Abstract
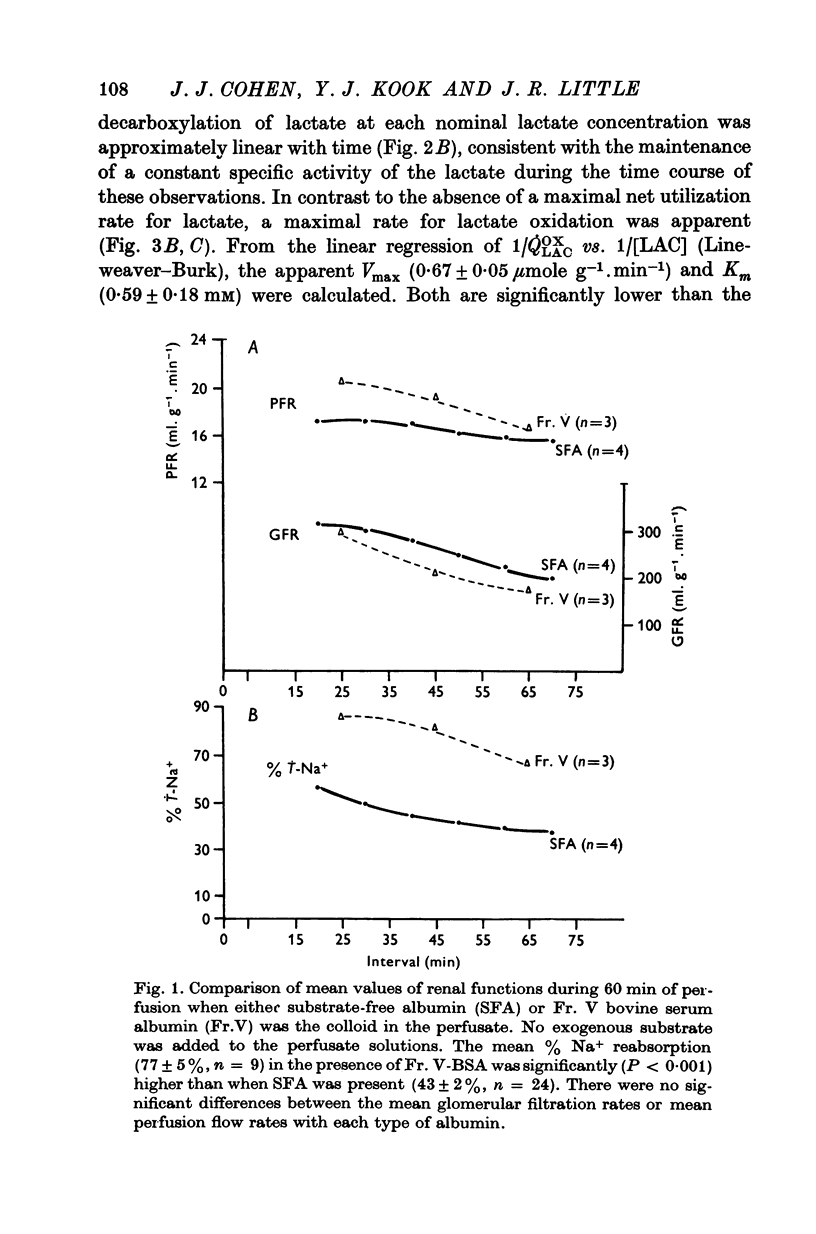
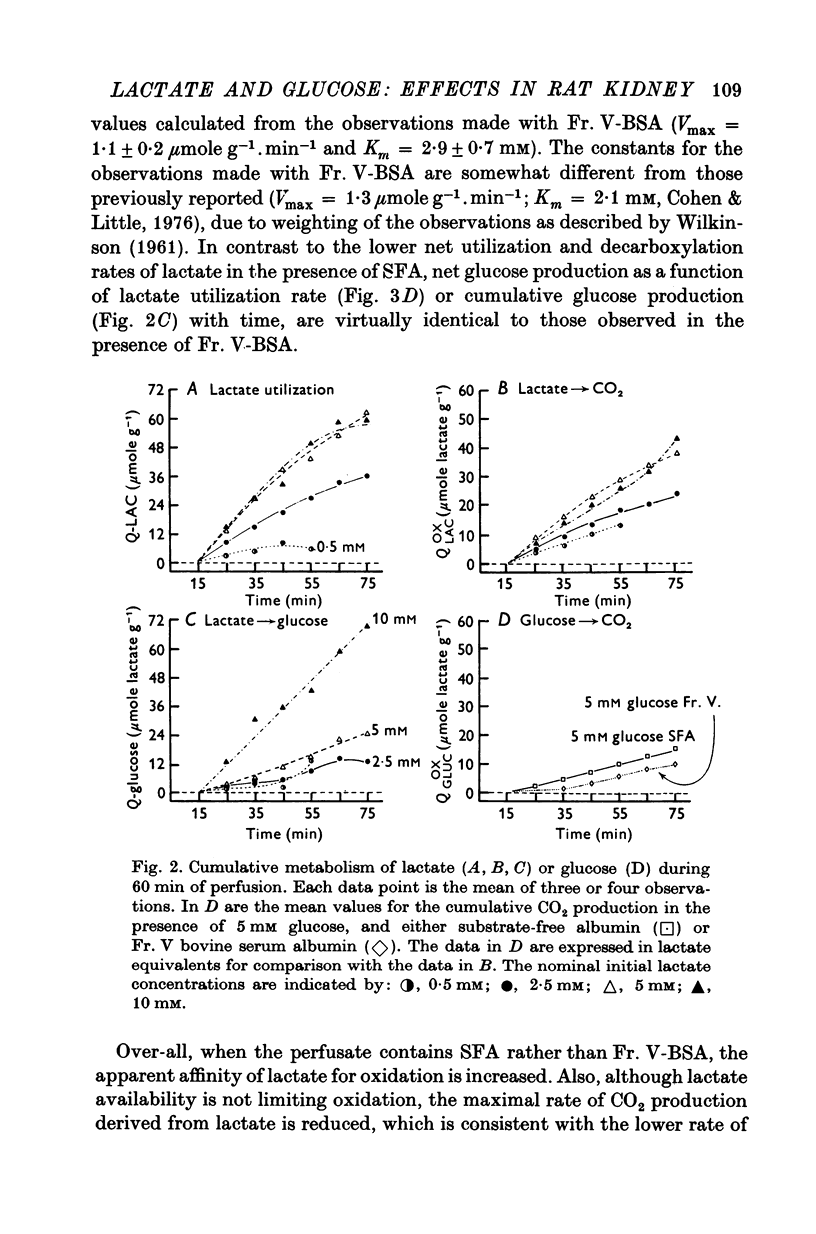
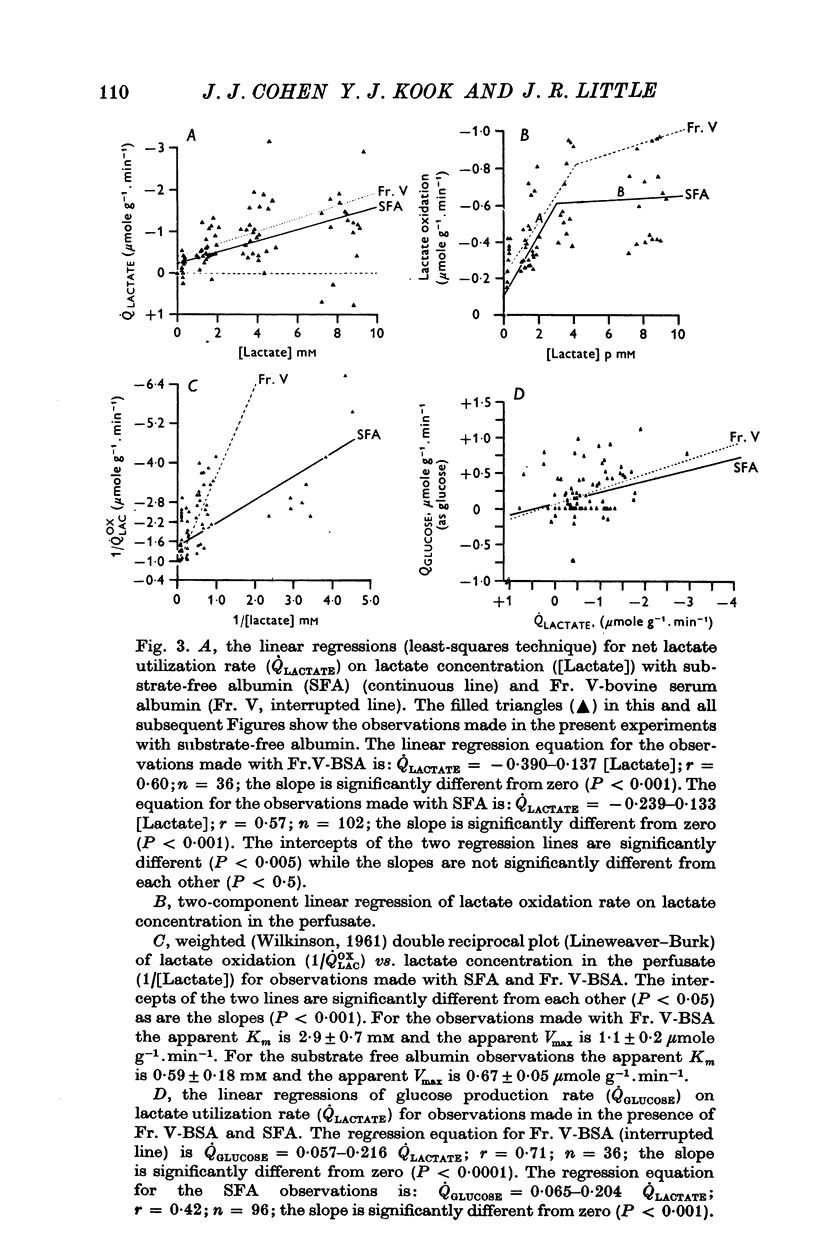
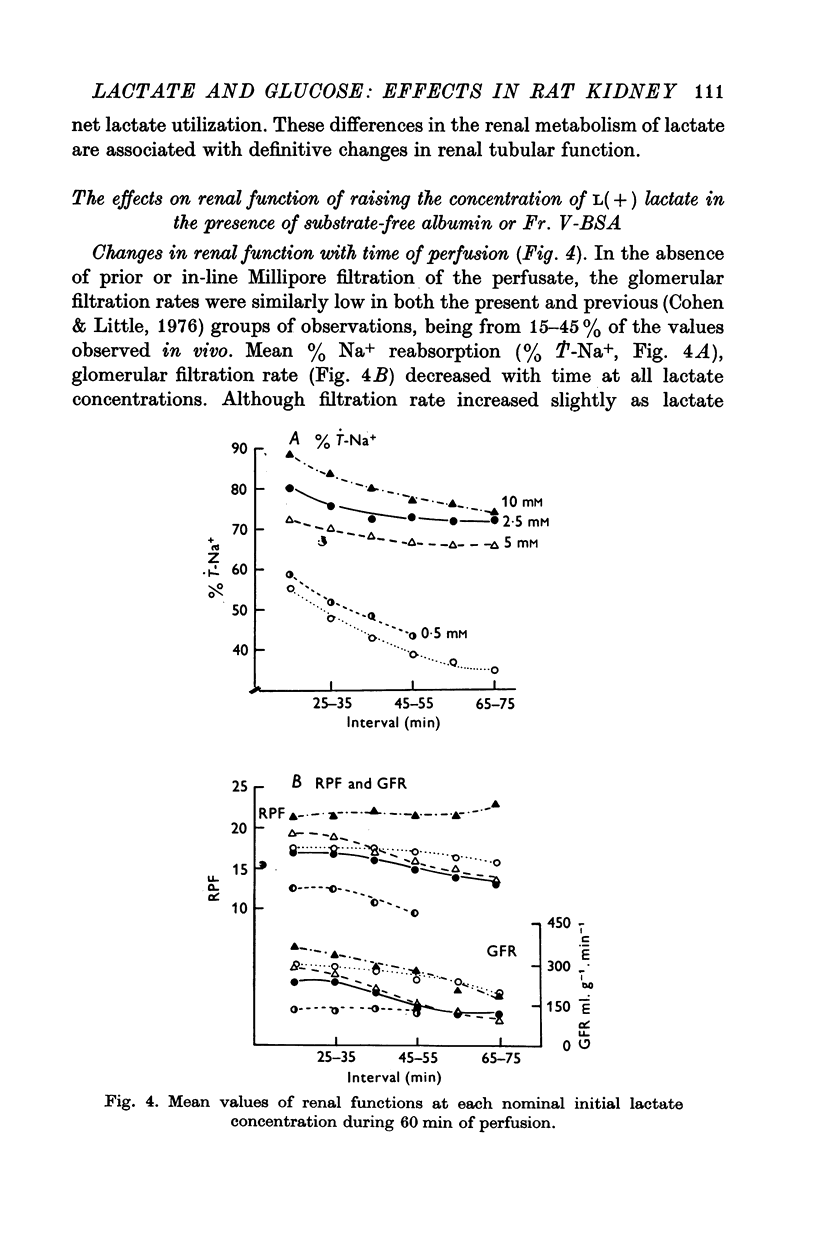
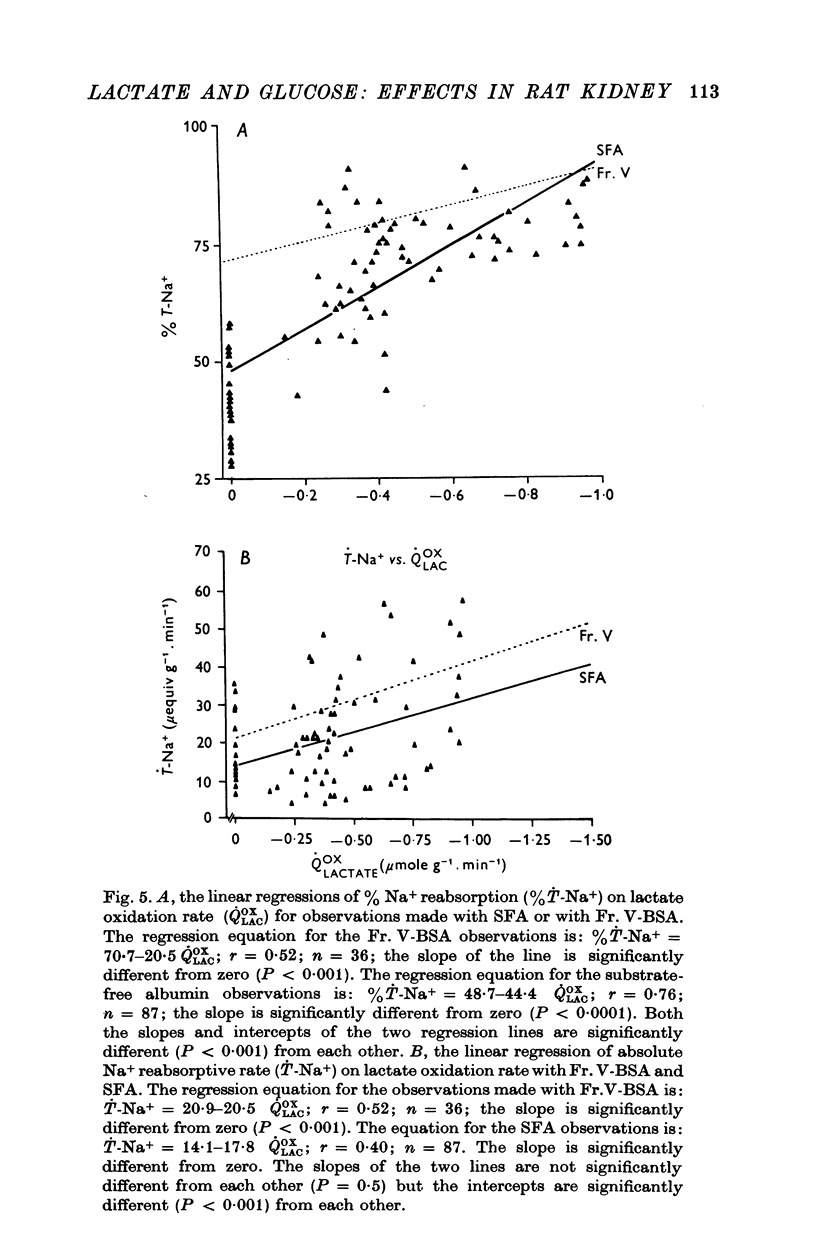
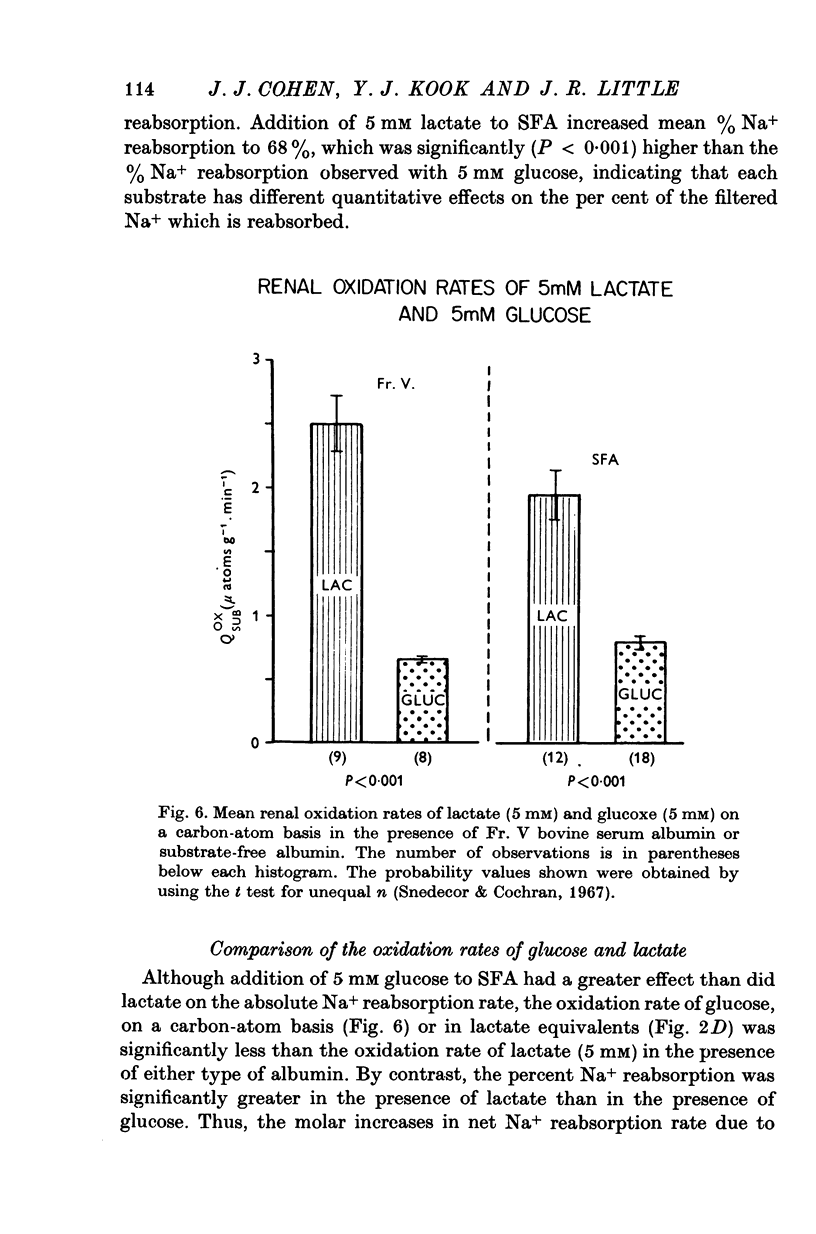
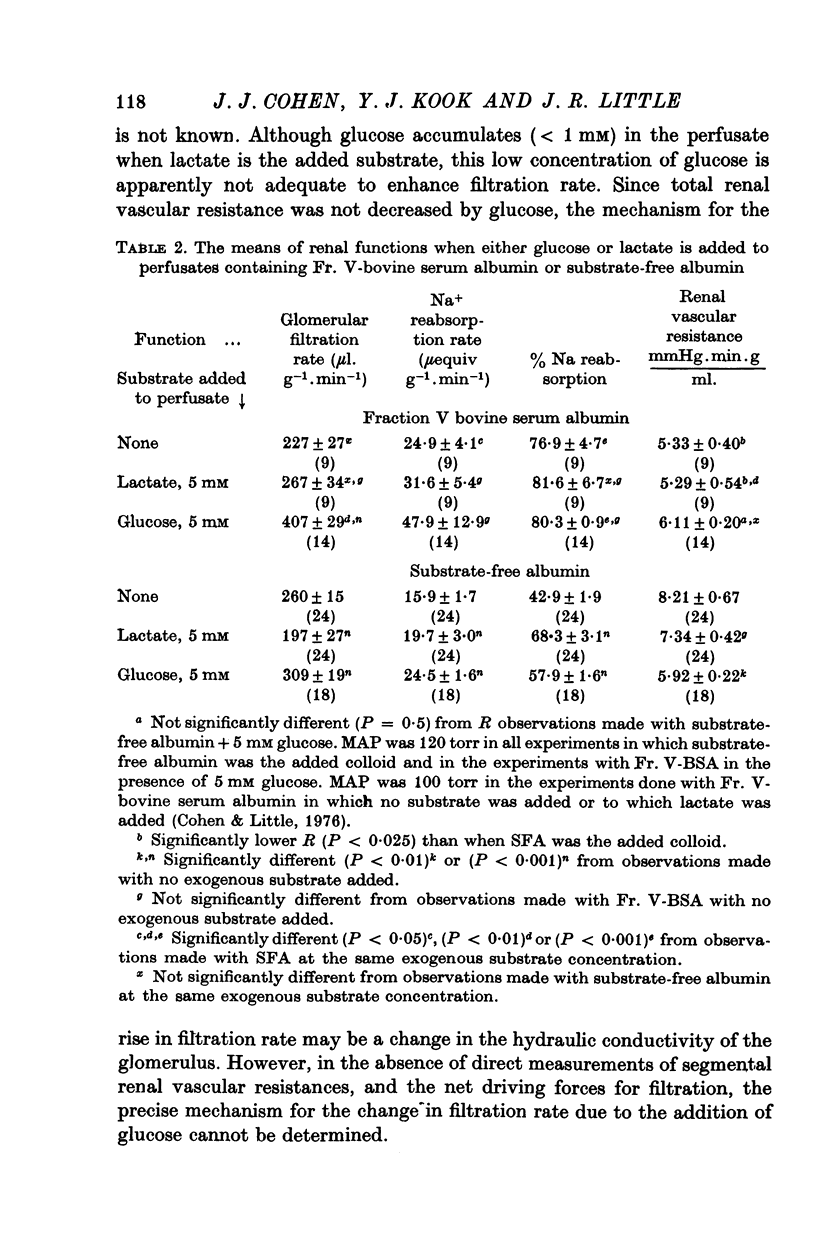
1. The objective of this study was to determine the separate contributions of exogenous substrate and of kidney tissue to the support of function and metabolism in the isolated perfused rat kidney. The effects of the addition of L(+) [U-14C]lactate or D[U-14C]glucose either to a specially prepared substrate-free albumin (SFA) or to Fr. V bovine serum albumin (Fr. V-BSA) were compared. The Fr. V-BSA has significant quantities of lactate, citrate and free fatty acids associated with it. 2. Perfusion of the rat kidney with the Krebs-Ringer bicarbonate solution containing SFA, without addition of