturer's directions. A rabbit polyclonal antibody to Texas red was purchased from Molecular Probes. The antimycobacterial reactivity of this commercial antiserum was completely removed by five consecutive overnight incubations with acetone-treated M. tuberculosis bacterial pellets (10:1 [vol/wet vol]). This and all other antisera were cleared of aggregates by ultracentrifugation (100,000 × g for 1 h) and filtration (0.2-μm-pore-size filter) prior to use. Protein A-colloidal gold conjugates (5, 10, and 15 nm) were provided by G. Posthuma (Utrecht University, Utrecht, The Netherlands). Texas red-conjugated goat anti-rabbit IgG, Oregon green-conjugated goat anti-mouse IgG, and Alexa fluor 350-conjugated goat anti-mouse IgG were purchased from Molecular Probes. Rhodamine-conjugated goat Fab antibody fragments directed against rabbit Ig were purchased from ICN Pharmaceuticals. All animal studies were approved by the University of California, Los Angeles (UCLA) Institutional Review Board.
Bacteria.
M. tuberculosis Erdman strain (ATCC 35801), a highly virulent strain, was obtained from the American Type Culture Collection (Manassas, Va.). The organism was passaged through guinea pig lung to maintain virulence, and infecting inocula were prepared as described previously (5). M. tuberculosis Erdman expressing a UV-optimized green fluorescence protein (GFPUV-M. tuberculosis) under the control of the BCG hsp60 promoter (pNBV1-GFPUV) was prepared as previously described (25), and virulence was assured by passage twice through monolayers of THP-1 cells. Aliquots of GFPUV-M. tuberculosis from the second passage were stored frozen at −80°C.
Wild-type L. monocytogenes strain 10403S was a gift from Jeff Miller (UCLA). These bacteria were passaged twice through monolayers of THP-1 cells to assure virulence. Aliquots of bacteria from the second passage were stored frozen at −80°C.
Human peripheral blood mononuclear cells.
Heparinized blood from healthy blood donors was diluted 1:1 with 0.9% saline, and the mononuclear-cell fraction was obtained by centrifugation at 800 × g for 30 min at 24°C over a Ficoll-sodium diatrizoate solution (Ficoll-Paque; Pharmacia Fine Chemicals, Inc.). The layer containing the mononuclear-cell fraction was removed and diluted 1:1 with RPMI 1640, and the mononuclear cells were collected by centrifugation at 400 × g for 10 min at 4°C. The mononuclear cells were washed twice by centrifugation at 115 × g for 10 min at 4°C. The cells were resuspended in RPMI 1640, counted in a hemocytometer (Clay Adams Division, Becton Dickinson and Co.), and adjusted to a concentration of 1.5 × 106 cells/ml in RPMI 1640 containing 10% heat-inactivated (HI) FBS and 10% autologous serum, and 1.0 ml was added to CELLocate glass (Brinkmann Instruments, Inc.) or plastic (Wako Pure Chemical Industries, Ltd.,) coverslips in 2-cm2 tissue culture wells. For experiments in which peripheral blood monocytes were cultured in screw cap Teflon wells, the cells were adjusted to a concentration of 1.5 × 106/ml in RPMI 1640 containing 10% HI FBS and 10% autologous serum. The participation of normal human blood donors in our research was approved by the UCLA Institutional Review Board.
THP-1 cells.
THP-1 cells (ATCC TIB 202) were added to CELLocate glass or plastic coverslips in 2-cm2 tissue culture wells (5 × 105 cells/well) and differentiated for 2 days with phorbol myristic acid (PMA; 0.16 nM) in RPMI 1640 at 37°C in air containing 5% CO2.
Preparation of bacteria for infection of phagocytic cells.
Before an infection experiment involving M. tuberculosis, the bacteria were thawed, grown for 7 to 8 days on plates of 7H9 plus oleic acid-albumin-dextrose-catalase (with 50 μg of hygromycin/ml for GFPUV-M. tuberculosis), and suspended in PBS. A suspension containing predominantly single bacilli was prepared by sonicating the bacteria in a water bath (model 9; Astrason) for eight periods of 15 s each. The bacterial suspension was centrifuged at 200 × g for 10 min to remove aggregates. The supernatant was then removed and centrifuged again under the same conditions, and an aliquot of the almost exclusively single-bacillus suspension was withdrawn from the top of the tube. The concentration of organisms was determined by measurement of optical density at 540 nm and by counting in a Petroff-Hausser chamber. The viability of the organisms was determined by plating serial dilutions of the infecting inoculum on 7H11 agar. Viability ranged from 70 to 84% in these experiments. Before an infection experiment involving L. monocytogenes, the bacteria were thawed, inoculated into brain heart infusion broth, and grown to mid-log phase at 37°C in an incubator shaking at 200 rpm.
Electroporation.
Human peripheral blood monocytes were isolated with Ficoll-Paque and maintained in Teflon wells for 5 days in RPMI 1640 with 10% HI FBS. The Teflon wells were placed on ice for 30 min, and the cells were collected, centrifuged at 4°C, and resuspended in ice-cold RPMI 1640 containing 2 mg of Texas Red-conjugated anti-LAM Fab/ml. The cells were electroporated at 750 V and 25 μF, incubated 15 min on ice, washed, and allowed to adhere to glass coverslips pretreated with 0.1 mg of poly-l-lysine/ml in RPMI 1640 with 10% HI FBS and 10% autologous serum. Twelve hours later, the cells were infected with GFPUV-M. tuberculosis (multiplicity of infection [MOI] of 30:1) for 30 min. Monolayers were washed, incubated for 24 h, fixed in 4% paraformaldehyde in 0.1 M sodium phosphate, pH 7.4, containing 6% sucrose for 2 h, washed in PBS, mounted in Prolong antifade mounting medium (Molecular Probes), and viewed by epifluorescence microscopy with a Fluoriphot Olympus microscope equipped with a mercury lamp light source.
