Abstract
In eukaryotic ribosome, the N domain of polypeptide release factor eRF1 is involved in decoding stop signals in mRNAs. However, structure of the decoding site remains obscure. Here, we specifically altered the stop codon recognition pattern of human eRF1 by point mutagenesis of the invariant Glu55 and Tyr125 residues in the N domain. The 3D structure of generated eRF1 mutants was not destabilized as demonstrated by calorimetric measurements and calculated free energy perturbations. In mutants, the UAG response was most profoundly and selectively affected. Surprisingly, Glu55Arg mutant completely retained its release activity. Substitution of the aromatic ring in position 125 reduced response toward all stop codons. This result demonstrates the critical importance of Tyr125 for maintenance of the intact structure of the eRF1 decoding site. The results also suggest that Tyr125 is implicated in recognition of the 3d stop codon position and probably forms an H-bond with Glu55. The data point to a pivotal role played by the YxCxxxF motif (positions 125–131) in purine discrimination of the stop codons. We speculate that eRF1 decoding site is formed by a 3D network of amino acids side chains.
INTRODUCTION
High fidelity of termination of protein synthesis ensures the formation of normal-sized polypeptide chains as encoded in the genome. Translational termination is known to be mediated by class-1 polypeptide release factors (RF1 and RF2 in prokaryotes, eRF1 and aRF1 in eukaryotes and Archaea, respectively) [reviewed in (1–5)]. When a nucleotide triplet (referred to as termination, or stop, or nonsense codon) in mRNA occupies the ribosomal A site, it is decoded by RFs at the small ribosomal subunit.
For prokaryotes, it has been suggested that the second and third nucleotides of the stop codons are decoded directly by a linear ‘protein anticodons’ (PAT in RF1 and SPF in RF2) (6–8).
For eukaryotes, it is now established that the specificity of stop codon recognition is associated with the eRF1 rather than the ribosome (9) and the recognition site is located in the N domain of eRF1 as is evident from genetic in vivo data (10), biochemical studies in vitro (11,12) and ‘molecular chimera’ approach (13). Data on multiple alignments of eRF1 amino acid sequences with different stop codon specificities are also consistent with this conclusion (14). The computer-assisted hypothesis suggesting the existence of so-called ‘terminator’ tRNAs inside the large ribosomal RNA (15) is inconsistent with the experimental data (9,13,16). Cross-linking experiments showing the close proximity of the A-site-located stop codon and eRF1 (17,18) and in particular cross-linking with a NIKS tetrapeptide of the N domain (19) are also consistent with the above mentioned conclusion.
There are significant differences between the data obtained by various approaches regarding the nature and position of amino acid residues of eRF1 implicated in decoding of the stop codons within the ribosome (10–14). This diversity of opinions is probably not associated with the origin of eRF1 since the eRF1 protein family is known to be highly conserved (20–22) and, for instance, the data obtained with yeast and human eRF1 should be fully compatible. Rather, the apparent controversies may be derived by a methodological distinction between in vivo and in vitro strategies or between genetic versus biochemical approaches.
Here, we introduced various amino acid substitutions into four positions in the N domain of human eRF1. Two of them, 55 and 125, are invariant in all class 1 eRF1s and aRFs and occupied by Glu and Tyr, respectively. Two other positions, 51 and 126, are variable in eRF1s from different species. All these positions were mentioned earlier as potentially essential for stop codon recognition based mostly on indirect evidence (10,14,22,23). The generated mutants were examined in vitro with respect to alterations of their functional and physical properties. Ability of mutated forms of eRF1 to trigger hydrolysis of fMet–tRNA (mimicking peptidyl–tRNA) in the ribosome was tested. Mutants were also studied by differential scanning calorimetry to evaluate stability of the protein globule after mutagenesis.
MATERIALS AND METHODS
Cloning and mutagenesis of human eRF1
The full-length cDNA encoding eRF1 with C-terminal His6-tag fusion was cloned into pET23b(+) vector (Novagen) under the control of phage T7 RNA polymerase promoter as described previously (11,24).
The mutagenesis procedure was simplified by introducing into human eRF1 cDNA a unique Bst98I site affecting neither amino acid sequence nor the reading frame of human eRF1. GeneEditor in vitro site-directed mutagenesis kit (Promega) was used with the RFBst primer 5′-CCATTCTTAAGCGGGCAAAACGCAAGG-3′ (the Bst98I site underlined). The resulting construct pERF4B containing the unique Bst98I site within the gene encoding human eRF1 at positions 576–581 (T576C substitution) from the start ATG codon was used for mutagenesis of human eRF1.
