Abstract
Several lines of work have shown that astrocytes release glutamate in response to receptor activation, which results in a modulation of local synaptic activity. Astrocytic glutamate release is Ca2+-dependent and occurs in conjunction with exocytosis of glutamate containing vesicles. However, astrocytes contain a millimolar concentration of cytosolic glutamate and express channels permeable to small anions, such as glutamate. Here, we tested the idea that astrocytes respond to receptor stimulation by dynamic changes in cell volume, resulting in volume-sensitive channel activation, and efflux of cytosolic glutamate. Confocal imaging and whole-cell recordings demonstrated that astrocytes exhibited a transient Ca2+-dependent cell volume increase, which activated glutamate permeable channels. HPLC analysis revealed that glutamate was released in conjunction with other amino acid osmolytes. Our observations indicate that volume-sensitive channel may constitute a previously uncharacterized target for modulation of astrocyte-neuronal interactions.
Keywords: electrophysiology, exocytosis, neurotransmitters, osmolarity, synapses
Astrocytes have in the past been viewed as passive support cells, which perform important but perfunctory housekeeping tasks to optimize the environment for neural transmission. New evidence has questioned this concept by demonstrating that astrocytes can actively modulate neuronal function. Astrocytes are required for synapse formation and stability and can actively modulate synaptic transmission by release of glutamate (1). A flurry of studies has shown that astrocytes can release glutamate by exocytosis (2). Astrocytes express several proteins that are required for exocytosis, and neurotoxins inhibit astrocytic glutamate release in cultures. Astrocytes also express functional vesicular glutamate transporters VGLUT1/2 and pharmacological inhibition of VGLUT1/2 reduced Ca2+-dependent glutamate release (3, 4). However, these findings do not exclude the existence of other mechanisms by which astrocytes release glutamate. Astrocytes possess multiple mechanisms for several key functions. For example, the important task of K+ buffering is undertaken by several K+ channels expressed by astrocytes, including KIR4.1 and rSlo K(Ca) (5), but also by the K+-Na+-Cl- cotransporter (6).
We here asked whether Ca2+-dependent astrocytic glutamate release occurs via channel-mediated efflux. This idea is based on the observations that astrocytic cytosolic glutamate concentration is high, averaging 2-10 mM and can increase to as high as 70 mM in cultures (7). Glutamate is a small anion that permeates through several channels, including volume-sensitive channels (VSCs) (8). Furthermore, glutamate functions as an osmolyte and is released in large quantities by astrocytes in response to external hypotonicity (9). Cellular swelling leads to activation of VSCs and to the release of glutamate and other amino acids, including aspartate, glutamine, and taurine, as a part of the regulatory volume decrease (10). Ca2+-dependent astrocytic glutamate release has not been linked previously to the opening of VSCs, because these channels are activated by Ca2+-independent processes. Here, we have tested the hypothesis that receptor-induced Ca2+ increase is associated with an increase in astrocytic cell volume, which leads to the activation of VSCs and, thereby, results in the Ca2+-dependent release of glutamate.
Materials and Methods
Cell Volume Measurements. Cortical astrocyte cultures were made from P1 Sprague-Dawley rat pups. Heterozygotes of the Cx43 knockout line were obtained from The Jackson Laboratory (11). Astrocytes were loaded with calcein/acetoxymethyl ester (AM) (5 μM for 30 min) and visualized by confocal microscopy (12). The fluorescence dilution technique was performed on astrocytes loaded with fura-2/AM (5 μM for 30 min). The volume of astrocytes in suspension was analyzed with a Coulter counter (see Supporting Materials and Methods, which is published as supporting information on the PNAS web site).
Glutamate Measurements, Slice Preparation, and Electrophysiology. An enzymatic fluorescence detection assay for monitoring glutamate was used (13). For analysis by high-performance liquid chromatography (HPLC), confluent cultures were mounted in a perfusion chamber. The amino acid content was analyzed after reaction with o-phthaldialdehyde by using fluorometric detection (14). Acutely isolated cortical or hippocampus slices prepared from 14- to 18-day-old Sprague-Dawley rats were used for electrophysiological recordings (15) (Supporting Materials and Methods).
Results
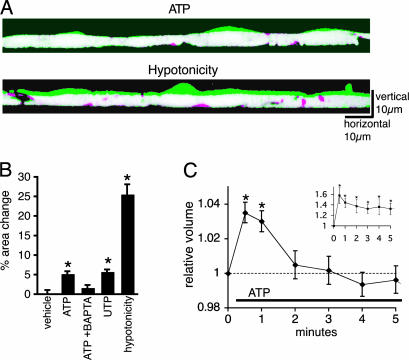
Receptor-Mediated Ca2+-Dependent Astrocytic Swelling. To test the hypothesis that a Ca2+ increase is associated with a transient increase in astrocytic cell volume, we measured relative changes in cell volume by using three different approaches. First, we used a confocal x-z laser scanning microscope (12). Vertical sections of cultured astrocytes loaded with calcein/AM (5 μM for 30 min) were constructed from repetitive x-z line scans (Fig. 1A). As several lines of work have indicated that astrocytic Ca2+ waves are mediated by ATP (16), we measured vertical section areas of the cells before and after purinergic receptor stimulation. The addition of ATP (100 μM) caused a small but significant increase of 5.19 ± 0.66% in the vertical sectional areas, which was attenuated to 1.63 ± 0.73% by cheletion with 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate (BAPTA)/AM (20 μM) (Fig. 1 A and B). Assuming that the swelling occurs only in vertical direction in confluent cell culture, these results suggest that ATP stimulation causes 5.2% volume increase.
