Abstract
Protein synthesis has long been known to be required for associative learning to consolidate into long-term memory. Here we demonstrate that PKC isozyme activation on days before training can induce the synthesis of proteins necessary and sufficient for subsequent long-term memory consolidation. Bryostatin (Bryo), a macrolide lactone with efficacy in subnanomolar concentrations and a potential therapeutic for Alzheimer's disease, is a potent activator of PKC, some of whose isozymes undergo prolonged activation after associative learning. Under normal conditions, two training events with paired visual and vestibular stimuli cause short-term memory of the mollusc Hermissenda that lasts ≈7 min. However, after 4-h exposures to Bryo (0.25 ng/ml) on two preceding days, the same two training events produced long-term conditioning that lasted >1 week and that was not blocked by anisomycin (1 μg/ml). Anisomycin, however, eliminated long-term memory lasting at least 1 week after nine training events. Both the nine training events alone and two Bryo exposures plus two training event regimens caused comparably increased levels of the PKC α-isozyme substrate calexcitin in identified type B neurons and enhanced PKC activity in the membrane fractions. Furthermore, Bryo increased overall protein synthesis in cultured mammalian neurons by up to 60% for >3 days. The specific PKC antagonist Ro-32-0432 blocked much of this Bryo-induced protein synthesis as well as the Bryo-induced enhancement of the behavioral conditioning. Thus, Bryo-induced PKC activation produces those proteins necessary and sufficient for long-term memory on days in advance of the training events themselves.
Keywords: bryostatin, PKC isozymes
The requirement of protein synthesis for long-term memory has been demonstrated over several decades for a variety of memory paradigms (1-14). It was originally shown that drug-induced inhibition of protein synthesis (e.g., with 5-propyluracil or anisomycin) blocked long-term memory when this inhibition occurred during a critical time interval after the training paradigm (9). It has remained a mystery as to what specific, critical proteins were so essential for memory consolidation and how their molecular regulation was so necessary for long-lasting memory storage.
In many species the formation of long-term associative memory has also been shown to depend on translocation, and thus activation, of protein kinase C (PKC) isozymes to neuronal membranes. PKC activation has been shown to occur in single identified type B cells of the mollusc Hermissenda (15) with Pavlovian conditioning and a variety of mammalian associative learning protocols (16-18). Furthermore, a high-affinity substrate of the α-isozyme of PKC, calexcitin (CE) (19), was found within single identified type B cells to show Pavlovian-conditioning-dependent increases of phosphorylation and absolute quantity (20).
Consistent with these findings, administration of the potent PKC activator bryostatin (Bryo) enhanced rat spatial maze learning (21). Bryo, a macrolide lactone, has efficacy in subnanomolar concentrations (22-24). Like phorbol esters and the endogenous activator diacylglycerol, Bryo binds to the C1 domain within PKC and causes its translocation to membranes, which is then followed by down-regulation (25, 26). However, unlike most other identified exogenous PKC activators, Bryo is not tumorigenic, but instead shows anti-neoplastic properties and has been investigated in cancer clinical trials (22, 27).
Here, we hypothesized that periods of drug-induced PKC activation on days before initiation of training might have long-lasting impact on memory consolidation, perhaps through PKC-dependent synthesis of those proteins critically required for long-term memory. Sufficient PKC activation and prior PKC-mediated synthesis of critical proteins might reduce the number of training events (TEs) required and eliminate the requirement for further protein synthesis to produce long-term memory. The report described here confirms these predictions and supports the hypothesis that PKC activation on days preceding training induces protein synthesis necessary and sufficient for consolidation of long-term memory.
Materials and Methods
Behavioral Conditioning. Pavlovian conditioning of Hermissenda involves repeated pairings of a neutral stimulus, light, with an unconditioned stimulus, orbital shaking [see Lederhendler et al. (28) and Epstein et al. (29)]. A rotation/shaking stimulus excites the statocyst hair cells and thereby elicits an unconditioned response: a brisk contraction of the muscular undersurface called a foot, accompanied by adherence or “clinging” to the surface that supports the foot. Before conditioning, light elicits a weakly positive phototaxis accompanied by lengthening of the foot. After sufficient light-rotation pairings, light no longer elicits phototaxis but instead elicits a new response (28): the “clinging” and foot shortening previously elicited only by the unconditional stimulus (see Fig. 1). This conditioned response to light can last for weeks, is not produced by randomized light and rotation, is stimulus-specific, and shares the other defining characteristics of mammalian Pavlovian conditioning.