In additional electroporation experiments, human peripheral blood monocytes or THP-1 cells were allowed to adhere to Cytodex-1 beads (Pharmacia) at a density of 4 × 107 cells per ml of beads in RPMI 1640 containing 10% AB serum (monocytes) or 10% HI FBS and 0.16 nM PMA (THP-1 cells). Two to 3 days later, the beads were mixed 1:1 (vol/vol) with Texas red-conjugated anti-LAM Fab (2 mg/ml) in PBS at 0°C. The suspension was electroporated at 750 V and 25 μF, incubated on ice for 15 min, washed three times to remove extracellular Texas red-conjugated Fab, and incubated for 8 h with RPMI 1640 containing 10% HI FBS and 5% autologous human serum (monocytes) or AB serum (THP-1 cells). The cells on the Cytodex-1 beads were incubated with GFPUV-M. tuberculosis at an MOI of 30:1 for 30 min and washed three times to remove noningested bacteria, and the suspension was transferred to 1-cm-diameter wells containing glass coverslips pretreated with poly-l-lysine (0.1 mg/ml; Sigma Chemical Company). Many of the cells moved from the Cytodex-1 to the poly-l-lysine-coated coverslips, facilitating subsequent microscopic visualization. At 1 day postinfection, the cells were fixed in 4% paraformaldehyde-0.1 M sodium phosphate, pH 7.4, containing 6% sucrose, washed with PBS, mounted, and viewed by epifluorescence microscopy as described above.
In a control experiment, 0.2 ml of THP-1 cells on Cytodex-1 beads was mixed with 0.2 ml of 2-mg/ml Texas-red conjugated anti-LAM Fab and electroporated as described above. The suspension was incubated on ice for 15 min, washed, and cultured in RPMI 1640 containing 10% FBS and 5% AB serum at 37°C in air containing 5% CO2. Two days later, the beads were washed in PBS, transferred to a conical 15-ml tube, and sonicated with a 2-mm probe tip at setting 5 with a 50% work cycle with continuous cooling on ice for three 5-min periods (with 5-min periods of cooling between the periods of sonication) in 1.0 ml containing 50 mM HEPES, 0.15 M NaCl, 0.1% bovine serum albumin (BSA), 0.01% fish skin gelatin, 10 μg of soy bean trypsin inhibitor/ml, 10 μg of pepstatin/ml, 10 μg of leupeptin/ml, and 0.05% sodium azide. Particulate material of the cell lysate was sedimented by ultracentifugation (100,000 × g for 1 h), and the supernatant was concentrated to 0.2 ml with a Centricon Plus-20 concentrator (PL-10; Millipore). The capacity of the Texas red-Fab to bind M. tuberculosis in the final sample was verified by fluorescence and immunoelectron microscopy.
Microinjection and immunofluorescence microscopy.
Monolayers of differentiated THP-1 cells or human monocytes on CELLocate glass coverslips were incubated for 30 min with GFPUV-M. tuberculosis at an MOI of 30:1 in RPMI 1640 containing 10% fresh AB serum (THP-1 cells) or autologous serum (monocytes) and 10% HI FBS. Monolayers were washed with culture medium, incubated in fresh medium at 37°C for an additional 1 to 3 days, fixed in 0.4% paraformaldehyde in 0.1 M PIPES, pH 7.4-6% sucrose (PIPES-sucrose) for 30 min, washed twice in PIPES-sucrose buffer, and incubated for 10 min at room temperature in PIPES-sucrose containing 10 mM glycine. Extracellular M. tuberculosis cells were stained by incubation for 30 min with CS35 mouse anti-LAM (10 μg/ml) in PIPES-sucrose containing 1% BSA and 5% goat serum, washed, and incubated with goat anti-mouse Alexa 350 (1:50; Molecular Probes) in the same buffer for 30 min. Coverslips were washed with PIPES-sucrose and microinjected with mouse anti-EEA1 IgG (0.2 mg/ml; Transduction Laboratories) and rabbit anti-LAM Fab (0.2 mg/ml; either unconjugated or conjugated to Texas red) in PBS. Microinjections were performed with Femtotip microcapillary needles (Eppendorf/Brinkmann Instruments) and a Microinjector 5242 controlled by a Micromanipulator 5171 (Eppendorf/Brinkmann Instruments) and monitored with an Axiovert 135 fluorescence microscope (Zeiss). The cells were allowed to incubate at 4°C for 1 h after the last cell was microinjected to permit additional interaction between the intracellular antigen and microinjected antibody. The microinjected cells were then fixed for 30 min in 2% paraformaldehyde in 0.1 M PIPES, pH 7.4-6% sucrose, permeabilized in acetone at −20°C for 30 min, air dried completely, rehydrated by being swirled briefly in three consecutive beakers containing 50 ml of PBS, blocked with 5% goat serum-1% BSA in PBS for 30 min at room temperature, and incubated with a combination of Oregon green-conjugated goat anti-mouse IgG and Texas red-conjugated goat anti-rabbit IgG (both diluted 1:50) in 5% goat serum-1% BSA in PBS. Cells were washed, postfixed in 2% paraformaldehyde, mounted, and viewed by epifluorescence microscopy as described above.
As a positive control, human peripheral blood monocytes or THP-1 cells were infected with GFPUV-M. tuberculosis and fixed and permeabilized with acetone as described above, air dried, rehydrated in PBS, blocked with 5% goat serum-1% BSA in PBS, incubated with the antibody mixture used for microinjection diluted 50-fold, and finally incubated with Oregon green-conjugated goat anti-mouse IgG and Texas red-conjugated goat anti-rabbit IgG. In these experiments, we found that the microinjected antibody solutions retained their capacity to stain M. tuberculosis and EEA1 in the permeabilized cells when diluted 50-fold (i.e., final Fab and IgG concentrations of 4 μg/ml).