Fig. 1.
Astrocytic Ca2+ increases are associated with a transient increase in cell volume. (A) Exposure to ATP (100 μM) induces swelling of cultured astrocytes. Confocal vertical cross-sectional images of confluent astrocyte cultures with 3-5 cells in the field of view loaded with calcein/AM (5 μM for 30 min) were constructed from repetitive x-z line scans at 488 nm excitation. Two images of cross-sectional area before (red) and 1 min after the exposure to ATP (green) are overlapped to indicate the change in cell volume. Overlapped areas (no change before and after ATP exposure) are displayed as white. Hypotonicity (214 mOsM) also induced cellular swelling. (B) Quantification of relative changes in cross-sectional areas 1 min after addition of vehicle (control, n = 12); ATP (100 μM, n = 23); ATP to cultures preloaded with BAPTA (20 μM for 30 min, n = 11); UTP (100 μM, n = 15) and hypotonicity (214 mOsM, n = 6). *, P < 0.01 compared with control, Tukey-Kramer test. (C) Coulter counter analysis of relative changes in astrocytic cell volume evoked by ATP. ATP exposure of astrocytes in suspension triggered a transient increase in cell volume at 30 and 60 sec. (C Inset) Hypotonicity induced a large and sustained increase in astrocytic cell volume. n = 5; *, P < 0.05 compared with control, t test. mean ± SEM.
Second, the use of the fluorescence-dilution method (17) detected a 9.6 ± 1.3% decrease in fluorescence emission in fura-2-loaded cultured astrocytes 1 min after ATP (100 μM) exposure compared with a 1.1 ± 1.3% increase in emission in vehicle-controls (n = 5; P < 0.001, t test).
Third, exposure of astrocytes in suspension culture to ATP and subsequent assay of cell volume with a Coulter counter (18) showed a significant, reversible increase in astrocytic cell volume, averaging 3.5 ± 0.6% at 30 sec (P < 0.0003) and 3.0 ± 0.5% at 60 sec (P < 0.001); cell volume returned to prestimulation values 2 min after stimulation (Fig. 1C). In comparison, reducing external osmolarity from 314 to 214 mOsM resulted in a 58 ± 15% increase in cell volume, which was only partly reversible (Fig. 1C Inset). Thus, each of these independent approaches to measure cell volume demonstrated a transient increase in astrocytic cell volume in response to purinergic activation. ATP-induced swelling was modest, in the range of 3-10%, compared with the 25-60% increase in cell volume in response to hypotonicity.
Pharmacological Characterization of Astrocytic Glutamate Release. To determine whether ATP and hypotonicity induce glutamate release by the same mechanism, glutamate release from cultured rat cortical astrocytes was analyzed by using a highly sensitive enzymatic assay (13). Application of 100 μM ATP resulted in the release of 2.49 ± 0.21 fmole glutamate per cell. ATP-induced glutamate release depended on increases of cytosolic Ca2+, because BAPTA/AM (20 μM for 30 min) and thapsigargin (1 μM for 10 min) attenuated the release, whereas removal of extracellular Ca2+ had no effect. In comparison, BAPTA and thapsigargin failed to affect glutamate release evoked by a hypoosmotic challenge (Fig. 2 A and B). Three different anion channel blockers, including 5-nitro-2-(3-phenylpropylamino)-benzoic acid (NPPB, 100 μM), flufenamic acid (FFA, 100 μM), and gossypol (10 μM), all decreased ATP- and swelling-induced glutamate release (0.22 ± 0.14, 0.00 ± 0.00, and 0.07 ± 0.07 fmole per cell, respectively), whereas a noncompetitive glutamate transporter inhibitor, dl-threo-β-benzyloxyaspartic acid (TBOA, 100 μM), was without effect (Fig. 2 A and B). The application of 1 μM bafilomycin A1, an inhibitor of vesicular proton pumps, for 1 h, or 2 μg/ml tetanus neurotoxin (TeNT), which inhibits exocytosis by cleaving synaptobrevin, for 24 h, had no effect on glutamate release evoked by ATP or by the hypotonic challenge (Fig. 2B). However, the protocol used for application of bafilomycin A1 effectively blocked exocytosis in hippocampal slices. Within 1 h, bafilomycin A1 reduced the frequency of inhibitory postsynaptic currents in interneurons from 2.35 per sec to 0.2 per sec (n = 4; P < 0.001, t test). Lastly, a 2-h application of methionine sulfoximine (MSO, 1.5 mM) (9), an inhibitor of glutamine synthetase that elevates the concentration of cytosolic glutamate, increased glutamate release after both ATP and hypotonicity (Fig. 2B). Together, these observations demonstrate that ATP and swelling-induced glutamate release share many characteristics but differ with regard to the dependency on cytosolic Ca2+: BAPTA and thapsigargin completely suppressed glutamate release evoked by ATP but had no effect on glutamate release triggered by hypotonicity.
Fig. 2.