exogenous substrate, resulted in a lower % Na+ reabsorption (approximately 43%) than when the perfusions contained Fr. V-BSA (approximately 80%). Thus, kidney tissue can support at most 45% of Na+ reabsorption, while the substrates associated with the Fr. V-BSA can support approximately 30% of Na+ reabsorption. When the initial concentration of L(+)lactate in the perfusate containing SFA was progressively raised from 0 to 10 mM, % Na+ reabsorption increased to between 85 and 90%. 3. The apparent Km (0-59 mM) and the Vmax (0-67 micronmole g-1. min-1) for lactate oxidation in the presence of SFA were both significantly lower than when Fr. V-BSA was present (Km = 2-0 mM; Vmax = 1-1 micronmole g-1. min-1). The lower Km is interpreted as being due to the removal of substances from the Fr. V-BSA which competitively inhibit either the uptake or oxidation of lactate; the lower Vmax is considered to be related to the lower rate of Na+ reabsorption when SFA is present. 4. Addition of glucose enhanced gomerular filtration rate in the presence of both types of albumin. The resulting increase in the filtered load of Na+ in the presence of glucose was associated with either no change (Fr. V-BSA) or an increase (SFA) in fractional Na+ reabsorption. Although absolute Na+ reabsorptive rate was greater in the presence of glucose than in the presence of lactate, the oxidation rate of glucose, on a carbon-atom basis, was less than 50% of the oxidation rate of lactate. 5. The metabolism of glucose may regulate the permeability characteristics of the glomerulus and the tubular epithelium: by contrast, the high oxidation rate of lactate suggests it can provide direct support for a major fraction of the Na+ actively absorbed.