Immunoelectron microscopy.
Plastic coverslips were scratched with a sterile 25-gauge needle to facilitate subsequent orientation in microinjection, embedding, and thin sectioning of a designated area of the monolayers. Human peripheral blood monocytes or THP-1 cells were cultured on plastic coverslips as described above for 2 days in RPMI 1640 with 10% autologous human serum or 10% FBS containing 0.16 nM PMA prior to infection with M. tuberculosis or L. monocytogenes at an MOI of 30:1 for 30 min at 37°C. The monolayers were washed three times with RPMI 1640 and grown for an additional 1 to 3 days (M. tuberculosis) or 3 to 4 h (L. monocytogenes) in RPMI 1640 with 10% HI FBS. The monolayers were fixed with 0.4% paraformaldehyde in 0.1 M PIPES, pH 7.40, containing 6% sucrose for 30 min at room temperature, washed with the same buffer, and microinjected with rabbit anti-LAM Fab-Texas red (2 mg/ml) or rabbit anti-L. monocytogenes Fab-Texas red (2 mg/ml) in PBS. After 1 h at 4°C, the cells were fixed with 0.5% glutaraldehyde-2% paraformaldehyde in 0.1 M PIPES, pH 7.4, containing 6% sucrose for 2 h at 4°C, washed with PBS, and dehydrated in a graded series of ethanol (70, 80, 90, 95, and 100%). The coverslips were immersed for 1 min in propylene oxide to denature and immobilize proteins, rinsed briefly in 100% ethanol, and embedded in medium-grade LR White embedding resin (Polysciences), and ultrathin sections were collected on Formvar-coated nickel grids. Nonspecific antibody sites on the thin sections were blocked by incubating the grids for 30 min at room temperature on drops of 1% BSA-0.1% fish skin gelatin in 50 mM HEPES buffer, pH 7.4, containing 0.3 M NaCl and 0.05% sodium azide (blocking buffer). Immunogold double labeling was performed by a modification of the sequential protein A-gold (PAG) technique as described by Slot et al. (22). Sections were stained by incubation for 1 h at room temperature with a rabbit anti-Texas red antibody (Molecular Probes) diluted 1:500 in blocking buffer, washed on seven consecutive drops of 50 mM HEPES buffer, pH 7.4, containing 0.3 M NaCl and 0.05% sodium azide (washing buffer), and stained with 15 nm PAG in blocking buffer. Free protein A and Fc sites are destroyed by incubating the sections with 2% glutaraldehyde for 10 min. Aldehydes were quenched with 10 mM glycine in wash buffer, and the sections were incubated with rabbit anti-LAM or rabbit anti-L. monocytogenes followed by staining with 5 nm PAG. Sections were stabilized with 2% glutaraldehyde for 10 min, washed in water, and stained with saturated uranyl acetate. Consecutive phagosomes within microinjected cells were photomicrographed with a JEOL 100 CX II electron microscope. Measurements of gold particles per unit area were made with a Numonics 2210 digitizer tablet and SigmaScan software (Jandel Scientific Co.).
RESULTS
M. tuberculosis is not accessible to Fab fragments electroporated into live macrophages prior to infection.
To determine whether M. tuberculosis permeabilizes its phagosome to molecules of 50,000 Da, we prepared rabbit anti-mycobacterial LAM Fab fragments and covalently coupled these to the fluorescent probe, Texas Red-X (anti-LAM Fab-Texas red). We electroporated the anti-LAM Fab-Texas red into the human THP-1 macrophage-like cell line and human peripheral blood monocytes and, 8 to 12 h later, infected the cells with GFPUV-M. tuberculosis. One day later, the macrophages were fixed and examined by fluorescence microscopy to determine whether the intracellular M. tuberculosis cells were stained by the intracellular Texas red-conjugated anti-LAM Fab antibody fragments. Of 200 consecutive GFPUV-M. tuberculosis cells within Texas red-positive macrophages, none of the M. tuberculosis cells stained positive for Texas red. This result indicated that intracellular M. tuberculosis was inaccessible to the Fab fragments. However, it remained possible that the intracytoplasmic Texas red-Fab was degraded within the macrophages. To explore this possibility, we determined whether the Texas red-Fab retained anti-LAM activity 2 days after electroporation into the macrophages. Macrophages adherent to Cytodex-1 beads and containing the probe were lysed by sonication, and the supernatant fluid was examined for its capacity to stain GFPUV-M. tuberculosis. Immunofluorescence microscopy and immunoelectron microscopy confirmed that the antibody retained its activity (data not shown). Thus, intracellular M. tuberculosis is inaccessible to Fab fragments electroporated into live macrophages prior to infection.
M. tuberculosis is not accessible to Fab fragments microinjected into macrophages after infection as assessed by immunofluorescence microscopy.