Pharmacology of Ca2+-dependent glutamate release from astrocytes. (A) ATP-induced glutamate release from astrocytic cultures detected by fluorescence enzymatic assay. BAPTA/AM (20 μM for 30 min) and the anion channel blocker NPPB (100 μM) attenuated the glutamate efflux. (B Upper) Comparison of ATP-induced and hypotonic-induced glutamate release. ATP-induced release was inhibited by BAPTA/AM (20 μM for 30 min) and thapsigargin (1 μM). Anion channel blockers, NPPB (100 μM), FFA (100 μM), and gossypol (10 μM) all eliminated ATP-induced glutamate release, whereas removal of extracellular Ca2+ had no effect. A glutamate transport blocker, dl-threo-β-benzyloxyaspartic acid (TBOA) (100 μM), had no effect. Similarly, the inhibition of vesicular release by bafilomycin A1 (1 μM for 1 h) or tetanus neurotoxin (TeNT; 2 μg/ml for 24 h) had no effect. A glutamine synthetase inhibitor, methionin sulfoximine (MSO; 1.5 mM for 2 h), increased ATP-induced glutamate releases. (n = 5; *, P < 0.01 compared with control, Tukey-Kramer test). The release from cultured astrocytes from Cx43 KO mice was not significantly different from the release from matched wild-type littermates (n = 4; P = 0.64, t test). (B Lower) Hypoosmotic stimulation (214 mOsM) induced glutamate release that was Ca2+-independent, but otherwise had the same pharmacological profile as ATP-induced release (n = 5, *, P < 0.01 compared with control, Tukey-Kramer test). (C) Glutamate release was mediated by P2YR activation. UTP (100 μM; a P2Y agonist) induced glutamate release with a potency comparable to that of ATP. By contrast, αβ-meATP (100 μM; a P2X agonist) and Bz-ATP (100 μM) elicited little glutamate release. Similarly, Ox-ATP (300 μM for 1 h) did not significantly attenuate the release. Reactive Blue 2 (RB2, 30 μM; a P2Y antagonist) blocked the release. A cycoloxygenase inhibitor indomethacin (10 μM) also failed to inhibit ATP-induced glutamate release. n = 4; *, P < 0.01 compared with ATP, Tukey-Kramer test. (D Left) Glutamate release by 10 μM ATP is smaller than the release by 100 μM ATP but retains sensitivity to NPPB (100 μM) and TeNT (10 μg/ml overnight) (n = 3-5). *, P < 0.01, Tukey-Kramer test, mean ± SEM. (Right) Dose-response curve of ATP-induced glutamate release (n = 3). (E) Cell swelling is required for ATP-induced glutamate release. ATP (100 μM) was added at the same time as the osmolarity change, which was accomplished by adding sucrose (for hypertonicity) or distilled water (for hypotonicity). Hyperosmolarity >15% completely inhibited glutamate release (n = 3-5).
Connexin (Cx) hemichannels have been implicated in astrocytic glutamate release after removal of extracellular divalent cations (such as Ca2+ and Mg2+) (7). To evaluate the role of Cx43 (the predominant member of the Cx family expressed by astrocytes), we compared ATP-induced glutamate release from cultured astrocytes prepared from Cx43 KO mice and matched wild-type littermates. ATP (100 μM) induced glutamate release of 3.02 fmole per cell from Cx43 KO astrocytes, which was 90.7% of astrocytes prepared from wild-type littermates (Fig. 2B). Therefore, astrocytes prepared from Cx43 KO mice responded similar (n = 4; P = 0.64, t test) to astrocytes that express Cx43.
To further characterize the mechanism of ATP-induced glutamate release, we next evaluated the potency with which ATP agonists triggered glutamate release (16). UTP (an agonist for several P2Y receptor subtypes that are expressed by astrocytes, 100 μM) triggered glutamate release with a potency that was roughly equivalent to that of ATP, and broad-spectrum P2Y receptor antagonist Reactive Blue 2 (30 μM) blocked the release (Fig. 2C). By contrast, two P2X receptor agonists, αβ-meATP (100 μM) and 2′,3′-O-(4-benzoylbenzoyl)-ATP (Bz-ATP, 100 μM) were without effect (Fig. 2C). Preincubation with P2X1 and 7 receptor antagonist, oxidized ATP (OxATP; 300 μM for 1 h), did not significantly reduce ATP-induced glutamate release from cultured astrocytes (Fig. 2C). Similarly, P2X7 receptor antagonist Brilliant Blue G (BBG; 1 μM) did not reduce the glutamate release (106.4 ± 19.1% of control). Furthermore, UTP (100 μM) caused 5.75 ± 0.57% cell swelling, similar to ATP (Fig. 1B), whereas Bz-ATP failed to induce the swelling (1.35 ± 1.05%, n = 22). These observations indicate that ATP-induced astrocytic cell swelling and glutamate release are primarily evoked by activation of P2Y receptors. Indomethacin (10 μM) did not reduce ATP-induced glutamate release, suggesting that PGE2 production was not necessary for ATP-induced glutamate release (Fig. 2C). We next found that lowering the concentration of ATP caused a dose-dependent reduction in glutamate release (Fig. 2D). To test the idea that vesicular release contribute more significantly to glutamate release, when astrocytes were stimulated with lower and more physiological ATP concentration, we next found that NPPB (100 μM) caused 72.6 ± 3.9% reduction after the exposure to 10 μM ATP, whereas TeNT (10 μg/ml overnight) had no effect (Fig. 2D). Thus, the relative potency of NPPB and TeNT did not significantly change when the concentration of ATP was lowered from 100 μM to 10 μM.