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. E., Synder F. Quantitative collection of 14CO2 in the presence of labeled short-chain acids. Anal Biochem. 1969 Feb;27(2):311–314. doi: 10.1016/0003-2697(69)90038-4. [DOI] [PubMed] [Google Scholar]
- Bertermann H., Franke H., Huland H., Weiss C. CO2-production by the isolated perfused rat kidney from 14C-labelled substrates. Res Exp Med (Berl) 1973 Jul 26;161(1):1–14. doi: 10.1007/BF01851017. [DOI] [PubMed] [Google Scholar]
- Bowman R. H. Gluconeogenesis in the isolated perfused rat kidney. J Biol Chem. 1970 Apr 10;245(7):1604–1612. [PubMed] [Google Scholar]
- Brand P. H., Cohen J. J., Bignall M. C. Independence of lactate oxidation from net Na+ reabsorption in dog kidney in vivo. Am J Physiol. 1974 Dec;227(6):1255–1262. doi: 10.1152/ajplegacy.1974.227.6.1255. [DOI] [PubMed] [Google Scholar]
- COHEN R. D., PROUT R. E. STUDIES ON THE RENAL TRANSPORT OF CITRATE USING 14C-CITRATE. Clin Sci. 1965 Jun;28:487–497. [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Cohen J. J., Little J. R. Lactate metabolism in the isolated perfused rat kidney: relations to renal function and gluconeogenesis. J Physiol. 1976 Feb;255(2):399–414. doi: 10.1113/jphysiol.1976.sp011286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J. Metabolic support for renal sodium reabsorption. Med Clin North Am. 1975 May;59(3):523–538. doi: 10.1016/s0025-7125(16)32006-5. [DOI] [PubMed] [Google Scholar]
- Cunningham V. J., Hay L., Stoner H. B. The binding of L-tryptophan to serum albumins in the presence of non-esterified fatty acids. Biochem J. 1975 Mar;146(3):653–658. doi: 10.1042/bj1460653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLE V. P., MEINERTZ H. Microdetermination of long-chain fatty acids in plasma and tissues. J Biol Chem. 1960 Sep;235:2595–2599. [PubMed] [Google Scholar]