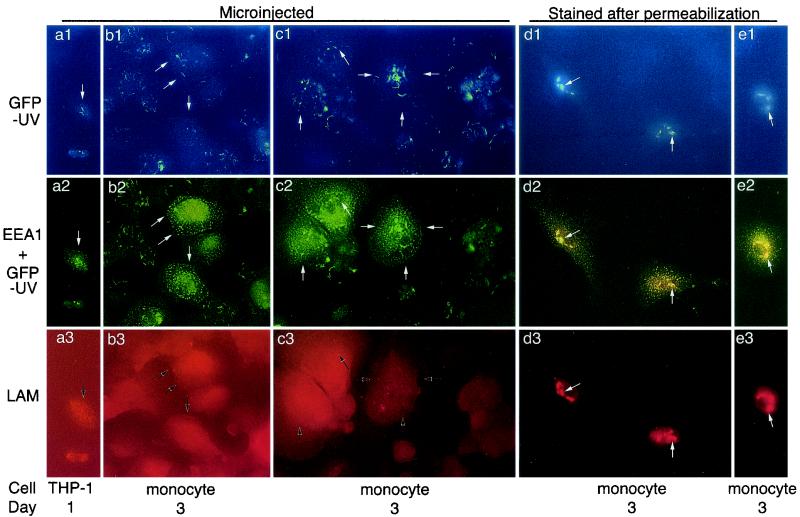
We considered the possibility that the intracytoplasmic anti-LAM Fab-Texas Red might alter the capacity of M. tuberculosis to permeabilize its phagosome and the possibility that M. tuberculosis might permeabilize its phagosome at times later than 1 day after infection. To explore these possibilities, we examined the capacity of anti-LAM Fab to bind to M. tuberculosis when microinjected 1 to 3 days after infection (Fig. 1). Human macrophage-like THP-1 cells and peripheral blood-derived monocytes were allowed to adhere to glass coverslips, infected with GFPUV-M. tuberculosis, fixed gently with 0.4% paraformaldehyde, and microinjected with unconjugated or Texas red-conjugated anti-LAM Fab antibody fragments. As a positive control, mouse IgG directed against EEA1 was microinjected simultaneously with the anti-LAM Fab. After fixation and permeabilization, the microinjected antibodies were immunolocalized with goat anti-rabbit and goat anti-mouse IgG-conjugated secondary antibodies. For each of days 1, 2, and 3 following infection, we examined over 100 consecutive M. tuberculosis-infected microinjected cells and found none that stained positive for Texas red. We observed intense staining of the cytoplasmically exposed EEA1 by the microinjected mouse antibody (Fig. 2a2, a3, and b2), despite the absence of staining of the M. tuberculosis cells by the rabbit Fab (Fig. 3 and b3). These results were obtained regardless of whether the second antibody used to immunolocalize the rabbit anti-LAM Fab was an intact goat IgG molecule or a Fab fragment. Thus, although the microinjected antibodies were capable of diffusing throughout the cells and binding to cytoplasmically exposed EEA1, the intracytoplasmically injected anti-LAM antibodies were unable to stain M. tuberculosis. These results indicate that the phagosome is not permeable to Fab molecules, which are 50,000 Da in size. In control experiments using cells fixed and permeabilized prior to incubation with antibodies, both EEA1 and mycobacterial LAM were stained by the microinjected antibody solution, even when diluted 50-fold (Fig. 1d1 to d3 and e1 to e3). Microinjection generally results in approximately a 10-fold dilution of the microinjected solution within the microinjected cells.
FIG. 1.
Microinjected Fab fragments detect EEA1, but not GFPUV-M. tuberculosis, in human macrophages. Monolayers of THP-1 cells and human peripheral blood monocytes were infected with GFPUV-M. tuberculosis and fixed 1 or 3 days after infection, as indicated. (a and b) THP-1 cells (a) and monocytes (b) were microinjected with rabbit anti-LAM Fab fragments and mouse anti-EEA1 IgG, postfixed for 30 min with 2% paraformaldehyde, permeabilized with acetone, air dried, rehydrated in a large volume of PBS, blocked with 57% goat serum-1% BSA in PBS, and stained with Texas red-conjugated goat anti-rabbit IgG and Oregon green-conjugated goat anti-mouse IgG in PBS with 57% goat serum-1% BSA. Coverslips were washed, mounted in Molecular Probes antifade mounting medium, and viewed by fluorescence microscopy. White and black arrows indicate the corresponding positions in micrographs within the same column. The GFPUV-M. tuberculosis cells are visualized by excitation with either violet (a1 and b1) or fluorescein (a2 and b2) filters. The microinjected cells show abundant Oregon green staining of EEA1 (a2 and b2) but no staining of M. tuberculosis by the microinjected anti-LAM Fab (a3 and b3). (c) Monocytes were fixed 3 days after infection with GFPUV-M. tuberculosis and microinjected with rabbit anti-LAM Fab and mouse anti-EEA1 IgG as in panels a and b. The monocytes were then microinjected a second time with rhodamine-conjugated goat Fab directed against rabbit IgG and Oregon green-conjugated goat anti-mouse IgG. Again, EEA1 stains well in the microinjected cells (c2) but the GFPUV-M. tuberculosis cells are not stained by the microinjected anti-LAM Fab (c3). (d and e) As positive controls, monocytes were fixed and permeabilized 3 days after infection with GFPUV-M. tuberculosis, incubated with a 1:50 dilution of the rabbit anti-LAM Fab and mouse anti-EEA1 IgG solution used for microinjection, washed, and stained with secondary antibodies as for panels a and b. In panels e, to confirm the stability of the staining of LAM by anti-LAM Fab fragments, after incubation with primary antibodies, we postfixed the monolayers for 30 min and then subjected them to a second treatment with acetone at −20°C, air dried them, rehydrated them, and washed and incubated them with secondary antibodies (as was done after microinjection of primary antibodies in panels a and b). In these acetone-permeabilized control cells, both LAM on the M. tuberculosis cell wall (e3) and EEA1 (e2) are readily stained. Magnification, ×157.