Cell Swelling Is Required for Astrocytic Ca2+-Dependent Glutamate Release. To determine whether cell swelling was required for Ca2+-dependent astrocytic glutamate release, ATP was applied simultaneously with either increasing extracellular osmolarity (positive shift inhibits cell swelling) or decreasing osmolarity (negative shift potentiates cell swelling). ATP-induced glutamate release from cultured astrocytes was an inverse function of extracellular osmolarity shift (regression curve: y = -0.181x + 2.656, R2 = 0.994) and completely attenuated when osmolarity was raised by 15% (Fig. 2E). This set of data confirms previous studies showing that ATP enhances swelling-induced release of excitatory amino acids released from astrocytes (8, 19) and indicates that volume increase is a prerequisite for ATP-induced astrocytic glutamate release. Furthermore, because hypertonicity is known to trigger vesicular release (20), our data does not support the idea that exocytosis of glutamate containing vesicles plays a predominant role in astrocytic glutamate release.
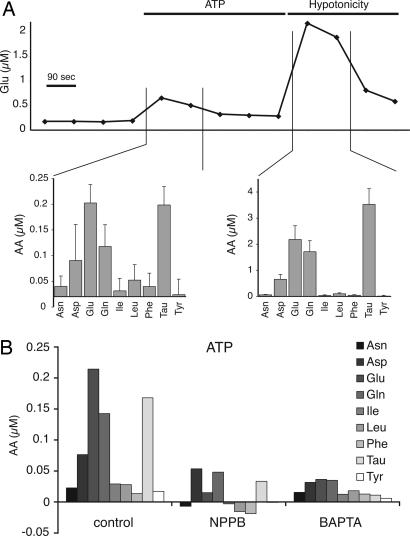
Joint Release of Amino Acid Osmolytes Evoked by Increases in Astrocytic Ca2+. One of the characteristics of swelling-induced glutamate release is that other osmolytes, including taurine, aspartate and glutamine, are also released in parallel (9). To compare the mechanism of ATP-induced, Ca2+-dependent astrocytic glutamate release with swelling-induced release, we analyzed the extracellular concentrations of amino acids released from cultured astrocytes by using HPLC (Fig. 3A). Interestingly, glutamate was not released in isolation, but in conjunction with taurine, aspartate, and glutamine. The profile of amino acid release in response to ATP was strikingly similar, if not identical, to swelling-induced release. In essence, purinergic stimulation induced efflux of amino acids that are regarded as osmolytes, but not of other amino acids such as asparagine, isoleucine, leucine, phenylalanine, and tyrosine (Fig. 3A). These observations strongly support the notion that volume-sensitive channels are activated during receptor-stimulated Ca2+ increase, resulting in efflux of cytosolic glutamate along with other amino acids. Moreover, NPPB and BAPTA/AM inhibited glutamate, as well as aspartate, glutamine, and taurine releases evoked by ATP exposure (Fig. 3B).
Fig. 3.
Astrocytic Ca2+ increases are associated with a joint release of organic osmolytes. (A) Time course of glutamate level in extracellular perfusion buffer showed that ATP stimulation (100 μM) triggers a 2- to 5-fold increase in glutamate release, whereas a 10-fold elevation of extracellular glutamate is evoked by hypotonicity (214 mOsm). HPLC analysis of the amino acid profile revealed that ATP stimulation caused release of glutamate, aspartate, glutamine, and taurine but not of asparagine, isoleucin, leucine, phenelalanine, and tyrosine. The hypoosmotic challenge triggered amino acid release of a larger amplitude, but the profile was almost identical to that observed after ATP stimulation. Amino acid release is displayed as changes from baseline concentration (n = 9). (B) BAPTA and NPPB not only inhibited ATP-induced release of glutamate but also the release of aspartate, glutamine, and taurine.
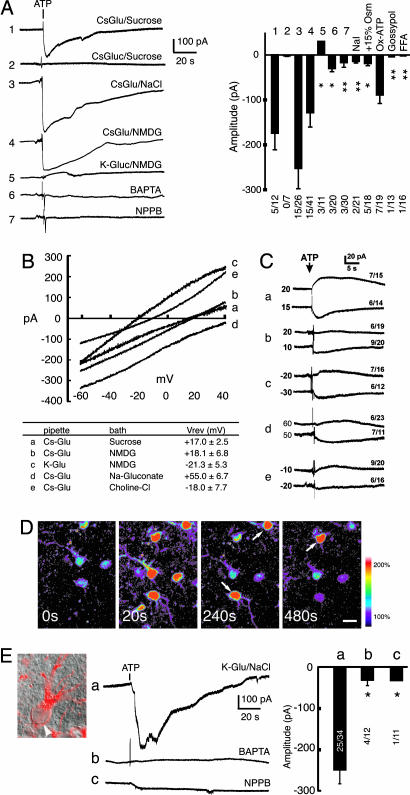
Ca2+-Mediated Activation of a Channel Permeable to Glutamate. To provide direct evidence for purinergic-mediated opening of a glutamate-permeable channel, we next recorded whole-cell currents in cultured astrocytes. To eliminate inward cation conductances, extracellular ions were replaced by sucrose (250 mM; osmolarity, 290 mEq) in the external solution, whereas the pipette solution contained 123 mM Cs-Glutamate (Cl--free). Under these ion conditions with a holding potential of -60 mV, glutamate is the only ion that can cause inward current due to its efflux. ATP (100 μM) triggered an inward current in 5 of 12 astrocytes, with average amplitude of 177 ± 37 pA (range of 90-260 pA) (Fig. 4A1). This observation suggests that purinergic-mediated Ca2+ increases are associated with the opening of a channel permeable to glutamate. Replacing glutamate with gluconate resulted in disappearance of the inward current (Fig. 4A2), demonstrating that glutamate was the sole ion responsible for the inward current, and Cs+, sucrose, and gluconate- were impermeable. With normal bath solution containing NaCl (126 mM), and Cs-glutamate in pipette solution, the amplitude of the current increased to 250 ± 33 pA (range of 70-670 pA), indicating that the channel was also permeable to Na+ (Fig. 4A3). When NMDG-Cl replaced NaCl in the external solution, ATP (100 μM) triggered an inward current