The mutagenesis procedure was performed according to the PCR-based ‘megaprimer’ method (25). Two rounds of PCR were performed with the same PCR mixture. The PCR primers used for the generation of eRF1 mutants are available on request (kissel@eimb.relarn.ru). For the M51 and E55 mutants the direct primer (RFNde), 5′-GAGATATACATATGGCGGACGACCC-3′ (the NdeI site underlined) together with one of the reverse primers for these mutants were used in the first round of PCR. In the second round the ‘megaprimer’ synthesized in the first round served as the direct primer together with the reverse primer (RFBst) 5′-CCATTCTTAAGCGGGCAAAACGCAAGG-3′ (the Bst98I site underlined). For the Y125 and L126 mutants the direct RFBst primer together with one of the reverse primers for these mutants was used in the first round. In the second round the ‘megaprimer’ synthesized in the first round served as the direct primer together with the reverse RFNde primer. The resulting 590 bp PCR product was purified in low-melting NuSieve GTG agarose (FMC Bioproducts) using DNA extraction kit (Bio-Rad), hydrolyzed with NdeI and Bst98I, and ligated with pERF4B plasmid, treated with the same endonucleases. The ligated mixture was used for the transformation of Escherichia coli, strain Z85. The cloned DNAs were sequenced and appropriate clones were used for the expression of the mutant eRF1s. The first rounds of DNA amplifications were carried out in 25 µl reaction mixtures containing 20 ng of pERF4B DNA, 0.48 µM direct primer and 0.6 µM reverse primer, 0.2 mM each of four deoxyribonucleoside triphosphates, 1× of Pfu DNA polymerase reaction buffer and 0.75 U of PfuTurbo DNA polymerase (Stratagene). The denaturation was carried out at 95°C (3 min) for the first cycle, and at 94°C (30 s) for the next 20–25 cycles. The amplification included 30 s of the primer annealing (60°C) and 40 s for elongation (72°C). The second round of PCR was performed after addition to the PCR mixture of 12 pmol of the reverse primer (RFBst/RFNde) and 1.25 U of PfuTurbo DNA polymerase. Amplification with ‘megaprimer’ was performed for 28 cycles at 94°C for 30 s, the appropriate annealing temperature Tm for the reverse primer for 40 s and at 72°C for 140 s for elongation.
Expression and purification of human eRF1 and eRF3Cp
The wild-type human eRF1 and its mutants and the human eRF3Cp containing His6-tags at the C-termini were produced in E.coli, strain BL21(DE3), and purified using Ni-NTA Superflow (Qiagen), as described previously (26,27). The N-terminal amino acids (1–138) were absent in eRF3Cp without any influence on its GTPase activity.
Ribosomes
Rabbit reticulocyte 80S ribosomes washed with 0.5 M KCl were treated with puromycin and GTP for dissociation into subunits which were subsequently resolved by centrifugation in a 10–25% (w/v) sucrose gradient containing 0.3 M KCl, 3 mM MgCl2, 1 mM DTT and 20 mM Tris–HCl, pH 7.6. Before addition to the incubation mixtures, the subunits were combined in an equimolar ratio.
In vitro RF activity assay
The eRF1 activity was measured as described previously (20,28) at saturating levels (50 µM) of one out of the three stop-codon-containing tetraplets. The incubation mixture (25 µl) contained 20 mM Tris–HCl, pH 7.5, 15 mM MgCl2, 8 mM NH4Cl, 1.5 pmol f[35S]Met––AUG–ribosome complex and 4 pmol of eRF1. The background was measured without tetraplet and subtracted from all values. AUG and ribotetraplets were synthesized by A. Veniaminova and M. Ryabkova (Institute of Chemical Biology and Fundamental Medicine, Novosibirsk).
GTPase activity assay
GTPase activity was followed by accumulation of [32P]Pi using a modified charcoal precipitation assay as described previously (29). Incubation mixture (50 µl) contained 20 mM Tris–HCl, pH 7.5, 30 mM NH4Cl, 15 mM MgCl2, 0.16 µM ribosomes, 0.16 µM human Cp and 10 µM [γ-32P]GTP (800–2000 c.p.m./pmol); human eRF1 was added in 0.04, 0.08 and 0.16 µM (final concentrations). Reaction was run at 30°C; 12.5 µl aliquots were withdrawn every 4 min and mixed with 0.5 ml of 5% activated charcoal suspension in NaH2PO4, cooled on ice. The mixture was vortexed and centrifuged at 9 300 g for 15 min at 4°C. Aliquot of supernatant (375 µl) was counted on scintillation counter.
Differential scanning calorimetry
Measurements were performed using differential adiabatic scanning microcalorimeter SCAL-1 (Scal Company, Pustchino, Russia) in 0.32 ml glass cells. The rate of heating was 1 K/min, in some cases 0.125, 0.5 and 2.0 K/min. Protein concentration ranged from 0.3 to 2.0 mg/ml. Partial molar heat capacity, Cp of proteins was determined as described previously (30). Partial molar protein volume was calculated from its amino acid composition as recommended (31). The precision of calorimetric enthalpy determination was 7 ± 1%; experimental error of Td value did not exceed ±0.2°C. For analysis of melting curves the MinScal programme (SCAL Company) was used. Other details could be found elsewhere (32).
Calculation of free energy changes in human eRF1 mutants
We performed the analysis of free energy perturbation (FEP) caused by point mutations in human eRF1 as described previously (33,34). Briefly, we implemented our analysis basing on NAMD (http://www.ks.uiuc.edu/Research/namd/) FEP module and self-designed script for ICM (www.molsoft.com/). We selected the N domain of wt-eRF1 (positions 5–146) from the eRF1 crystal structure (1dt9) for introducing point mutations. Free energy calculations were performed as described previously (35,36). The N129A mutant was modeled by 3D-JIGSAW programme (www.bmm.icnet.uk/servers/3djigsaw/) and used as the model for introduction of double mutation N129A + F131A.
RESULTS
Selection of amino acids positions for mutagenesis
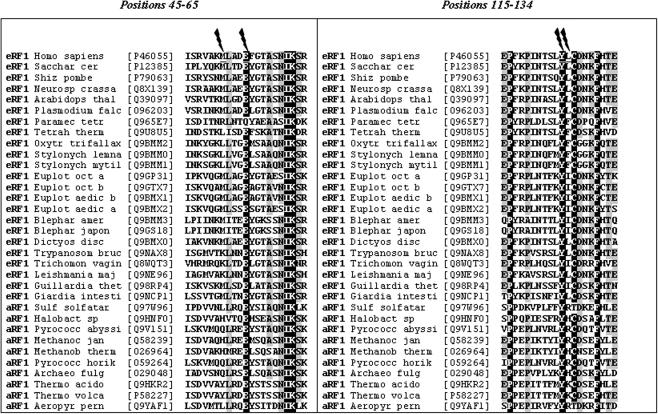
Multiple alignments of amino acid sequences of eRF1 and aRF1 are shown in Figure 1. The reasons to combine RFs from different kingdoms of living matter relay on the close structural homology between eRF1 and aRF1 (21). Evolutionarily distant eRF1s are selected including organisms with variant genetic codes, e.g. Euplotes, Tetrahymena, etc. (22).