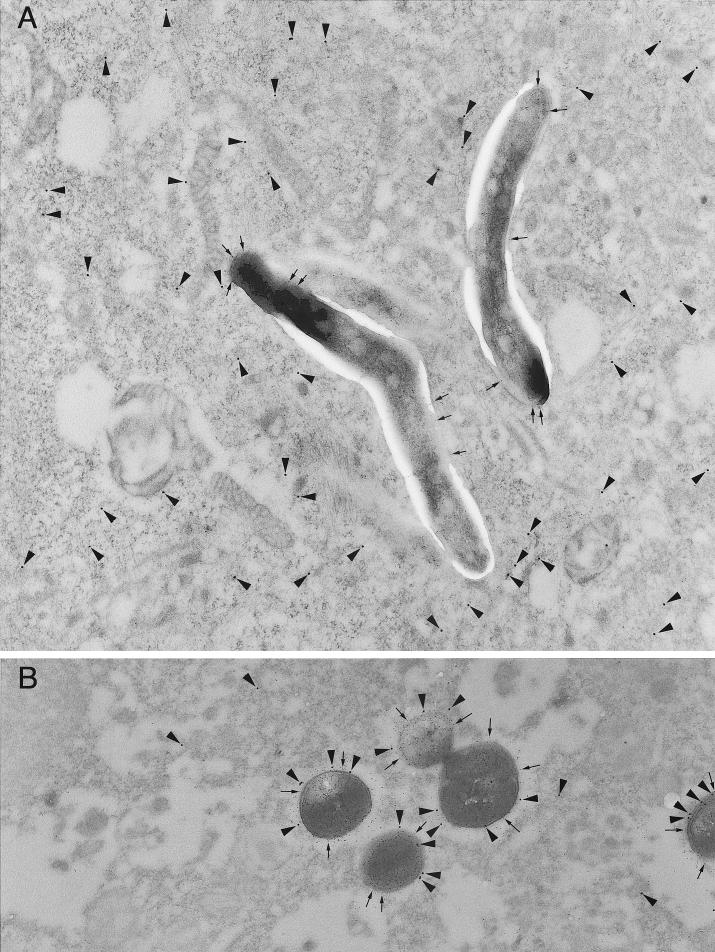
FIG. 2.
L. monocytogenes but not M. tuberculosis is accessible to cytoplasmically injected Fab antibody fragments as assessed by immunoelectron microscopy. Human peripheral blood mononuclear cells were infected with M. tuberculosis (A) or L. monocytogenes (B) and fixed after 3 days (A) or 4 h (B). Cells of the monolayer were microinjected with Texas red-conjugated-rabbit anti-LAM Fab or Texas red-conjugated rabbit anti-L. monocytogenes Fab. The monolayers were fixed, dehydrated, embedded in LR White resin, and thin sectioned, and the sections were incubated sequentially with rabbit anti-Texas red and 15-nm PAG to localize the microinjected Texas red-Fab conjugates. The sections were blocked and incubated with rabbit anti-LAM IgG or rabbit anti-L. monocytogenes and 5-nm PAG. (A) In the M. tuberculosis-infected macrophages, the cytoplasm of the microinjected macrophage has copious Texas red-conjugated anti-LAM Fab (15 nm gold; arrowheads) but there is no 15-nm gold associated with the M. tuberculosis. The M. tuberculosis cells are well stained postsectioning by the 5-nm anti-LAM immunogold (arrows). (B) In the L. monocytogenes-infected macrophages, the 15-nm gold (arrowheads) is abundantly associated with the L. monocytogenes bacteria (identified postsectioning with 5-nm gold; arrows). Magnification (A and B), ×22,400.
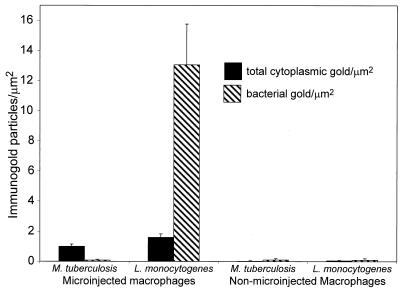
FIG. 3.
Exclusion of Fab fragments from M. tuberculosis but not L. monocytogenes within human macrophages quantitated by immunoelectron microscopy. Monolayers of peripheral blood mononuclear cells were infected with M. tuberculosis or L. monocytogenes for 3 days or 4 h, respectively, fixed, microinjected, and processed for immunoelectron microscopy as described for Fig. 2. Texas red-Fab immunogold staining was quantitated by counting the number of 15-nm gold particles per unit area of host cell cytoplasm and per unit of bacterial cross-sectional area. Texas red-conjugated anti-LAM Fab is excluded from the M. tuberculosis bacteria, in marked contrast to the increased concentration of Texas red-conjugated anti-L. monocytogenes Fab on the L. monocytogenes bacteria. The level of immunogold staining of M. tuberculosis in the microinjected cells is comparable to the background level observed in nonmicroinjected cells that are stained with the same secondary antibody and PAG. Values indicate the means ± standard errors for at least 20 bacteria on each of at least three separate electron microscope grids.
We considered the possibility that permeabilization of the cells with acetone at −20°C prior to staining with the secondary antibodies might extract LAM and its associated Fab fragments. Microinjection of Texas red-conjugated primary anti-LAM Fab fragments eliminates the need for secondary antibody staining and for acetone permeabilization. When we lightly fixed GFPUV-M. tuberculosis-infected cells 1 to 3 days postinfection and microinjected them with 0.2 mg of 2-mg/ml Texas red-conjugated anti-LAM Fab, we still observed no Texas red staining of GFPUV-M. tuberculosis, despite diffuse cytoplasmic Texas red staining of the microinjected host cells (data not shown). In additional experiments, we lightly fixed human peripheral blood monocytes 3 days after infection with GFPUV-M. tuberculosis and microinjected the cells with a rabbit anti-LAM Fab (0.37 mg/ml) together with mouse anti-EEA1 (0.2 mg/ml), followed by a second microinjection into the same cells with rhodamine-conjugated goat Fab fragments directed against rabbit IgG and Oregon green-conjugated goat anti-mouse IgG. These doubly injected cells (which had no exposure to acetone) showed intense endosomal staining of EEA1 but had no rhodamine staining of GFPUV-M. tuberculosis (Fig. 1c1 to c3).
In these experiments, mouse anti-EEA1 Oregon green fluorescence was employed to identify which cells are microinjected and to demonstrate that the microinjected antibody is able to move throughout the cell and bind to cytoplasmically exposed antigens. The GFPUV fluorescence can be distinguished from the Oregon green fluorescence because the former, but not the latter, fluoresces under UV excitation (Fig. 1, top row). However, since both GFPUV and Oregon green fluoresce green under visible-blue-light excitation (Fig. 1, middle row), the images are not intended to assess the degree of colocalization of EEA1 and M. tuberculosis.