in 15 of 41 astrocytes, with an average amplitude of 138 ± 36 pA (range 25-290 pA), similar to sucrose substitution (Fig. 4A4). When the pipette contained K-gluconate (glutamate-free solution), and NaCl was substituted by NMDG in the extracellular solution, no inward current was detected. Instead, ATP triggered a small outward current (Fig. 4A5), suggesting that K+ also could permeate the channel. The ATP-induced current (with NaCl in the external solution) was blocked by adding 10 mM BAPTA to the pipette solution (Fig. 4A6) in agreement with the observation that BAPTA attenuates glutamate release from astrocytic cultures (Fig. 2B). Similarly, three anion channel blockers that inhibited glutamate release, NPPB (100 μM, Fig. 4A7), FFA (100 μM) and gossypol (10 μM), all inhibited ATP-induced current (Fig. 4A). Taken together, these observations indicate that ATP activates a glutamate-permeable channel, and that channel opening is Ca2+-dependent and strongly inhibited by anion channel blockers. Replacing Cl- with I- (NaI) attenuated the inward current (Fig. 4A, NaI), in agreement with the recent observation that I- potently inhibited volume recovery of cultured astrocytes (21). Indeed, increasing the osmolarity of the bath solution by 15% decreased the amplitude of the ATP-induced current (Fig. 4A, +15% Osm). Preincubation with OxATP (300 μM for 1 h), had no significant effect on the frequency or amplitude of the ATP-induced current, suggesting that P2 × 7 does not play a significant role in the Ca2+-dependent glutamate release (Fig. 4A, Ox-ATP).
Fig. 4.
Astrocytic Ca2+ increases are associated with activation of a glutamate-permeable channel. (A Left) ATP induced an inward current in cultured astrocytes. Astrocytes were patched in the whole-cell voltage-clamp configuration with a holding potential of -60 mV. Continuous recording with 123 mM Cs-glutamate in the pipette and 250 mM sucrose in the extracellular solution showed that ATP evoked an inward current (1). When Cs-gluconate replaced intracellular Cs-glutamate and sucrose in extracellular solution, no currents were induced by ATP (2). When sucrose was replaced with 126 mM NaCl in the bath, the amplitude of the inward current increased (3). When 126 mM NMDG-Cl replaced sucrose, a similar inward current was induced by ATP (4). When K-gluconate replaced intracellular Cs-glutamate, a small outward current was recorded (5). The addition of 10 mM BAPTA in the pipette (same conditions as in 3) inhibited the inward current (6). When 100 μM NPPB was added to the bath (same conditions as in 3), the inward current was inhibited (7). (A Right) Mean amplitude of ATP-induced currents. Replacing Cl- with I- (NaI) potently inhibited the inward current. Increasing the bath osmolarity by 15% (+15% Osm) by adding sucrose to the bath solution attenuated the ATP-induced current, whereas OxATP (300 μM for 1 h) was without effect. The ATP-induced current was inhibited by gossypol (10 μM) and FFA (100 μM). *, P < 0.05 and **, P < 0.01 compared with (1). The numbers indicate responding cells/total cells in each experiment. (B Upper) The ramp I-V currents (ATP-induced net currents) with [CsGlu]in/[sucrose]out (a), [CsGlu]in/[NMDG]out (b), [KGlu]in/[NMDG]out (c), [CsGlu]in/[NaGluconate]out (d), [CsGlu]in/[CholineCl]out (e). (B Lower) Summary table of the reversal potentials (mean ± SEM in mV). (C) Measurements of reversal potential by using steady-state holding potentials in the same conditions as in B (a-e labeling as in B). The numbers to the left side of each trace show the holding potential, whereas the numbers on the right show the number of responding cells/the total number of tested cells. (D) ATP-induced Ca2+ increases in astrocytes in hippocampal slices (P14). Ca2+ normalized within 1 or 2 min, but some cells continued to display oscillatory increases in Ca2+ (white arrows). (Scale bar: 10 μm.) (E) ATP-evoked inward currents in astrocytes in hippocampal slices. (Right) Astrocytes in hippocampus (stratum radiatum) were identified under DIC optics by their small cell bodies (white arrowhead), which stained positive for GFAP (red), and by their high resting membrane potential, and absence of depolarization-evoked action potential. (Middle) Representative recordings of ATP-induced currents. (a) 50 mM K-glutamate/73 mM K-gluconate in the pipette and 126 mM NaCl in the bath. (b) 10 mM BAPTA in the pipette with the same solutions as in a.(c) NPPB (100 μM) was added to the bath. Tetrodotoxin (1 μM) was present in the bath. (Left) Summary histogram showing the mean amplitude of the ATP-induced currents with the number of responding cells/the total number of tested cells. *, P < 0.05 compared to a. Mean ± SEM.