Fig. 1.
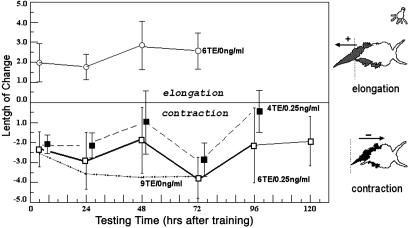
Effect of Bryo on long-term retention. Animals were trained with four and six paired conditioned stimulus/unconditioned stimulus TEs with Bryo (0.25 ng/ml). Bryo was added during dark adaptation (10 min) before training and continued for 4 h. All animals were tested with the conditioned stimulus alone at 4 h, then at 24-h intervals after training. Animals trained suboptimally but treated with Bryo all demonstrated long-term retention (n = 8-16, ANOVA, P < 0.01). Filled squares (dashed lines) are displaced slightly to the right for clarity. Lowest dashed line indicates previously observed effect of nine training trials.
Graphs in the figures shown below depict behavioral changes in terms of percentage of length change. However, graphic representations of all behavioral effects were virtually identical, producing the same statistically significant differences, when using percentage of change of foot length or using the actual length measurements acquired from video camera recordings. For example, in Fig. 4, at 96 h, the length increment responses in trained animals preexposed to Bryo were -0.50 and -0.52 mm. These points correspond to -2.6% and -2.9% change, respectively.
Fig. 4.
Effect of three successive days of 4-h Bryo exposure (0.25 ng/ml) followed 1 day later by two TEs. Retention was measured over 96 h after training. Nonexposed animals (same as in Fig. 3) did not demonstrate any behavioral modification (no conditioned response to conditioned stimulus testing) (Upper). Anisomycin (1 μg/ml) administered immediately and remaining for 4 h after training to animals receiving the 3-day Bryo treatments did not prevent long-term retention (Lower). (n = 16; ANOVA, P < 0.01.) Note that the control data (Upper) are the same as in Fig. 3.
Long-term Pavlovian conditioning of Hermissenda usually reverses after 1-2 weeks. This reversal, demonstrated in many previous articles, is, for the most part, not illustrated in the figures presented here.
Behavioral Pharmacology: Bryo Exposure. Specimens of Hermissenda crassicornis were purchased from Sea Life Supply (Sand City, CA) and maintained in artificial sea water (ASW) at 15°C for 3 days in perforated 50-ml conical centrifuge tubes before starting experiments. Bryo (Calbiochem), purified from the marine Bryozoan Bugula neritina, was dissolved in ethanol and diluted to its final concentration in ASW. Animals were incubated with Bryo in ASW for 4 h, then rinsed with normal ASW. For selected experiments lactacystin (10 μM) or anisomycin was added to the ASW. Bryo is stable for at least 28 days at room temperature in saline solutions at neutral pH with no measurable decomposition, provided that exposure to UV light is minimized (30). Bryo effects on Hermissenda behavior were produced by adding the drug to the bathing medium within an 8-cm-long, 1-cm-diameter test tube housing each individual animal. For effects on PKC activity, Hermissenda were exposed to Bryo in their individual 50-ml conical centrifuge tubes, thereby eliminating possible effects caused by transferring the animals to separate containers.
Immunostaining Methods. CNSs were removed and fixed in 4% paraformaldehyde in 20 mM Tris-buffered (pH 8) natural sea-water (0.2-μm micropore-filtered), embedded in polyester wax (20), sectioned (6 μm) and immunostained by using a biotinylated secondary antibody coupled to avidin-bound microperoxidase (ABC method, Vector Laboratories). Aminoethylcarbazole was used as the chromogen. The primary polyclonal antibody (designated 25U2) was raised in rabbits from the full-length CE protein extracted from squid optic lobes. Grayscale intensity measures were done from digital photomicrographs by using image-j software on circumscribed cytoplasmic areas of the B-photoreceptors minus the same background area (nonstaining neuropile).