Figure 1.
Alignment of the N domain fragments from eRF1 and aRF1 primary structures. Residue numbering is based on human wild-type eRF1. The shortened Latin names are given. Accession numbers are in brackets. Hundred percent identical positions are black-shadowed; 80% identical positions are gray-shadowed. Similarity scoring is disabled.
In recently suggested models for decoding site of eRF1 several residues are mentioned (10,14,22,23), including positions 51, 55, 125 and 126 (Figure 1). Some of these positions are located on the surface of the eRF1 molecule as is evident from crystal structure (37) and for these reasons amino acid side chains in these positions are exposed to interaction with ligands, e.g. mRNA. Moreover, positions 125 and 126 are proximal to Cys127 which is known to be essential for decoding function of eRF1 (12). Positions 55 and 125 are proximal in space in eRF1 crystals (37). Collectively, these data point to the necessity to analyze possible involvement of positions mentioned above in decoding function of eRF1.
Positions 51 and 55
Position 51 is occupied in human eRF1 by Met residue which is not conserved and varies considerably in both eRF1 and aRF1 families (Figure 1). The RF activities of the M51L, M51Y, M51D, M51R and M51E mutants have been tested in an in vitro assay. All these eRF1 mutants retain full activity with any of the three stop codons indistinguishable from that of the wild-type eRF1 (data not shown). Since the substituting amino acids are very different from the initial Met residue and at the same time distinct among themselves, it implies that the eRF1 function is tolerant to the nature of amino acid in position 51.
In a sharp contrast with Met51, position 55 is occupied exclusively by Glu (Figure 1). This may point to an essential structural and/or functional role of this residue in eRF1/aRF1 protein superfamily. In fact, in E55A, E55S and E55Y mutants the UAG response was entirely abolished, whereas only about 2- to 3-fold reduction of the RF activity took place with UGA (Table 1). The UAG-selective inactivation was not due to inability of the mutant proteins to bind to the ribosome as the GTPase activity of eRF3 which was a measure of eRF1 codon-independent binding to the ribosome (12,26,29) was not affected at all (data not shown). Relatively high remaining RF activity (40–80%) toward UGA (Table 1) also spoke against loss of binding capacity as a cause of UAG-specific loss of function. Three E55 mutants seem to be the very first cases when a complete inactivation toward one stop codon response was achieved though the mutant factor remained partially active toward two other stop codons. The E55R mutant will be discussed below.
Table 1.
In vitro release activity of human Glu55 and Tyr125 eRF1 mutants
| Mutant eRF1 | Release activity (%) | ||
|---|---|---|---|
| UAAA | UAGA | UGAA | |
| E55A | 59 | 0 | 48 |
| E55D | 72 | 21 | 78 |
| E55Q | 75 | 35 | 80 |
| E55R | 98 | 97 | 104 |
| E55Y | 11 | 2 | 56 |
| E55S | 27 | 1 | 36 |
| Y125A | 5 | 6 | 8 |
| Y125E | 12 | 14 | 19 |
| Y125F | 100 | 34 | 100 |
| Y125S | 17 | 2 | 54 |
The release activity of mutant eRF1s was measured according to the in vitro Caskey's assay as described previously (20,28). Data are given in % versus the wt-eRF1. Average values from three independent experiments run in duplicates are presented. One-letter amino acid code is used. An error in RF activity measurements varied from 8.5 to 10% for different mutants. Background values (without any stop codon) were subtracted everywhere.
Positions 125 and 126
We have shown earlier that two invariant amino acids, Cys127 and Phe131 (Figure 1), are critical for the functional integrity of human eRF1 and presumably are implicated in the recognition of the 2d position of the stop codon (12). We have now mutagenized two other amino acid residues from the same region, Tyr125 and Leu126. The first amino acid, as is evident from the alignment, is invariant while the second one, which neighbors Tyr125 from the C side, is variable (Figure 1).
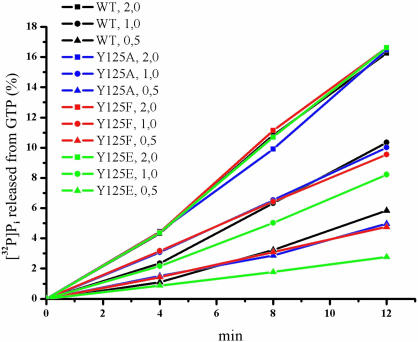
Functional activity of the Tyr125 mutants is given in Table 1. Most remarkably, a single point mutation, Y125A, causes loss of function toward all three stop codons. Introduction of Glu instead of Tyr also causes a profound and unspecific loss of activity. The Tyr125Ser mutant is silent toward UAG like Tyr125Ala mutant but retains half of its initial activity with UGA (Table 1). The most interesting result has been obtained with a mild substitution Tyr → Phe, i.e. one aromatic amino acid is exchanged with another one. Here, the decrease of the RF activity is highly selective: no change in response to UAA and UGA but a 3-fold loss of function toward UAG. Clearly, this selective inactivation is not due to damage of binding to the ribosome: two other activities are not affected at all and the GTPase activity of eRF3 which is entirely dependent on the binding of eRF1 and eRF3 to the ribosome as mentioned above remains as in the presence of the wild-type eRF1 (Figure 2). Most likely, invariant Tyr125 is essential for the UAG stop codon recognition.