- Franke H., Huland H., Weiss C., Unsicker K. Improved net sodium transport of the isolated rat kidney. Z Gesamte Exp Med. 1971;156(4):268–282. doi: 10.1007/BF02045828. [DOI] [PubMed] [Google Scholar]
- Garland P. B., Newsholme E. A., Randle P. J. Regulation of glucose uptake by muscle. 9. Effects of fatty acids and ketone bodies, and of alloxan-diabetes and starvation, on pyruvate metabolism and on lactate-pyruvate and L-glycerol 3-phosphate-dihydroxyacetone phosphate concentration ratios in rat heart and rat diaphragm muscles. Biochem J. 1964 Dec;93(3):665–678. doi: 10.1042/bj0930665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza-Quintero R., Cohen J. J., Brand P. H., Kook Y. J. Steady-state glucose oxidation by dog kidney in vivo: relation to Na+ reabsorption. Am J Physiol. 1975 Feb;228(2):549–555. doi: 10.1152/ajplegacy.1975.228.2.549. [DOI] [PubMed] [Google Scholar]
- HERRIN R. C., LARDINOIS C. C. Renal clearance of citric acid in the dog. Proc Soc Exp Biol Med. 1958 Feb;97(2):294–297. doi: 10.3181/00379727-97-23720. [DOI] [PubMed] [Google Scholar]
- Hanson R. W., Ballard F. J. Citrate, pyruvate, and lactate contaminants of commercial serum albumin. J Lipid Res. 1968 Sep;9(5):667–668. [PubMed] [Google Scholar]
- Kokko J. P. Proximal tubule potential difference. Dependence on glucose on glucose, HCO 3 , and amino acids. J Clin Invest. 1973 Jun;52(6):1362–1367. doi: 10.1172/JCI107308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Källskog O., Lindbom L. O., Ulfendahl H. R., Wolgast M. Kinetics of the glomerular ultrafiltration in the rat kidney. An experimental study. Acta Physiol Scand. 1975 Nov;95(3):293–300. doi: 10.1111/j.1748-1716.1975.tb10053.x. [DOI] [PubMed] [Google Scholar]