Biochemistry. PKC assay. Cells were homogenized by sonication (5 sec, 25 W) in 100 μl of 10 mM Tris-HCl (pH 7.4) buffer containing 1 mM EGTA, 1 mM PMSF, and 50 mM NaF. Homogenate was transferred to a polyallomer centrifuge tube and was centrifuged at 100,000 × g for 10 min at 4°C. The supernatant was removed and immediately frozen on dry ice. The particulate fraction was resuspended by sonication in 100 μl of the same buffer and stored at -80°C. To measure PKC, 10 μl of cytosol or particulate fraction was incubated for 15 min at 37°C in the presence of 10 μM histones, 4.89 mM CaCl2, 1.2 μg/μl phosphatidyl-l-serine, 0.18 μg/μl 1,2-dioctanoyl-snglycerol, 10 mM MgCl2, 20 mM Hepes (pH 7.4), 0.8 mM EDTA, 4 mM EGTA, 4% glycerol, 8 μg/ml aprotinin, 8 μg/ml leupeptin, and 2 mM benzamidine. [γ-32P]ATP (0.5 μCi) was added, and 32P-phosphoprotein formation was measured by adsorption onto phosphocellulose as described previously (31). This assay, used with slight adjustments for either Hermissenda nervous system homogenates or cultured mammalian neuron homogenates, measures the calcium and diacylglycerol-dependent phosphorylation of histones by protein kinase. Other protein kinases that act on histones and are dependent on calcium and diacylglycerol potentially could also be measured by this assay. However, protein kinase D (formerly known as PKCμ) also binds diacylglycerol, but it is not calcium-dependent (32). Other major kinases, such as Ca2+/calmodulin kinase II, protein kinase A, casein kinase, mitogen-activated protein kinases, and tyrosine kinases, would not be detected in our assay.
Cell culture. Rat hippocampal H19-7/IGF-IR cells (American Type Culture Collection) were plated onto poly-l-lysine-coated plates and grown at 35°C in DMEM/10% FCS for several days until ≈50% coverage was obtained. The cells were then induced to differentiate into a neuronal phenotype by replacing the medium with 5 ml of N2 medium containing 10 ng/ml basic fibroblast growth factor at 39°C and grown in six-well plates (33). Various concentrations of Bryo (0.01-1.0 nM) were then added in 10 μl of aqueous solution. After a specified interval the medium was removed and the cells were washed with PBS, removed by gentle scraping, and collected by centrifugation at 1,000 rpm for 5 min.
Results
Bryo-Induced Prolongation of Associative Memory. Two TEs of paired light and orbital shaking (see Materials and Methods) induce a learned, Pavlovian-conditioned response (light-elicited foot contraction or shortening) that persists without drug treatment for ≈7 min. Four to six TEs induce a conditioned response that persists up to a few hours, whereas nine TEs produce long-term associative memory lasting as long as 2 weeks (Fig. 1 Lower, dotted line). However, two TEs plus Bryo produced memory retention lasting hours (vs. minutes without Bryo), four TEs plus Bryo extended retention beyond 24 h (Fig. 1), and six TEs plus Bryo produced retention lasting 1 week or longer. Randomized presentations of light and rotation, with or without Bryo, produced no conditioned response (Fig. 2A), i.e., light-elicited foot contraction.
Fig. 2.
Effects of blockers on retention. (A) Four-hour retention effects of Bryo under control and antagonist experimental regimes (NSW, normal sea water controls). R032, Ro-32-0432 blocks retention with six TE plus Bryo. (n = 4-8; ANOVA, P < 0.01.) (B) Effects of Bryo and anisomycin on retention after 4 h. Animals received two paired TEs in normal sea water (NSW), 0.25 ng/ml Bryo, or 0.25 ng/ml Bryo plus 1 ng/ml anisomycin. (n = 12, each group; two-way ANOVA, P < 0.01.)
Preexposure to Bryo on Days Before Training Enhances Memory Retention. A 4-h exposure to Bryo on the day before training as well as on the day of training, followed by two TEs, prolonged memory retention to >24 h after training. Two successive days of 4-h Bryo exposures followed on the subsequent day by two TEs alone produced long-term memory lasting at least 6 days (Fig. 3). A third day of exposure to the 4-h Bryo treatment caused a similar enhanced retention of the Pavlovian-conditioned response (Fig. 4). A single more prolonged Bryo exposure, i.e., for 8-20 h, followed by two TEs was not sufficient itself to produce memory retention equivalent to that accompanied the two 4-h exposures on successive preceding days. Sufficiently prolonged Bryo exposure (e.g., 8-12 h) is known in other cell systems to cause prolonged PKC down-regulation. Similarly, sufficiently increased concentrations of Bryo ultimately blocked memory retention (Fig. 5) presumably also because of PKC down-regulation.
Fig. 3.
Effect of two successive days of 4-h Bryo exposure (0.25 ng/ml) followed 1 day later by two TEs. Retention was measured up to 144 h after training. (n = 16; ANOVA, P < 0.01.)