Figure 2.
Kinetics of GTP hydrolysis catalyzed by human eRF3 in the presence of the ribosomes and eRF1 mutants. Amount of wt-eRF1 and its mutants are indicated in pmoles. Each tube contained 125 pmol of GTP (800–2000 c.p.m./pmol), 2 pmol of the ribosomes, 2 pmol of eRF3Cp and varying amounts of wt- or mutant eRF1. An average from three independent experiments is shown. The experimental error was ±5%.
The neighboring variable L126 has been found to be non-essential for function since L126E, L126F and L126I mutants possess the same activity toward all three stop codons as the wild-type eRF1 (data not shown).
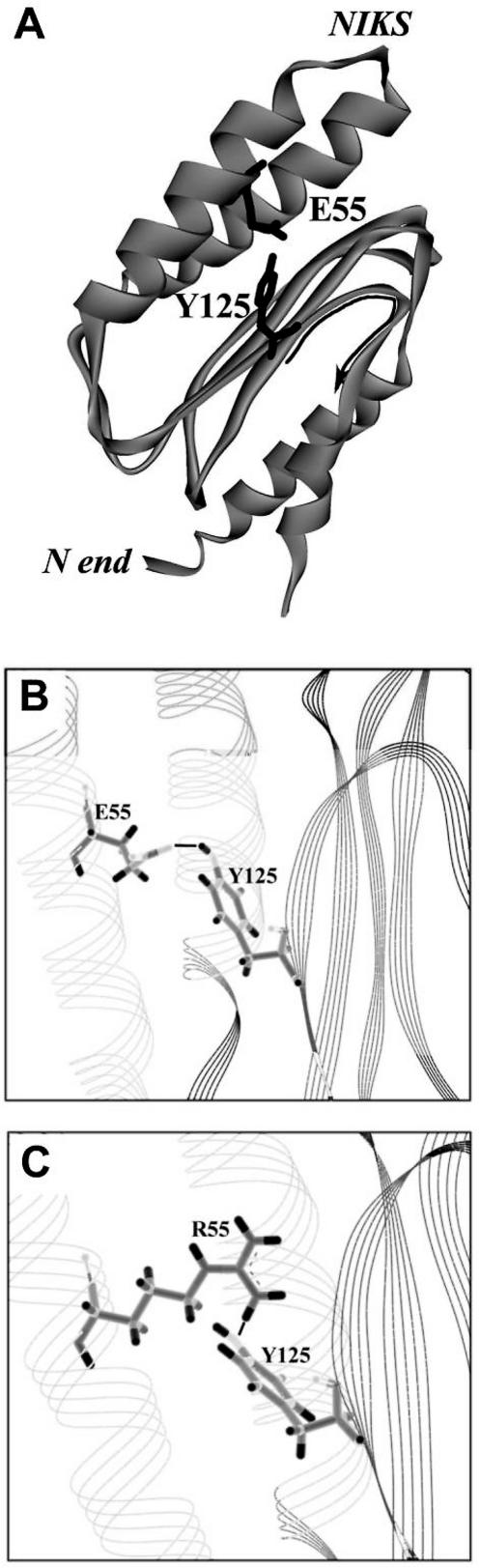
Hydrogen bonding between Glu55 and Tyr125
As follows from the structural analysis (Figure 3A) positions 55 and 125 are close in space and can form a hydrogen bond between the hydrogen atom of OH group of Tyr125 and the oxygen atom of the COO group of Glu55. The length of the bond is equal to 1.73 Å (Figure 3B). This suggestion is consistent with the functional alterations observed with mutant eRF1. When in the E55A mutant the acceptor group disappears, the RF activity is completely lost toward the UAG stop codon (Table 1). It is also five times diminished if Glu is substituted with a shorter amino acid residue (Asp) leading to increase in the distance between the acceptor and donor groups forming the H-bond. The E55Q mutant has also a 3-fold reduced RF activity, possibly because the acceptor COO group is converted into a potential donor CONH2 group. This group theoretically is able to form an H-bond with OH group of Tyr125 causing an increase of the distance between the interacting atoms (not <2 Å). Moreover, due to the neighborhood of the aromatic ring the donor property of the Tyr125 OH group is significantly weakened making an H-bond between the Tyr125 and Gln55 unstable.
Figure 3.
Structure of the N domain of human eRF1 (37). (A) The ribbon model is represented. The YxCxxxF loop is shown by thin arrow. The side-chains of essential residues Glu55 and Tyr125 are shown as sticks. The H-bond is shown as a black line. (B) Human wt-eRF1. (C) The Glu55Arg mutant.
Our suggestion that Glu55 and Tyr125 form an H-bond is consistent also with other observations. All Tyr125 mutants tested so far (Table 1) reduce their RF activity if the donor group of the amino acid side chain is lost. To explain a rather surprising observation that the E55R mutant is completely active (Table 1), we have inserted Arg instead of Glu in the 3D structure of eRF1 (Figure 3C). It turned out from the model that an ‘inversion’ of the H-bond formation takes place. Instead of H-bond acceptor in position 55, Arg becomes a donor of H while oxygen of the Tyr OH group becomes an acceptor. The distance between H of the NH group (Arg55) and O of Tyr125 is equal to 1.82A (Figure 3C) which permits H-bond formation. This is possible because the strong NH group of Arg55 is able to bind even to weak OH acceptor group of Tyr125.
Thermal denaturation of wild-type eRF1 and its mutants
In principle, point mutations of invariant amino acids if they play an essential role in maintenance of 3D structure of the protein may cause changes in protein stability. The destabilization of protein conformation can, in turn, causes a decrease or even abolishment of RF activity.