As a positive control, we permeabilized infected macrophages with acetone before applying antibodies and then stained them with a 50-fold dilution of the primary and secondary antibody solutions removed from the microinjection needles. Under this condition, both EEA1 and M. tuberculosis were positively stained (Fig. 1d1 to d3). In additional control experiments, exposure of acetone-fixed cells to a second round of −20°C acetone treatment had no discernible effect on the fluorescent staining of GFPUV-M. tuberculosis or EEA1 (Fig. 1e1 to e3). This result is consistent with the fact that, in the purification of LAM, the addition of −20°C acetone is routinely used to precipitate LAM after it has been solubilized by Triton X-100 detergent (4). In parallel experiments, we also microinjected rabbit anti-LAM IgG molecules into lightly fixed GFPUV-M. tuberculosis cells and found no staining of the M. tuberculosis by the anti-LAM IgG (data not shown).
L. monocytogenes but not M. tuberculosis is accessible to microinjected Fab fragments, as assessed by immunoelectron microscopy.
To examine at a higher resolution the interaction of microinjected anti-LAM Fab antibody fragments with M. tuberculosis-infected cells, we gently fixed monolayers of human peripheral blood monocytes 3 days after infection with M. tuberculosis, microinjected the cells with a high concentration (2 mg/ml) of Texas red-conjugated anti-LAM Fab fragments, and examined the distribution of the Fab fragments by electron microscopy (Fig. 2A). We quantitated the number of immunogold particles per square micrometer of macrophage cytoplasm and per square micrometer of bacterium and found that the immunogold particles were excluded from M. tuberculosis (Fig. 3). Nonmicroinjected cells and M. tuberculosis cells within nonmicroinjected cells had negligible levels of immunogold staining. None of the M. tuberculosis cells in over 40 consecutive microinjected macrophages in each of two separate experiments showed appreciable staining by the anti-LAM Fab fragments: 160 of 166 M. tuberculosis cells had no immunogold particles, 5 bacteria had a single immunogold particle, and only 1 of the M. tuberculosis cells had two immunogold paticles. This level of staining is comparable to the background level of immunogold staining of M. tuberculosis in macrophages that are not microinjected (Fig. 3). As a positive control, we found that even a 200-fold dilution of the Texas red-conjugated anti-LAM Fab (10 μg/ml) yielded intense staining of mycobacteria in thin sections (data not shown). As an additional control, we found that microinjected Texas red-conjugated Fab fragments directed against L. monocytogenes do stain L. monocytogenes in the cytoplasm of host macrophages (Fig. 2B). Of 64 consecutive L. monocytogenes cells in microinjected cells, 59% had more than two immunogold particles. In sharp contrast to the case with M. tuberculosis, we found that the anti-L. monocytogenes immunogold particles were relatively concentrated on the bacteria compared with their concentration in the host cell cytoplasm (Fig. 3).
DISCUSSION
In summary, we have found that M. tuberculosis within human cells is inaccessible to microinjected Fab fragments. This indicates that the M. tuberculosis phagosome is not permeable to proteins with a size of 50,000 Da or greater. Our results differ from those of Teitelbaum et al. (24), who studied the related but avirulent mycobacterium M. bovis BCG and reported that approximately 30% of phagosomes containing GFP-expressing M. bovis cells in mouse macrophages were permeable to fluorescent dextrans of less than 75,000 Da. Aside from the different species studied, methodological differences may account for the different observations. The fluorescent-dextran technique used by Teitelbaum et al. relied on a determination of the color of the untargeted fluorescent dextran probe in the vicinity of the phagosome. An erroneous result might occur in the case of very tight-fitting phagosomes. It has been observed that M. tuberculosis and Mycobacterium avium phagosomes can be extremely tight (7, 29). Microinjected fluorescent dextrans can be expected to move freely within the cytoplasm and to surround these tight phagosomes. The resolving power of fluorescence microscopy, even confocal microscopy, is limited by the wavelength of light used to detect the fluorescent probes. Judging from published electron micrographs of tight-fitting M. tuberculosis phagosomes, the distance between the mycobacterium and the host cell cytoplasm (50 to 75 nm) may be considerably less than the wavelength of the light used to detect the fluorescent probes (450 nm). This would result in color mixing and potentially misleading results. Further loss in resolution of the fluorescent-dextran technique may occur if the mycobacteria are moving within the microinjected cell. The combined mobility of the fluorescent dye and the bacterial phagosome can be expected to increase the measured degree of color mixing. Note also that the use of fluorescent beads immobilized on a poly-l-lysine-coated microscope slide (as described by Teitelbaum et al.) would not define the resolution that is achieved when observing GFP-expressing M. bovis BCG within living macrophages. This is because the beads are stationary, whereas the living specimen is not, and because light is scattered at multiple interfaces of differing refractive indices that are present in the living cell but that are absent from a microscope slide with adherent fluorescent beads. Differences in refractive index occur at the following interfaces: extracellular medium-plasma membrane, plasma membrane-cytosol, cytosol-phagosomal membrane, phagosomal membrane-phagosomal space, phagosomal space-mycobacterial cell wall, and mycobacterial cell wall-GFP-containing bacterial cytoplasm. Refractile structures or organelles between the objective and the plane of focus, for example, granules or lipid globules, can also scatter light, further degrading resolution. Such refractile structures and interfaces are absent when the microscope resolving power is assessed with stationary fluorescent microspheres.
Furthermore, the different results obtained with different sizes of dextran probe (24) could reflect the different viscosities of the probes injected. Smaller probes are less viscous and greater volumes are likely to be injected into the host cell cytoplasm. This could result in a greater fluorescence signal, a greater osmotic shift, and greater osmotic shrinkage of the phagosome. Smaller volumes of more-viscous higher-molecular-weight dextran probes might be injected into the host cell cytoplasm, resulting in a smaller fluorescence signal and less osmotic shrinkage of the phagosome.