Fig. 5.
Dose-response curves for four and nine paired conditioned stimulus/unconditioned stimulus TEs. Bryo concentrations <0.50 ng/ml augmented acquisition and memory retention with suboptimal (four TE) training conditions. Those concentrations had no demonstrable effects on retention performance with nine paired TEs. However, with all training conditions tested, Bryo ≥1.0 ng/ml inhibited acquisition and behavioral retention (n = 16). Retention of four paired TE conditioning with 20-h preexposure to Bryo persisted for 24 h (n = 8; ANOVA at 48 h, P < 0.01).
Preexposure to Bryo Obviates the Requirement for Protein Synthesis During Training. A single 4-h exposure to Bryo together with two TEs produced long-term memory retention lasting several hours that was entirely eliminated when anisomycin was present along with the Bryo (Fig. 2B). Similar blocking effects of anisomycin on long-term memory were also observed with six TEs plus Bryo or nine TEs alone (27). However, when we blocked protein synthesis for 4 h with anisomycin immediately after two TEs for animals that on each of three preceding days had been first exposed to 4 h of Bryo, anisomycin did not prevent memory retention that lasted at least 4 days (Fig. 4). Finally, if two TEs were given 1 day after three successive days of 4-h exposures to Bryo that was accompanied each time by anisomycin, long-term memory was eliminated.
Preexposure to Proteasome Inhibition Enhances Bryo Effects on Memory. The ubiquitin-proteasome pathway (34, 35) is known to be a major route for degradation of the α-isozyme of PKC. Therefore, to prolong PKC activation, we added lactacystin (10 μM), a known proteasome inhibitor (34, 35), during a single 4-h exposure to Bryo followed 24 h later by two TEs. Lactacystin, in this case, transformed the short-term memory produced by the single Bryo exposure (followed by two TEs) to long-term memory lasting 4 days (Fig. 6).
Fig. 6.
Behavioral effects of Bryo and lactacystin. Animals were incubated simultaneously for 4 h with Bryo (0.25 ng/ml) with and without lactacystin (10 μM), and then 24 h later were conditioned with two paired conditioned stimulus/unconditioned stimulus TEs. Animals were subsequently tested with the conditioned stimulus alone at 4 h after training and then at 24-h intervals (n = 28, combined Bryo/lactacystin; n = 20, Bryo alone; n = 16, lactacystin alone).
Immunohistochemistry: CE Immunostaining After PKC Activation. CE, a low-molecular-weight calcium and GTP-binding protein and a high-affinity substrate for the α-PKC isozyme, is phosphorylated during Hermissenda conditioning (19) and increases within single identified type B cells (20). The intensity of CE immunostaining (Figs. 7 and 8A) increased within the type B cells with the number of TEs, reached a plateau (four to five times baseline) of 60-70 units (see Materials and Methods) with nine TEs (sufficient to induce memory retention lasting several days), and was prevented by the specific PKC blocker Ro-32-0432. This conditioning-induced CE label increase represents an increase in the actual amount of the protein because the immunostaining antibody reacts with both the phosphorylated and unphosphorylated forms of the protein. Twenty-four hours after two Bryo exposures on each of two successive days, there was greater residual CE immunostaining, and even greater CE levels after three successive days of Bryo exposure. With two TEs on the day after these three exposures, CE immunostaining 24 h later approached the levels previously observed immediately after nine TEs (Fig. 8B). Finally, this CE immunostaining was not reduced when anisomycin followed two TEs preceded by 3 days of 4-h Bryo exposures (n = 8 animals with and 8 animals without anisomycin, t test, P > 0.5, not statistically significant).
Fig. 7.
Representative tissue sections from Hermissenda eyes immunolabeled with CE antibody. (A) Random presentations of the two stimuli (TEs) did not produce behavioral modifications or a rise in CE above normal background levels. Basement membrane and lens staining are artifacts associated with using vertebrate polyclonal antibodies. (B) Positive CE immunostaining occurred in B cell photoreceptors (*B-Cell) of trained animals (two TEs) after 2 days' Bryo exposure intervals. (C) Intensity measures after nine random TEs or two exposures on successive days to Bryo (0.25 ng/ml), and then associatively followed by two paired TEs. Two exposures of Bryo coupled with two TEs significantly increased CE to levels associated with nine paired TEs and consolidated (long-term) memory (n = 4-8; t test, P < 0.01). CE immunostaining also resolved boutons within synaptic fields of photic-vestibular neurites (D). Arrows indicate arborization field between an interneuron (a), axon from a contralateral neuron (b), and terminal boutons of neurites from a putative photoreceptor (c). (Scale bars, 10 μm.) CPG, cerebropleural ganglion.