To examine this possibility, we have measured a thermal stability of eRF1 mutants in comparison with wild-type protein. As shown in Table 2, melting temperatures of the mutants differ insignificantly from the Td of wt-eRF1. The results mean that protein globule after point mutagenesis remains stable enough and the functional alterations (Table 1) are not associated with a destabilization of protein 3D structure. This conclusion is supported by analogous analysis performed with other eRF1 mutants (32).
Table 2.
Melting temperatures of wild-type and mutant forms of human eRF1
The only apparent exception is Y125A mutant with ΔTd = 2.8°C (Table 2). However, most likely, it is not the cause of functional damage because, for example, a double mutant N129P + K130Q exhibits strong destabilization (ΔTd = 7.9°C) (32), whereas its RF activity was only partially diminished toward UAG and was fully preserved versus two other stop codons (12).
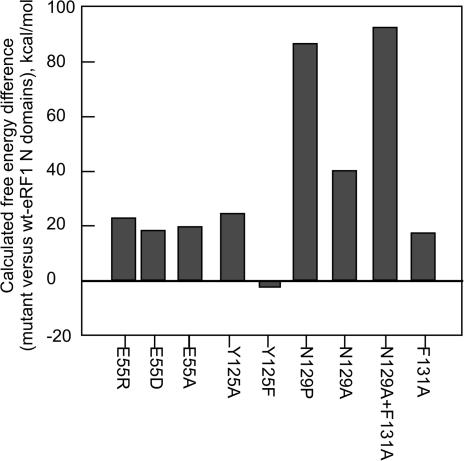
Changes in the calculated free energy of eRF1 mutants
We have calculated the changes in free energy induced by point mutations in eRF1 as described in Materials and Methods. In addition to E55 and Y125 mutants, we have performed similar analysis for some previously described (12) mutants (positions 129 and 131). As seen from Figure 4, changes in free energy of mutants in positions 55 and 125 are very small or negligible and do not exceed 20 kcal/mol. This result means that mutations in these positions do not destabilize the 3D structure of eRF1 in full agreement with calorimetric measurements (Table 2). At the same time the strong alteration of free energy in double N129AF131A mutant (Figure 4) is consistent with known involvement of these residues in intramolecular interactions (37) and with known profound changes of functional activity of this mutant (12). The case of this double mutant demonstrates the sensitivity of the used methods: free energy change calculated for the N129AF131A mutant is 90 kcal/mol (Figure 2), which correlates with ΔTd = −6°C (Table 2, N 10). In contrast, for E55 and Y125 mutants this free energy difference is ∼20 kcal/mol and consistent with very small ΔTd values (Table 2). The Y125F is the only mutation tested so far that slightly stabilizes eRF1 molecule (Figure 4).
Figure 4.
The molecular modeling analysis of free energy changes in eRF1 virtual mutants. The procedure included the whole molecule side-chain minimization (100 calls, Newton method) followed by 1000 calls of both backbone and side-chain energy minimization in 10 Å sphere around the Cα-atom of the target residue. The ICM molecular modeling program was used for minimization procedures.
DISCUSSION
Invariant amino acids, such as Glu55 and Tyr125, may be essential for maintenance of spatial protein structure and/or for decoding function of eRF1. We attempted to follow the consequences of point mutagenesis of Glu55 and Tyr125 using three strategies. First, we measured the thermal stability of eRF1 mutants by differential scanning microcalorimetry and found that the destabilization of protein conformation is in most cases negligible with one exception for Y125A mutant where decrease in melting temperature is meaningful (Table 2). This observation implies that these positions in protein structure are not critical for protein stability as anticipated from surface location of both residues in protein globule (37). Second, we calculated possible changes of free energy of eRF1 mutants in comparison with wild-type protein and found very small alterations (Figure 4). This indicates that the introduced substitutions do not disturb the protein conformation. These data are fully consistent with the calorimetric data discussed above. Third, we measured the ability of eRF1 mutants to induce GTPase activity of eRF3 within the ribosome (Figure 2). We have shown earlier that this assay reflects the ability of mutated eRF1 to bind to the ribosome (29) and is sensitive to some mutations in the N domain of eRF1 (12,24). For all Glu55 and Tyr125 mutants tested so far, their ability to activate eRF3 GTPase within the ribosome was the same as for wt-eRF1. It means that codon-independent binding ability of mutated eRF1 forms toward the ribosome remains undamaged.
Collectively, all three approaches speak against the possibility that functional consequences of eRF1 mutagenesis are associated with protein destabilization or distortion of the 3D structure.
Substitution of the aromatic ring in position 125 makes the eRF1 almost silent toward two or three stop codons (Table 1). This result shows the initial importance of Tyr125 for maintenance of the intact structure of the decoding site.
The UAG response is affected most strongly in the Glu55 and Tyr125 mutants (Table 1). This is the only stop codon which has G in the third position while it has common U with two other stop codons and shares A with UAA. Consequently, Glu55 and Tyr125 are implicated in the recognition of the G base in the UAG stop codon.
We assume that between Glu55 and Tyr125 an H-bond is formed. This interaction may help to preserve close proximity of the two remote regions of the N domain. Parallel UAG-selective loss of RF activity in E55A and Y125F mutants suggests that preservation of the H-bond between these residues is a prerequisite for UAG-dependent RF activity. The role of Glu55 is less essential than Tyr125 because some other amino acids in position 55 are able to partially substitute Glu and, most surprisingly, Arg55 is as active as Glu55 (Table 1). Therefore, we suggest that in the Glu-Tyr pair the major role is played by Tyr, whereas Glu is most likely required for H-bond formation with Tyr125 to fix it in a proper orientation.