Ion Permeability of the ATP-Activated Channel. Because ATP-activated glutamate-permeable channel also exhibited permeability to Na+ and K+, we next further characterized the ion permeability of the channel. Reversal potentials under different ionic conditions were measured. We first applied ramp commands before and after the application of ATP. The net I-V current was obtained by subtracting the I-V current before ATP application from the I-V current after ATP application (Fig. 4B). This step was taken to eliminate the large leak current of cultured astrocytes. Because subtraction of the leak currents might interfere with the measurement of reversal potential in the ramp experiments, we used an alternative approach to confirm the reversal potential of the ATP-induced current. For each ion substitution condition, we used two different holding potentials, one below and one above the reversal potential that we obtained from the ramp experiments. Astrocytes were patched in the voltage-clamp configuration, and once a stable baseline was obtained, the cells were exposed to ATP. The currents reversed between the test holding potentials (Fig. 4C), confirming the reversal potential values from the ramp experiments.
With 123 mM Cs-glutamate in the pipette and sucrose outside, the reversal potential of the ATP-induced current was +17.0 ± 2.5 mV (Fig. 4 Ba and Ca), indicating that glutamate indeed permeated the channel. With 100 mM Cs-glutamate/23 mM Cs-gluconate in the pipette and NMDG outside, the reversal potential of the ATP-induced current was +18.1 ± 6.8 mV (Fig. 4 Bb and Cb). Together with the observation that no current was recorded in response to ATP, when the pipette contained Cs-gluconate and extracellular NaCl was replaced by sucrose (Fig. 4A2), this set of information indicates that gluconate and NMDG both are impermeable. When Cs+ was replaced by K+ (100 mM K+ glutamate/23 mM K+-gluconate in the pipette and NMDG outside), the reversal potential shifted leftward to -21.3 ± 5.3 mV (Fig. 4 Bc and Cc), suggesting that the channel is permeable to K+. When NMDG was replaced by Nagluconate, the reversal potential shifted rightward to +55 ± 6.7 mV (Fig. 4 Bd and Cd), indicating that the channel is permeable to Na+. Of note, the tail of ramp command was 40 mV, and the reversal potential for Na+-gluconate was therefore calculated by extrapolation. Last, when choline chloride replaced NMDG (100 mM Cs-glutamate/23 mM Cs-gluconate in the pipette and 126 mM choline chloride outside), the reversal potential shifted leftward to -18.0 ± 7.7 mV (Fig. 4 Be and Ce), as compared with recordings made in the presence of Cs-glutamate/sucrose or Cs-glutamate/NMDG (Fig. 4 B and C, a and b), suggesting that the channel is also permeable to Cl-.
Together, these observations on ion permeability are consistent with the recordings in Fig. 4A and add further strength to the conclusion that ATP activates glutamate-permeable channels.
ATP Activates Glutamate-Permeable Channel in Astrocytes in Acute Slices. To determine whether stimulation of ATP is associated with a transient increase in astrocytic Ca2+ concentration and activation of glutamate-permeable channels in intact tissue, ATP-induced activation of astrocytes in situ was next characterized by using two-photon microscopy and whole-cell current-clamp approach. Astrocytes in hippocampal slices were loaded with Ca2+ indicator dye, Fluo-4 a.m (15). (22). Application of ATP (100 μM) evoked a 131 ± 13% increase in the fluo-4 signal over baseline that lasted an average of 8.7 ± 1.4 sec in the vast majority of cells (>95%, n = 250) (Fig. 4D). Thus, ATP potently increased astrocytic Ca2+ in acute slices, similar to previous observations in cultured astrocytes (16). In the presence of 50 mM glutamate in the pipette solution, bath application of ATP (100 μM) induced an inward current in 25 of 34 cells (254 ± 31 pA, range: 53-560) (Fig. 4Ea). Similar to the observations in cultured astrocytes, the ATP-induced inward current was attenuated by either 10 mM intracellular BAPTA (Fig. 4Db) or 100 μM NPPB (Fig. 4Ec) in bath solution. BBG (1 μM) did not affect the amplitude of the inward current (106.8 ± 22.0%; n = 14; P = 0.8, t test).
Discussion
The main observation of this study is that receptor-mediated astrocytic Ca2+ increases are associated with transient cell swelling, resulting in the activation of volume-sensitive channels and the release of cytosolic glutamate, thus demonstrating that glutamate release is intimately linked to dynamic changes in astrocytic cell volume and activation of VSC. Another important observation is that cytosolic glutamate can be released in a regulated, Ca2+-dependent manner and, therefore, constitute a potential transmitter pool.
Direct evidence for channel-mediated efflux of glutamate was obtained by whole-cell recordings of cultured astrocytes. ATP activated a glutamate-permeable channel. The property of channel opening closely mimicked the characteristics of astrocytic glutamate release. BAPTA, NPPB, FFA, and glossypol potently inhibited both channel activation and glutamate release. Importantly, increasing bath osmolarity by 15% strongly inhibited channel activation and eliminated glutamate release (Figs. 2 and 4). The role of VSC in receptor-mediated glutamate release was illustrated by the striking similarity of the profiles of amino acids released between the receptor stimulation and the hypotonic activation of VSC (Fig. 3). Both stimulation paradigms were associated with the selective release of amino acid osmolytes, including aspartate, glutamate, glutamine, and taurine, whereas other amino acids, such as leucine, phenylalanine, and tyrosine, were not released (Fig. 3). It has previously been demonstrated that ATP potentiates hypotonicity-induced release of amino acid osmolytes (8, 19), but direct evidence for receptor-mediated opening of a channel permeable to glutamate has been lacking. Taken together, our observations indicate that astrocytes release glutamate through a regulated pathway that requires mobilization of intracellular Ca2+ stores and activation of volume sensitive glutamate-permeable channels.