Another potential problem with the use of confocal imaging to assess the integrity of phagosomal membranes in living cells is that the intense laser light of a confocal microscope can cause photo-oxidation and can be toxic to the specimen. Excitation of a fluorophore inevitably produces nonradiative transitions of the fluorophore to the ground state with generation of reactive products, including singlet oxygen, that can oxidize membrane lipids and cause membranes to become permeable (17, 26). Indeed, this principle has been employed deliberately to destroy organelles and to permeabilize cells.
Alternatively, it is possible that the differences between our results and those of Teitelbaum et al. (24) reflect the properties of a putative pore. It is possible that a pore such as that in the M. tuberculosis phagosome is selectively permeable to uncharged dextrans but not to ionically charged Fab fragments or that the formaldehyde fixation employed in our microinjection experiments blocked a channel in such a pore. We believe that this is unlikely, considering the relative dimensions of the 30-Da formaldehyde molecule (4 Å) versus the dimensions of a channel that would be needed to allow passage of a 50,000-Da Fab fragment (40 Å wide, 60 Å long).
Our experiments and those of Teitelbaum et al. (24) have examined the movement of antigens from the cytosol into the phagosome. These experiments do not directly address the movement of mycobacterial antigens from the phagosome into the cytosol, the direction of antigenic flow that would be relevant to the MHC-I antigen-processing and presentation pathway.
The possible existence of macromolecule-sized pores in another mycobacterial species has also been examined. Schaible et al. (21) used immunoelectron microscopy to demonstrate that intracytoplasmic epitope-tagged dextrans (10,000 Da) do not enter the M. avium vacuole. In contrast, intracytoplasmic epitope-tagged high-molecular-weight dextrans enter the Leishmania mexicana vacuole, possibly via an autophagic process (21).
Our findings are not consistent with escape of M. tuberculosis into the cytoplasm of human host cells. Interestingly, when Hess et al. (12) infected macrophages with an M. bovis BCG that expressed recombinant listeriolysin (Hly), they found no evidence that the M. bovis BCG escaped from its phagosome into the cytoplasm, although they did find evidence of Hly outside of the phagosome and evidence of improved MHC-I presentation of cophagocytosed soluble protein by the infected macrophages. The Hly-expressing M. bovis BCG had a reduced capacity to persist in macrophages compared with the parental strain (12). The extraphagosomal, intracytoplasmic compartment may not be a permissive environment for bacteria that have not specifically adapted to intracytoplasmic growth and replication. Goetz et al. (10) microinjected individual bacteria into Caco-2 cells and examined their capacity to grow and multiply within the cytosol. Whereas L. monocytogenes and Shigella were able to grow in the intracytoplasmic compartment, many other bacteria were unable to do so, including Legionella pneumophila, Salmonella enterica, and Bacillus subtilis (10). Growth and multiplication in the cytoplasmic compartment require specific adaptation to that compartment. It is not known whether M. tuberculosis is adapted to or capable of growth within the extraphagosomal, cytoplasmic compartment.
While M. tuberculosis clearly resides within a membrane-bound phagosome in human macrophages, it remains possible that the phagosomal membrane contains small pores that allow the passage of molecules less than 50,000 Da. In this regard, contact-dependent hemolytic activity has been described for M. tuberculosis (14) and Mycobacterium haemophilum (8), but the mechanism underlying this activity and whether or not it is associated with formation of discrete pores have not been reported. It also is not clear whether this lytic capacity is confined to red blood cells or can also be exerted on macrophages. Contact-dependent hemolytic activity was not observed for M. bovis BCG (14), the organism studied by Teitelbaum et al. (24), and it is unclear whether there is any relation between mycobacterial hemolytic activity and any putative pore-forming capacity. Many microbial pathogens induce pores of discrete sizes in the membranes of mammalian cells (2). The sizes and mechanisms of the pores that are formed by microbial pathogens are quite variable. Whereas streptolysin O creates pores that are permeable to large proteins, such as lactate dehydrogenase (140,000 Da), the pores that are formed by Legionella pneumophila are smaller than 3 nm and impermeable to dextrans of 3,000 Da or larger (15). While it is certainly possible that M. tuberculosis creates pores in its phagosome, our data indicate that any such pores are not permeable to proteins of 50,000 Da or larger.
Acknowledgments
We thank Birgitta Sjostrand and Chalermchai Chaloyphian for expert technical assistance, Meisheng Jiang and Lutz Birnbaumer for advice and assistance with microinjection, and Michael Tullius for providing GFPuv-M. tuberculosis.