Fig. 8.
Effects of Bryo and training on CE immunostaining. (A) Immunointensity measurements (0-255) of CE antibody labeling. CE levels (0-255 relative units) increased >4.3 times with paired training (mean ± SE, n = 5 animals per treatment). 4RanTE, random control, four trials with random light and rotation; 6PTE, paired trials, six trials with paired light and rotation. 6PTE-0Bry vs. 6PTE-0.25Bry: P < 0.001; 4RTE-0.25Bry vs. 6PTE-0.25Bry: P < 0.001 (t test). (B) Effect of Bryo alone (without associative conditioning) administered for 4-h over each of 1, 2, and 3 days with and without two TEs on CE levels in the B photoreceptors of Hermissenda 24 h after each of the periods of Bryo exposures. (n = 16; ANOVA, P < 0.01.)
Biochemistry. Bryo is known to transiently activate PKC by increasing PKC association with the cellular membrane fraction. In addition, Bryo exposures (4 h each) on two successive days were followed 24 h later by increased PKC activity in both the membrane and cytosol fractions (Fig. 9). Consistent with an increased synthesis of PKC as well as PKC activation, the presence of anisomycin during each of three successive days of Bryo completely eliminated PKC increases (n = 9, P < 0.01) as well as the subsequent memory consolidation produced by two TEs.
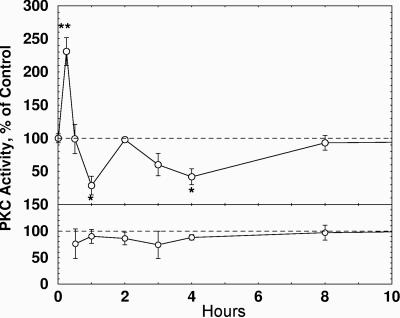
Fig. 9.
PKC activity in Hermissenda nervous systems after Bryo. Intact Hermissenda were exposed for 4-h intervals to Bryo (0.28 nM) on successive days as described in the text. PKC activity in isolated circumesophageal nervous systems was then measured in the cytosol fraction. (Left) Cytosol. (Right) Particulate fraction. (n = 6-12 for each measurement; *, P < 0.05; **, P < 0.01, two-tailed t test).
To further examine biochemical consequences of repeated exposures to Bryo, we used rat hippocampal neurons that had been immortalized by retroviral transduction of temperature-sensitive tsA58 SV40 large T antigen (31). These differentiate to a neuronal phenotype when induced by basic fibroblast growth factor in N2 medium (33) and express a normal complement of neuronal proteins, including PKC. Exposure of cultured hippocampal neurons to a single activating dose of Bryo (0.28 nM) for 30 min produced a brief translocation of PKC from the cytosol to the particulate fraction (evidenced by a 60% increase in activity in the membrane fraction) followed by a prolonged down-regulation (Fig. 10). Exposing the cultured hippocampal neurons to one 30-min period of Bryo, followed by a second 30-min exposure, at intervals ranging from 30 min to 8 h, caused the membrane-bound PKC to rebound more quickly. Thus, a second exposure after a 2- to 4-h delay eliminated the significant down-regulation that a single Bryo exposure produced (Fig. 10). In the cytoplasmic fraction, no significant alteration of PKC activity was detected within the first 4 h after Bryo exposure. In contrast, if cells were exposed to Bryo twice within a 2-h period, there was a significant reduction of PKC activity in response to the second exposure. However, if the second exposure was delayed until 4 h after the first, activity was increased above baseline to a degree that was significantly greater compared with a second exposure delivered after 2 h or less (see Fig. 10 legend). These results are consistent with the interpretation that the initial Bryo activation of PKC followed by down-regulation (34-36) leads to increased synthesis (via de novo protein synthesis) of PKC isozymes (as well as CE, described earlier). In fact, we found here that a single 30-min exposure to 0.28 nM Bryo increased overall protein synthesis (Fig. 11), measured by incorporation of [35S]methionine in the last half-hour before collecting the neurons, by 20% within 24 h, increasing to 60% by 79 h after the Bryo exposure, but increasing significantly less in the presence of the PKC inhibitor Ro-32-0432. (Fig. 11).
Fig. 10.