We considered four other possible mechanisms of glutamate release to explain our data. First, opening of Ca2+-activated Cl- channels may provide a pathway for glutamate efflux. However, the inner pore diameter of Ca2+-activated Cl- channels may not be large enough to allow permeation of glutamate (6.5 × 10.8 Å), because diphenylamine-2-carboxylic acid (DPC, 6.0 × 9.4 Å) failed to permeate (23). Also, the dependence of astrocytic glutamate release upon medium osmolarity (Fig. 2E) does not support the role of Ca2+-activated Cl- channels in efflux of glutamate. However, ATP may trigger opening of several types of channels, which may include both glutamate permeable and impermeable channels.
Second, P2X7 receptor-gated channels have been implicated in Ca2+-independent efflux of glutamate from astrocytes (24). The lack of action of BzATP and OxATP lend no support to a significant contribution of P2X7 receptors in Ca2+-dependent glutamate release (Figs. 2 and 4). Also, P2X7-linked channels are characterized by their cation selectivity and are not gated by cytosolic Ca2+.
Third, Ransom and coworkers (7) have recently reported that removal of divalent cations opens Cx-hemichannels, resulting in efflux of cytosolic glutamate. We confirmed that removal of both extracellular divalent cations Mg2+ and Ca2+ resulted in sustained basal release but failed to potentiate ATP-induced glutamate release (data not shown). Furthermore, astrocytes prepared from Cx43 KO and wild-type mice released comparable amount of glutamate, lending no support for the idea that Cx-hemichannels play a role in Ca2+-dependent glutamate release from astrocytes. This observation does not exclude that Cx-hemichannel may play important roles in glutamate release in pathological conditions, including ischemia and epilepsy (7, 25).
Fourth, Ca2+-dependent exocytosis of glutamate from cultured astrocytes has been demonstrated by several groups (3, 4, 26, 27). Although our observations do not directly address the role of exocytosis in glutamate release, a number of our observations are not consistent with exocytosis constituting the primary pathway of astrocytic glutamate release. First, several anion channel blockers attenuated Ca2+-dependent glutamate release (Fig. 2B). Second, astrocytes did not release glutamate in isolation, but in conjunction with the release of other amino acids osmolytes, including aspartate, taurine, and glutamine (Fig. 3). VGLUT1/2 are highly specific for glutamate and do not transport other amino acid osmolytes. Thus, the joint release of aspartate, glutamate, glutamine, and taurine indicates that channel-mediated efflux play a predominant role in Ca2+-mediated glutamate release. Culturing can induce astrocytes to express proteins that in situ are neuron specific. For example, synaptic vesicular protein 2 is abundantly expressed by cultured astrocytes but not by astrocytes in intact brain (28). In this regard, it is important to note that the ATP-induced inward current was of a similar magnitude, ≈250 pA, in astrocytes in slices and in cultures in the presence of extracellular Na+ (Fig. 4). Third, hyperosmotic solutions are known to trigger vesicle fusion (29), yet hyperosmotic solutions inhibited the glutamate release. In fact, glutamate release was an inverse function of osmolarity and was completely blocked by a 15% increase in medium osmolarity in agreement with previous reports (19) (Fig. 2E). Finally, another important argument against exocytosis as the principal pathway of glutamate release is the large quantity of the amino acid, which is released by cultured astrocytes in response to receptor activation. ATP and PGE2 stimulation trigger glutamate release in the order of 1 nM/mg protein or ≈3 fmole of glutamate released per astrocyte (ref. 13 and Fig. 2). VGLUT1/2 expressing vesicles in astrocytes have a diameter of 30 nm (4), and assuming that the concentration of glutamate is similar to synaptic vesicles (10-100 mM glutamate), each astrocyte must release 105 to 106 vesicles to account for the release observed (30). To the contrary, TIR-FM imaging of membrane fusions of acridine orange-filled vesicles detected a total of 120 exocytotic events per astrocyte (4).
The finding that astrocytes release glutamate by a regulated pathway that is sensitive to several anion channel inhibitors offers an opportunity to manipulate synaptic transmission both in normal physiology and in conditions that involve the pathological activation of astrocytes, including neurodegenerative diseases.
Supplementary Material
Author contributions: T.T., J.K., J.K.J., S.M.S., J.H.-C.L., G.D., H.R.Z., and M.N. designed research; T.T., J.K., J.K.J., J.H.-C.L., Y.Y., and Y.L. performed research; J.Y., G.D., and H.R.Z. contributed new reagents/analytic tools; and M.N. wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AM, acetoxymethyl ester; BAPTA, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate; Cx, connexin; FFA, flufenamic acid; NPPB, 5-nitro-2-(3-phenylpropylamino)benzoic acid; TeNT, tetanus neurotoxin; VSC, volume-sensitive channel.