Since the functioning eRF1 is located within the ribosome the Glu55-Tyr125 pair is probably surrounded by hydrophobic environment and therefore water molecules will probably not interfere with H-bond formation. Similar behavior of intramolecular H-bonds in proteins has recently been discussed (38,39).
Although we consider E55 as invariant amino acid (Figure 1) it appeared very recently that in ciliate Loxodes striatus eRF1 (Swiss-Prot accession no. Q5CD84) this residue is substituted by Ala (40). L.striatus eRF1 corresponds in this position to one of our mutated forms of human eRF1 which has no RF activity toward UAG (Table 1). Based on our results we infer that this ciliate eRF1 possesses an altered stop codon recognition pattern lacking UAG response. This prediction is fully consistent with the fact that in this organisms only UGA servers as a single stop codon while two other stop codons are reassigned from sense codons (40).
The sets of amino acids recognizing A and G are different, for example, Ser and Thr form H-bonds with A, while G prefers Asn, Glu and Gly (41). We suggest that A and G in the same position are recognized by non-identical sets of amino acids.
In the previous work (12), an omnipotent human eRF1 has been converted into unipotent factor recognizing only UGA stop codon. The major role in this conversion is played by Cys127 and/or Phe131. From these data, it is evident that these amino acid residues can be implicated in recognition of A in the second stop-codon position. The Cys residue can form an H-bond with A while the aromatic ring of Phe can be involved in van der Waals interactions with a purine heterocycle (41).
Thus, two purines in the UAG stop codon require for specific discrimination at least four amino acids probably with a dominant role of Cys127 and Tyr125. The U base should be discriminated by no less than two amino acids as predicted by structural analysis (42). Therefore, we assume that stop codon triplet requires for its specific recognition more than three amino acid residues.
In previously published recognition models (14,23) Glu55 has been assumed to be involved in recognition of U, the first stop codon base. Our data clearly show that this amino acid residue is not implicated in discrimination of U. The most striking example is the E55R mutant, which is as active as the wt-eRF1 toward all three stop codons (Table 1). Tyr125 and Cys127 have been also assumed to recognize the first U (14). This hypothesis contradicts our previous data (12) showing the role of Cys127 in 2nd base discrimination but not the first. As shown here, mutations at Tyr125 do not profoundly impair UGA response (Table 1) and therefore, most likely, Tyr125 is not involved in U discrimination.
Leu126 has been proposed as an amino acid residue which recognizes the second stop-codon base (10,14). However, substitutions in this position as shown here exhibit no effect on RF activity toward all three stop codons.
In summary, the suggested models (10,14,23) are not supported by previous (12) and new (this work) data obtained by directed point mutagenesis followed by in vitro determination of RF activity versus all three stop codons. Previous models were based on indirect genetic data (10), bioinformatics approach (14,22) or molecular modeling (10,14,23).
We suggest that stop codon recognition is controlled by many amino acid residues (at present only part of them are identified) via a 3D network rather then by one amino acid–one nucleotide interaction as proposed in the prokaryotic recognition model (6). It is noteworthy that amino acid residues identified as being implicated in stop codon recognition by eRF1s (Glu, Tyr, Cys and Phe) are distinct from those involved in stop codon recognition in bacteria (Pro, Ala, Thr, Ser and Phe) with one exception of Phe. This remarkable difference most likely reflects profound dissimilarities between eRF1/aRF1 and RF1/RF2 protein families in their amino acid sequences (20,21,37) and 3D structures (37,43).
Acknowledgments
The authors are grateful to Vladimir Mitkevich who took part in calorimetric measurements. The authors thank both referees for valuable and helpful critical remarks. This work was supported by the Presidium of the Russian Academy of Sciences (Programme on Molecular and Cell Biology), by the Presidential Programme of Supporting the Leading Scientific Schools (via Ministry of Education and Science of the Russian Federation), and by Novo-Nordic Foundation by grants 03-04-48943 and 05-04-49385 from the Russian Foundation for Basic Research. Funding to pay the Open Access publication charges for this article was provided by Danish Research Council.
Conflict of interest statement. None declared.