Effect of Bryo on membrane-bound PKC activity in hippocampal cultured IGF-IR cells after a single 30-min exposure (Upper) or two 30-min exposures separated by intervals of 30 min to 8 h (Lower) (n = 3-6 for each measurement; *, P < 0.05; **, P < 0.01, two-tailed t test). Two 30-min exposures separated by intervals of 2 or 3 h (but not 4 h) caused PKC down-regulation in the cytosol (n = 3-6 for each group; P < 0.05 and P < 0.01, respectively, two-tailed t test).
Fig. 11.
Effect of Bryo on protein synthesis. (Upper) Rat IGF-IR cells were incubated for 30 min with 0.28 nM Bryo for incubation times ranging from 1 to 79 h. [35S]Methionine (9.1 μCi) was then added to the medium followed by analysis of radiolabel as described in Materials and Methods. (Lower) Rat IGF-IR cells were incubated as above in the presence or absence of 100 nM Ro-32-0432. (n = 6 for each measurement; *, P < 0.05; **, P < 0.01, two-tailed t test.)
Discussion
The nontumorigenic PKC activator Bryo has undergone extensive testing in humans for the treatment of cancer in doses (20 μg/m2 to 120 μg/m2 per week) known to cause initial PKC activation followed by prolonged down-regulation (34-36). Phorbol esters and Bryo compete with diacylglycerol at the C1 domain of PKC. Other diacylglycerol-binding proteins containing closely related C1 domains, such as RasGRP (37, 38), and other C1 domain-containing proteins that bind phorbol esters such as chimaerins and munc13 (39, 40) are also potential targets for Bryo. Bryo has also recently been shown to activate the α-secretase (secondary to PKC activation) that cleaves the amyloid precursor protein (APP) to generate the nontoxic fragment soluble precursor protein sAPP from human fibroblasts (41). Bryo also enhances learning and memory retention of the rat spatial maze task (21), learning of the rabbit nictitating membrane paradigm (B. Schreurs and D.L.A., unpublished data), and, in a preliminary report, Hermissenda conditioning.§ Here, Bryo in low doses (0.1-0.25 ng/ml) markedly enhanced memory after two, four, or six TEs. Pavlovian conditioning with two TEs was prolonged from 7 min to several hours by Bryo, and with six TEs from hours to many days with Bryo. This memory enhancement was blocked by anisomycin or the PKC inhibitor Ro-32-0432. Bryo also increased the synthesis of CE (19, 42) within type B cells as occurs with Pavlovian conditioning of Hermissenda, both increases blocked by Ro-32-0432. Thus, Bryo, in lower doses (0.1-0.25 ng/ml), and with limited intervals of exposure, initially enhances PKC activation followed at first by down-regulation and then prolonged (days) enhancement of protein synthesis. Furthermore, Bryo-induced enhancement of protein synthesis apparently enhances the duration of the memory of Pavlovian-conditioned responses. Consistent with these findings, Bryo-induced PKC activation on days before training is sufficient, with minimal training trials, to cause long-term memory. This latter long-term memory does not require protein synthesis after the training (after PKC activation on preceding days). One of those proteins whose synthesis is induced by Bryo-induced PKC activation as well as conditioning trials is CE, as demonstrated by the immunostaining labeling. It is also possible that synthesis of PKC itself might also be increased (see Fig. 9). PKC, after activation induced by Bryo, has been shown to be down-regulated by two distinct pathways, one proteasome-mediated and the other mediated by the phosphatases PP1 and PP2A (31). With sufficient concentrations and/or durations of PKC activators, the PKC-degrading pathways create a deficit of PKC that is offset by de novo synthesis of PKC. With sufficient activation, however, PKC synthesis cannot compensate for in-activation and down-regulation, thereby causing depletion of available PKC of 95% or more, thereby explaining inhibition of Hermissenda learning with higher Bryo concentrations.
It is possible, therefore, that one primary requirement of protein synthesis after associative learning in many species may be to manufacture de novo PKC to replenish down-regulated PKC. Persistence of PKC as well as other proteins synthesized during this prior period of Bryo exposure would then eliminate the requirement for protein synthesis after learning to produce long-term memory. Apparently, PKC activation itself, independent of training, induces the synthesis of those proteins (e.g., calexcitin) that are necessary and sufficient to produce long-term memory after training. Those proteins whose synthesis is stimulated by PKC activation may be, therefore, uniquely critical for long-term memory storage.