References
- 1.Volterra, A. & Meldolesi, J. (2005) Nat. Rev. Neurosci. 6, 626-640. [DOI] [PubMed] [Google Scholar]
- 2.Haydon, P. G. (2001) Nat. Rev. Neurosci. 2, 185-193. [DOI] [PubMed] [Google Scholar]
- 3.Montana, V., Ni, Y., Sunjara, V., Hua, X. & Parpura, V. (2004) J. Neurosci. 24, 2633-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bezzi, P., Gundersen, V., Galbete, J. L., Seifert, G., Steinhauser, C., Pilati, E. & Volterra, A. (2004) Nat. Neurosci. 7, 613-620. [DOI] [PubMed] [Google Scholar]
- 5.Price, D. L., Ludwig, J. W., Mi, H., Schwarz, T. L. & Ellisman, M. H. (2002) Brain Res. 956, 183-193. [DOI] [PubMed] [Google Scholar]
- 6.Su, G., Kintner, D. B. & Sun, D. (2002) Am. J. Physiol. 282, C1136-C1146. [DOI] [PubMed] [Google Scholar]
- 7.Ye, Z. C., Wyeth, M. S., Baltan-Tekkok, S. & Ransom, B. R. (2003) J. Neurosci. 23, 3588-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mongin, A. A. & Kimelberg, H. K. (2005) Am. J. Physiol. 288, C204-C213. [DOI] [PubMed] [Google Scholar]
- 9.Kimelberg, H., Goderie, S., Higman, S., Pang, S. & Waniewski, R. (1990) J. Neurosci. 10, 1583-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jentsch, T. J., Stein, V., Weinreich, F. & Zdebik, A. A. (2002) Physiol. Rev. 82, 503-568. [DOI] [PubMed] [Google Scholar]
- 11.Lin, J. H., Yang, J., Liu, S., Takano, T., Wang, X., Gao, Q., Willecke, K. & Nedergaard, M. (2003) J. Neurosci. 23, 430-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schreiber, R., Nitschke, R., Greger, R. & Kunzelmann, K. (1999) J. Biol. Chem. 274, 11811-11816. [DOI] [PubMed] [Google Scholar]
- 13.Bezzi, P., Carmignoto, G., Pasti, L., Vesce, S., Rossi, D., Rizzini, B. L., Pozzan, T. & Volterra, A. (1998) Nature 391, 281-285. [DOI] [PubMed] [Google Scholar]
- 14.Shank, R. P., Leo, G. C. & Zielke, H. R. (1993) J. Neurochem. 61, 315-323. [DOI] [PubMed] [Google Scholar]
- 15.Kang, J., Jiang, L., Goldman, S. & Nedergaard, M. (1998) Nat. Neurosci. 1, 683-692. [DOI] [PubMed] [Google Scholar]
- 16.Cotrina, M. L., Lin, J. H.-C., Alves-Rodrigues, A., Liu, S., Li, J., Azmi-Ghadimi, H., Kang, J., Naus, C. C. & Nedergaard, M. (1998) Proc. Natl. Acad. Sci. USA 95, 15735-15740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansson, E. (1994) J. Biol. Chem. 269, 21955-21961. [PubMed] [Google Scholar]
- 18.Raat, N. J., De Smet, P., van Driessche, W., Bindels, R. J. & Van Os, C. H. (1996) Am. J. Physiol. 271, C235-C241. [DOI] [PubMed] [Google Scholar]
- 19.Mongin, A. A. & Kimelberg, H. K. (2002) Am. J. Physiol. 283, C569-C578. [DOI] [PubMed] [Google Scholar]
- 20.Sara, Y., Mozhayeva, M. G., Liu, X. & Kavalali, E. T. (2002) J. Neurosci. 22, 1608-1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parkerson, K. A. & Sontheimer, H. (2003) Am. J. Physiol. 284, C1460-C1467. [DOI] [PubMed] [Google Scholar]
- 22.Kang, J. & Nedergaard, M. (1999) Imaging Astrocytes in Acute Brain Slices (Cold Spring Harbor Lab. Press, Plainview, NY).
- 23.Qu, Z. & Hartzell, H. C. (2001) J. Biol. Chem. 276, 18423-18429. [DOI] [PubMed] [Google Scholar]
- 24.Duan, S., Anderson, C. M., Keung, E. C., Chen, Y. & Swanson, R. A. (2003) J. Neurosci. 23, 1320-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian, G. F., Azmi, H., Takano, T., Xu, Q., Peng, W., Lin, J., Oberheim, N., Lou, N., Wang, X., Zielke, H. R., et al. (2005) Nat. Med. 11, 973-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kreft, M., Stenovec, M., Rupnik, M., Grilc, S., Krzan, M., Potokar, M., Pangrsic, T., Haydon, P. G. & Zorec, R. (2004) Glia 46, 437-445. [DOI] [PubMed] [Google Scholar]
- 27.Zhang, Q., Pangrsic, T., Kreft, M., Krzan, M., Li, N., Sul, J. Y., Halassa, M., Van Bockstaele, E., Zorec, R. & Haydon, P. G. (2004) J. Biol. Chem. 279, 12724-12733. [DOI] [PubMed] [Google Scholar]
- 28.Wilhelm, A., Volknandt, W., Langer, D., Nolte, C., Kettenmann, H. & Zimmermann, H. (2004) Neurosci. Res. 48, 249-257. [DOI] [PubMed] [Google Scholar]
- 29.Pyle, J. L., Kavalali, E. T., Piedras-Renteria, E. S. & Tsien, R. W. (2000) Neuron 28, 221-231. [DOI] [PubMed] [Google Scholar]
- 30.Glavinovic, M. I. (1999) Pflügers Arch. 437, 462-470. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.