REFERENCES
- 1.Wilson D.N., Blaha G., Connell S.R., Ivanov P.V., Jenke H., Stelzl U., Teraoka Y., Nierhaus K.H. Protein synthesis at atomic resolution: mechanistics of translation in the light of highly resolved structures for the ribosome. Curr. Protein Pept. Sci. 2002;3:1–53. doi: 10.2174/1389203023380846. [DOI] [PubMed] [Google Scholar]
- 2.Inge-Vechtomov S., Zhouravleva G., Philippe M. Eukaryotic release factors (eRFs) history. Biol. Cell. 2003;95:195–209. doi: 10.1016/s0248-4900(03)00035-2. [DOI] [PubMed] [Google Scholar]
- 3.Kisselev L.L., Ehrenberg M., Frolova L.Yu. Termination of translation: interplay of mRNA, rRNAs and release factors. EMBO J. 2003;22:175–182. doi: 10.1093/emboj/cdg017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakamura Y., Ito K. Making sense of mimic in translation termination. Trends Biochem. Sci. 2003;28:99–105. doi: 10.1016/S0968-0004(03)00006-9. [DOI] [PubMed] [Google Scholar]
- 5.Poole E.S., Askarian-Amiri M.E., Major L.L., McCaughan K.K., Scarlett D.J., Wilson D.N., Tate W.P. Molecular mimicry in the decoding of translational stop signals. Prog. Nucleic Acid Res. Mol. Biol. 2003;74:83–121. doi: 10.1016/s0079-6603(03)01011-0. [DOI] [PubMed] [Google Scholar]
- 6.Ito K., Uno M., Nakamura Y. A tripeptide ‘anticodon’ deciphers stop codons in messenger RNA. Nature. 2000;403:680–684. doi: 10.1038/35001115. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura Y., Ito K., Ehrenberg M. Mimicry grasps reality in translation termination. Cell. 2000;101:349–352. doi: 10.1016/s0092-8674(00)80845-4. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura Y., Ito K. A tripeptide discriminator for stop codon recognition. FEBS Lett. 2002;514:30–33. doi: 10.1016/s0014-5793(02)02330-x. [DOI] [PubMed] [Google Scholar]
- 9.Kervestin S., Frolova L., Kisselev L., Jean-Jean O. Stop codon recognition in ciliates: Euplotes release factor does not respond to reassigned UGA codon. EMBO Rep. 2001;2:680–684. doi: 10.1093/embo-reports/kve156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertram G., Bell H.A., Ritchie D.W., Fullerton G., Stansfield I. Terminating eukaryote translation: domain 1 of release factor eRF1 functions in stop codon recognition. RNA. 2000;6:1236–1247. doi: 10.1017/s1355838200000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frolova L., Seit-Nebi A., Kisselev L. Highly conserved NIKS tetrapeptide is functionally essential in eukaryotic translation termination factor eRF1. RNA. 2002;8:129–136. doi: 10.1017/s1355838202013262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seit-Nebi A., Frolova L., Kisselev L. Conversion of omnipotent translation termination factor eRF1 into ciliate-like UGA-only unipotent eRF1. EMBO Rep. 2002;3:881–886. doi: 10.1093/embo-reports/kvf178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito K., Frolova L., Seit-Nebi A., Karamyshev A., Kisselev L., Nakamura Y. Omnipotent decoding potential resides in eukaryotic translation termination factor eRF1 of variant-code organisms and is modulated by the interactions of amino acid sequences within the domain 1. Proc. Natl Acad. Sci. USA. 2002;99:8494–8499. doi: 10.1073/pnas.142690099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inagaki Y., Blouin C., Doolittle W.F., Roger A.J. Convergence and constraint in eukaryotic release factor (eRF1) domain 1: the evolution of stop codon specificity. Nucleic Acids Res. 2002;30:532–544. doi: 10.1093/nar/30.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ivanov V., Beniaminov A., Mikheev A., Minyat E. A mechanism for stop codon recognition by the ribosome: a bioinformatics approach. RNA. 2001;7:1683–1692. [PMC free article] [PubMed] [Google Scholar]
- 16.Bulygin K.N., Demeshkina N.A., Frolova L.Y., Graifer D.M., Ven'yaminova A.G., Kisselev L.L., Karpova G.G. The ribosomal A site-bound sense and stop codons are similarly positioned towards the A1823-A1824 dinucleotide of the 18S ribosomal RNA. FEBS Lett. 2003;548:97–102. doi: 10.1016/s0014-5793(03)00755-5. [DOI] [PubMed] [Google Scholar]
- 17.Chavatte L., Frolova L., Kisselev L., Favre A. The polypeptide chain release factor eRF1 specifically contacts the s(4)UGA stop codon located in the A site of eukaryotic ribosomes. Eur. J. Biochem. 2001;268:2896–2904. doi: 10.1046/j.1432-1327.2001.02177.x. [DOI] [PubMed] [Google Scholar]
- 18.Bulygin K.N., Repkova M.N., Ven'yaminova A.G., Graifer D.M., Karpova G.G., Frolova L.Y., Kisselev L.L. Positioning of the mRNA stop signal with respect to polypeptide chain release factors and ribosomal proteins in 80S ribosomes. FEBS Lett. 2002;514:96–101. doi: 10.1016/s0014-5793(02)02304-9. [DOI] [PubMed] [Google Scholar]
- 19.Chavatte L., Seit-Nebi A., Dubovaya V., Favre A. The invariant uridine of stop codons contacts the conserved NIKSR loop of human eRF1 in the ribosome. EMBO J. 2002;21:5302–5311. doi: 10.1093/emboj/cdf484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frolova L., Le Goff X., Rasmussen H.H., Cheperegin S., Drugeon G., Kress M., Arman I., Haenni A.-L., Celis J.E., Philippe M., et al. A highly conserved eukaryotic protein family possessing properties of polypeptide chain release factor. Nature. 1994;372:701–703. doi: 10.1038/372701a0. [DOI] [PubMed] [Google Scholar]
- 21.Kisselev L.L., Oparina N.Yu., Frolova L.Yu. Class-1 polypeptide chain release factors are structurally and functionally similar to suppressor tRNAs and comprise different structural-functional families of prokaryotic/mitochondrial and eukaryotic/archaebacterial factors. Mol. Biol. (Moscow) 2000;34:427–442. [PubMed] [Google Scholar]