Author contributions: D.L.A. designed research; D.L.A. analyzed data; D.L.A. wrote the paper; and H.E., A.K., M.C.B., and T.J.N. performed research.
Conflict of interest statement: No conflicts declared.
Abbreviations: ASW, artificial sea water; TE, training event; Bryo, bryostatin; CE, calexcitin.
Footnotes
Scioletti, A. B., Kuzirian, A. M., Epstein, H. T., Nelson, T. J. & Alkon, D. L. (2004) Biol. Bull. 207, 159 (abstr.).
References
- 1.Agranoff, B. W., Davis, R. E., Casola, L. & Lim, R. (1967) Science 158, 1600-1601. [DOI] [PubMed] [Google Scholar]
- 2.Bergold, P. J., Sweatt, J. D., Winicov, I., Weiss, K. R., Kandel, E. R. & Schwartz, J. H. (1990) Proc. Natl. Acad. Sci. USA 87, 3788-3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavallaro, S., D'Agata, V., Pachiappan, M., Dufour, F. & Alkon, D. L. (2002) Proc. Natl. Acad. Sci. USA 99, 16279-16284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crow, T. & Forrester, J. (1990) Proc. Natl. Acad. Sci. USA 87, 4490-4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crow, T., Xue-Bian, J. J. & Siddiqi, V. (1999) J. Neurophysiol. 82, 495-500. [DOI] [PubMed] [Google Scholar]
- 6.Epstein, H. T., Child, F. M., Kuzirian, A. M. & Alkon, D. L. (2003) Neurobiol. Learn. Mem. 79, 127-131. [DOI] [PubMed] [Google Scholar]
- 7.Ezzeddine, Y. & Glanzman, D. L. (2003) J. Neurosci. 23, 9585-9594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farley, J. & Schuman, E. (1991) Proc. Natl. Acad. Sci. USA 88, 2016-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flexner, L. B. & Flexner, J. B. (1966) Proc. Natl. Acad. Sci. USA 55, 369-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hyden, H. & Lange, P. W. (1970) Proc. Natl. Acad. Sci. USA 65, 898-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson, T. J. & Alkon, D. L. (1990) Proc. Natl. Acad. Sci. USA 87, 269-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quattrone, A., Pascale, A., Nogues, X., Zhao, W., Gusev, P., Pacini, A. & Alkon, D. L. (2001) Proc. Natl. Acad. Sci. USA 98, 11668-11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao, W., Chen, H., Xu, H., Moore, E., Meiri, N., Quon, M. J. & Alkon, D. L. (1999) J. Biol. Chem. 274, 34893-34902. [DOI] [PubMed] [Google Scholar]
- 14.Zhao, W., Meiri, N., Xu, H., Cavallaro, S., Quattrone, A., Zhang, L. & Alkon, D. L. (2000) FASEB J. 14, 290-300. [DOI] [PubMed] [Google Scholar]
- 15.McPhie, D. L., Matzel, L. D., Olds, J. L., Lester, D. S., Kuzirian, A. M. & Alkon, D. L. (1993) J. Neurochem. 60, 646-651. [DOI] [PubMed] [Google Scholar]
- 16.Bank, B., DeWeer, A., Kuzirian, A. M., Rasmussen, H. & Alkon, D. L. (1988) Proc. Natl. Acad. Sci. USA 85, 1988-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olds, J. L., Anderson, M. L., McPhie, D. L., Staten, L. D. & Alkon, D. L. (1989) Science 245, 866-869. [DOI] [PubMed] [Google Scholar]
- 18.Olds, J. L., Golski, S., McPhie, D. L., Olton, D., Mishkin, M. & Alkon, D. L. (1990) J. Neurosci. 10, 3707-3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson, T. J., Collin, C. & Alkon, D. L. (1990) Science 247, 1479-1483. [DOI] [PubMed] [Google Scholar]
- 20.Kuzirian, A. M., Epstein, H. T., Buck, D., Child, F. M., Nelson, T. & Alkon, D. L. (2001) J. Neurocytol. 30, 993-1008. [DOI] [PubMed] [Google Scholar]
- 21.Sun, M. K. & Alkon, D. L. (2005) Eur. J. Pharmacol. 512, 45-51. [DOI] [PubMed] [Google Scholar]