This work was supported by a Research Grant from the American Lung Association and by grants AI-31338 and AI-35275 from the National Institutes of Health. M.A.H. is the Gordon MacDonald Scholar at UCLA. D.L.C. was supported by a Young Investigator Award from the Infectious Diseases Society of America.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Armstrong, J. A., and P. D. Hart. 1971. Response of cultured macrophages to Mycobacterium tuberculosis with observations on fusion of lysosomes with phagosomes. J. Exp. Med. 134:713-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhadki, S., M. Roth, A. Szsiegoleit, and J. Tranum-Jensen. 1987. Damage to mammalian cells by proteins that form transmembrane pores. Rev. Physiol. Biochem. Pharmacol. 107:147-223. [DOI] [PubMed] [Google Scholar]
- 3.Burton, P. R., N. Kordova, and D. Paretsky. 1971. Electron microscopic studies of the rickettsia Coxiella burnetii: entry, lysosomal response, and fate of rickettsial DNA in L-cells. Can. J. Microbiol. 17:143-150. [DOI] [PubMed] [Google Scholar]
- 4.Chatterjee, D., A. Roberts, K. Lowell, P. Brennan, and I. Orme. 1992. Structural basis of capacity of lipoarabinomannan to induce secretion of tumor necrosis factor. Infect. Immun. 60:1249-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clemens, D. L., and M. A. Horwitz. 1995. Characterization of the M. tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J. Exp. Med. 181:257-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clerc, P. L., A. Ryter, J. Mounier, and P. J. Sansonetti. 1987. Plasmid-mediated killing of eucaryotic cells by Shigella flexneri as studied by infection of J774 macrophages. Infect. Immun. 55:521-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Chastellier, C., and L. Thilo. 1997. Phagosome maturation and fusion with lysosomes in relation to surface property and size of the phagocytic particle. Eur. J. Cell Biol. 74:49-62. [PubMed] [Google Scholar]
- 8.Fischer, L., F. Quinn, E. White, and C. King. 1996. Intracellular growth and cytotoxicity of Mycobacterium haemophilum in a human epithelial cell line (Hec-1-B). Infect. Immun. 64:269-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaillard, J.-L., P. Berche, J. Mounier, S. Richard, and P. Sansonetti. 1987. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect. Immun. 55:2822-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goetz, M., A. Bubert, G. Wang, I. Chico-Calera, J.-A. Vazquez-Boland, M. Beck, J. Slaguis, A. A. Szalay, and W. Goebel. 2001. Microinjection and growth of bacteria in the cytosol of mammalian host cells. Proc. Natl. Acad. Sci. USA 98:12221-12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harlowe, E., and D. Lane. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 12.Hess, J., D. Miko, A. Catic, V. Lehmensiek, D. Russell, and S. Kaufmann. 1998. Mycobacterium bovis bacille Calmette-Guerin strains secreting listeriolysin of Listeria monocytogenes. Proc. Natl. Acad. Sci. USA 95:5299-5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horwitz, M. A. 1983. Formation of a novel phagosome by the Legionnaires' disease bacterium (Legionella pneumophila) in human monocytes. J. Exp. Med. 158:1319-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King, C., S. Mundayoor, J. Crawford, and T. Shinnick. 1993. Expression of contact-dependent cytolytic activity by Mycobacterium tuberculosis and isolation of the genomic locus that encodes the activity. Infect. Immun. 61:2708-2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirby, J., J. Vogel, H. Andrews, and R. Isberg. 1998. Evidence for pore-forming ability by Legionella pneumophila. Mol. Microbiol. 27:323-326. [DOI] [PubMed] [Google Scholar]
- 16.McDonough, K. A., Y. Kress, and B. R. Bloom. 1993. Pathogenesis of tuberculosis: interaction of Mycobacterium tuberculosis with macrophages. Infect. Immun. 61:2763-2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minamikawa, T., A. Sriratana, D. Williams, D. Bowser, J. Hill, and P. Nagley. 1999. Chloromethyl-X-rosamine (Mitotracker Red) photosensitizes mitochondria and induces apoptosis in intact human cells. J. Cell Sci. 112:2419-2430. [DOI] [PubMed] [Google Scholar]
- 18.Myrvik, Q. N., E. S. Leake, and M. J. Wright. 1984. Disruption of phagosomal membranes of normal alveolar macrophages by the H37Rv strain of Mycobacterium tuberculosis. Am. Rev. Respir. Dis. 129:322-328. [PubMed] [Google Scholar]
- 19.Noguiera, N., and Z. Cohn. 1976. Trypanosoma cruzi: mechanism of entry and intracellular fate in mammalian cells. J. Exp. Med. 143:1402-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sansonetti, P. J., A. Ryter, P. Clerc, A. T. Maurelli, and J. Mounier. 1986. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid mediated contact hemolysis Infect. Immun. 51:461-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaible, U., P. Schlesinger, T. Steinberg, W. Mangel, T. Kobayashi, and D. G. Russell. 1999. Parasitophorous vacuoles of Leishmania mexicana acquire macromolecules from the host cell cytosol via two independent routes. J. Cell Sci. 112:681-693. [DOI] [PubMed] [Google Scholar]
- 22.Slot, J., H. Geuze, S. Gigengack, G. Lienhard, and D. E. James. 1991. Immuno-localization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J. Cell Biol. 113:123-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanowitz, H., M. Wittner, Y. Kress, and B. Bloom. 1975. Studies of in vitro infection by Trypanosoma cruzi. I. Ultrastructural studies on the invasion of macrophages and L-cells. Am. J. Trop. Med. Hyg. 24:25-33. [DOI] [PubMed] [Google Scholar]
- 24.Teitelbaum, R., M. Cammer, M. L. Maitland, N. E. Freitag, J. Condeelis, and B. R. Bloom. 1999. Mycobacterial infection of macrophages results in membrane permeable phagosomes. Proc. Natl. Acad. Sci. USA 96:15190-15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tullius, M. V., G. Harth, and M. A. Horwitz. 2001. High extracellular levels of M. tuberculosis glutamine synthetase and superoxide dismutase in actively growing cultures are due to high expression and extracellular stability rather than to a protein-specific export mechanism. Infect. Immun. 69:6348-6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White, J. G., J. M. Squirrell, and K. W. Eliceri. 2001. Applying multiphoton imaging to the study of membrane dynamics in living cells. Traffic 2:775-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winkler, H. H. 1990. Rickettsia species (as organisms). Annu. Rev. Microbiol. 44:131-153. [DOI] [PubMed] [Google Scholar]
- 28.Wyrick, P. B., and E. A. Brownridge. 1978. Growth of Chlamydia psittaci in macrophages. Infect. Immun. 19:1054-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu, S., A. Cooper, S. Sturgill-Koszycki, T. van Heyningen, D. Chatterjee, I. Orme, P. Allen, and D. Russell. 1994. Intracellular trafficking in Mycobacterium tuberculosis and Mycobacterium avium infected macrophages. J. Immunol. 153:2568-2578. [PubMed] [Google Scholar]