- 22.Liang H., Wong J.Y., Bao Q., Cavalcanti A.R., Landweber L.F. Decoding the decoding region: analysis of eukaryotic release factor (eRF1) stop codon-binding residues. J. Mol. Evol. 2005;60:337–344. doi: 10.1007/s00239-004-0211-8. [DOI] [PubMed] [Google Scholar]
- 23.Muramatsu T., Heckmann K., Kitanaka C., Kuchino Y. Molecular mechanism of stop codon recognition by eRF1: a wobble hypothesis for peptide anticodons. FEBS Lett. 2001;488:105–109. doi: 10.1016/s0014-5793(00)02391-7. [DOI] [PubMed] [Google Scholar]
- 24.Seit-Nebi A., Frolova L., Justesen J., Kisselev L. Class-1 translation termination factors: invariant GGQ minidomain is essential for release activity and ribosome binding but not for stop codon recognition. Nucleic Acids Res. 2001;29:3982–3987. doi: 10.1093/nar/29.19.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarkar G., Sommer S.S. The ‘megaprimer’ method of site-directed mutagenesis. Biotechniques. 1990;8:404–407. [PubMed] [Google Scholar]
- 26.Frolova L.Y., Merkulova T.I., Kisselev L.L. Translation termination in eukaryotes: polypeptide release factor eRF1 is composed of functionally and structurally distinct domains. RNA. 2000;6:381–390. doi: 10.1017/s135583820099143x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frolova L.Y., Simonsen J.L., Merkulova T.I., Litvinov D.Y., Martensen P.M., Rechinsky V.O., Camonis J.H., Kisselev L.L., Justesen J. Functional expression of eukaryotic polypeptide chain release factors 1 and 3 by means of baculovirus/insect cells and complex formation between the factors. Eur. J. Biochem. 1998;256:36–44. doi: 10.1046/j.1432-1327.1998.2560036.x. [DOI] [PubMed] [Google Scholar]
- 28.Caskey C.T., Beaudet A.L., Tate W.P. Mammalian release factor: in vitro assay and purification. Methods Enzymol. 1974;30:293–303. doi: 10.1016/0076-6879(74)30032-8. [DOI] [PubMed] [Google Scholar]
- 29.Frolova L., Le Goff X., Zhouravleva G., Davydova E., Philippe M., Kisselev L. Eukaryotic polypeptide chain release factor eRF3 is an eRF1- and ribosome-dependent guanosine triphosphatase. RNA. 1996;2:334–341. [PMC free article] [PubMed] [Google Scholar]
- 30.Privalov P.L., Potechin S.A. Scanning microcalorimetry in studding temperature-induced changes in proteins. Methods Enzymol. 1986;131:4–51. doi: 10.1016/0076-6879(86)31033-4. [DOI] [PubMed] [Google Scholar]
- 31.Makharadze G.I., Privalov P.L. Heat capacity of proteins. Partial molar heat capacity of individual amino acid residues in aqueous solution: hydration effect. J. Mol. Biol. 1990;213:375–384. doi: 10.1016/S0022-2836(05)80197-4. [DOI] [PubMed] [Google Scholar]
- 32.Mitkevich V., Kononenko A., Oparina N., Makarov A., Kisselev L. Thermal denaturarion of eukaryotic translation termination factor eRF1. Relation between protein stability and functional changes in eRF1 mutants. Mol. Biol. (Moscow) 2006;40 N (in press) [PubMed] [Google Scholar]
- 33.Chipot C., Pearlman D.A. Free energy calculations: the long and winding gilded road. Mol. Sim. 2002;28:1–12. [Google Scholar]
- 34.Lu N., Kofke D.A., Woolf T.B. Improving the efficiency and reliability of free energy perturbation calculations using overlap sampling methods. J. Comput. Chem. 2004;25:28–39. doi: 10.1002/jcc.10369. [DOI] [PubMed] [Google Scholar]
- 35.Wang L., Veenstra D.L., Radmer R.J., Kollman P.A. Can one predict protein stability? An attempt to do so for residue 133 of T4 lysozyme using a combination of free energy derivatives, PROFEC, and free energy perturbation methods. Proteins. 1998;32:438–458. [PubMed] [Google Scholar]
- 36.Weng Z., Delisi C., Vajda S. Empirical free energy calculation: comparison to calorimetric data. Protein Sci. 1997;6:1976–1984. doi: 10.1002/pro.5560060918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song H., Mugnier P., Webb H.M., Evans D.R., Tuite M.F., Hemmings B.A., Barford D. The crystal structure of human eukaryotic release factors eRF1—mechanism of stop codon recognition and peptidyl-tRNA hydrolysis. Cell. 2000;100:311–321. doi: 10.1016/s0092-8674(00)80667-4. [DOI] [PubMed] [Google Scholar]
- 38.Efimov A.V., Brazhnikov E.V. Relationship between intramolecular hydrogen bonding and solvent accessibility of side-chain donors and acceptors in proteins. FEBS Lett. 2003;554:389–393. doi: 10.1016/s0014-5793(03)01189-x. [DOI] [PubMed] [Google Scholar]
- 39.Morozov A.V., Kortemme T., Tsemekhman K., Baker D. Close agreement between the orientation dependence of hydrogen bonds observed in protein structures and quantum mechanical calculations. Proc. Natl Acad. Sci. USA. 2004;101:6946–6951. doi: 10.1073/pnas.0307578101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim O.T., Yura K., Go N., Harumoto T. Newly sequenced eRF1s from ciliates: the diversity of stop codon usage and the molecular surfaces that are important for stop codon interactions. Gene. 2005;346:277–286. doi: 10.1016/j.gene.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 41.Jones S., Daley D.T., Luscombe N.M., Bertram H.M., Thornton J.M. Protein–RNA interactions: a structural analysis. Nucleic Acids Res. 2001;29:943–954. doi: 10.1093/nar/29.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saenger W. New York, Berlin, Heidelberg, Tokyo: Springer-Verlag; 1984. Principles of nucleic acid structure, Chapter 18. Protein-Nucleic Acid Interactions; pp. 385–431. [Google Scholar]
- 43.Vestergaard B., Van L.B., Andersen G.R., Nyborg J., Buckingham R.H., Kjeldgaard M. Bacterial polypeptide release factor RF2 is structurally distinct from eukaryotic eRF1. Mol. Cell. 2001;8:1375–1382. doi: 10.1016/s1097-2765(01)00415-4. [DOI] [PubMed] [Google Scholar]