- 22.Marshall, J. L., Bangalore, N., El-Ashry, D., Fuxman, Y., Johnson, M., Norris, B., Oberst, M., Ness, E., Wojtowicz-Praga, S., Bhargava, P., et al. (2002) Cancer Biol. Ther. 1, 409-416. [DOI] [PubMed] [Google Scholar]
- 23.Talk, A. C., Muzzio, I. A. & Matzel, L. D. (1999) Neurobiol. Learn. Mem. 72, 95-117. [DOI] [PubMed] [Google Scholar]
- 24.Wender, P. A., Cribbs, C. M., Koehler, K. F., Sharkey, N. A., Herald, C. L., Kamano, Y., Pettit, G. R. & Blumberg, P. M. (1988) Proc. Natl. Acad. Sci. USA 85, 7197-7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wender, P. A., Lippa, B., Park, C. M., Irie, K., Nakahara, A. & Ohigashi, H. (1999) Bioorg. Med. Chem. Lett. 9, 1687-1690. [DOI] [PubMed] [Google Scholar]
- 26.Bogi, K., Lorenzo, P. S., Szallasi, Z., Acs, P., Wagner, G. S. & Blumberg, P. M. (1998) Cancer Res. 58, 1423-1428. [PubMed] [Google Scholar]
- 27.Baryza, J. L., Brenner, S. E., Craske, M. L., Meyer, T. & Wender, P. A. (2004) Chem Biol. 11, 1261-1267. [DOI] [PubMed] [Google Scholar]
- 28.Lederhendler, I. I., Gart, S. & Alkon, D. L. (1986) J. Neurosci. 6, 1325-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Epstein, H. T., Kuzirian, A. M., Child, F. M. & Alkon, D. L. (2004) Neurobiol. Learn. Mem. 81, 12-18. [DOI] [PubMed] [Google Scholar]
- 30.Cheung, A. P., Hallock, Y. F., Vishnuvajjala, B. R., Nguyenle, T. & Wang, E. (1998) Invest. New Drugs 16, 227-236. [DOI] [PubMed] [Google Scholar]
- 31.Morrione, A., Romano, G., Navarro, M., Reiss, K., Valentinis, B., Dews, M., Eves, E., Rosner, M. R. & Baserga, R. (2000) Cancer Res. 60, 2263-2272. [PubMed] [Google Scholar]
- 32.Johannes F. J., Prestle J., Eis, S., Oberhagemann, P. & Pfizenmaier, K. (1994) J. Biol. Chem. 269, 6140-6148. [PubMed] [Google Scholar]
- 33.Eves, E. M., Tucker, M. S., Roback, J. D., Downen, M., Rosner, M. R. & Wainer, B. H. (1992) Proc. Natl. Acad. Sci. USA 89, 4374-4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prevostel, C., Alice, V., Joubert, D. & Parker, P. J. (2000) J. Cell Sci. 113, 2575-2584. [DOI] [PubMed] [Google Scholar]
- 35.Leontieva, O. V. & Black, J. D. (2004) J. Biol. Chem. 279, 5788-5801. [DOI] [PubMed] [Google Scholar]
- 36.Lu, Z., Liu, D., Hornia, A., Devonish, W., Pagano, M. & Foster, D. A. (1998) Mol. Biol. Cell 18, 839-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lorenzo, P. S., Beheshti, M., Pettit, G. R., Stone, J. C. & Blumberg, P. M. (2000) Mol. Pharmacol. 57, 840-846. [PubMed] [Google Scholar]
- 38.Ebinu, J. O., Stang, S. L., Teixeira, C., Bottorff, D. A., Hooton, J., Blumberg, P. M., Barry, M., Bleakley, R. C., Ostergaard, H. L. & Stone, J. C. (2003) Blood 95, 3199-3203. [PubMed] [Google Scholar]
- 39.Geiger, M., Wrulich, O. A., Jenny, M., Schwaiger, W., Grunicke, H. H. & Uberall, F. (2003) Curr. Opin. Mol. Ther. 5, 631-641. [PubMed] [Google Scholar]
- 40.Kazanietz, M. G. (2002) Mol. Pharmacol. 61, 759-767. [DOI] [PubMed] [Google Scholar]
- 41.Etcheberrigaray, R., Tan, M., Dewachter, I., Kuiperi, C., Van der Auwera, I., Wera, S., Qiao, L., Bank, B., Nelson, T. J., Kozikowski, A. P., et al. (2004) Proc. Natl. Acad. Sci. USA 101, 11141-11146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nelson, T. J., Quattrone, A., Kim, J., Pacini, A., Cesati, V. & Alkon, D. L. (2003) Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 135, 627-638. [DOI] [PubMed] [Google Scholar]