- Little J. R., Cohen J. J. Effect of albumin concentration on function of isolated perfused rat kidney. Am J Physiol. 1974 Mar;226(3):512–517. doi: 10.1152/ajplegacy.1974.226.3.512. [DOI] [PubMed] [Google Scholar]
- Moellering H., Gruber W. Determination of citrate with citrate lyase. Anal Biochem. 1966 Dec;17(3):369–376. doi: 10.1016/0003-2697(66)90172-2. [DOI] [PubMed] [Google Scholar]
- Osswald H., Schmitz H. J., Heidenreich O. Adenosine response of the rat kidney after saline loading, sodium restriction and hemorrhagia. Pflugers Arch. 1975 Jun 26;357(3-4):323–333. doi: 10.1007/BF00585986. [DOI] [PubMed] [Google Scholar]
- Pashley D. H., Cohen J. J. Substrate interconversion in dog kidney cortex slices: regulation by ECF-pH. Am J Physiol. 1973 Dec;225(6):1519–1528. doi: 10.1152/ajplegacy.1973.225.6.1519. [DOI] [PubMed] [Google Scholar]
- Ross B. D., Epstein F. H., Leaf A. Sodium reabsorption in the perfused rat kidney. Am J Physiol. 1973 Nov;225(5):1165–1171. doi: 10.1152/ajplegacy.1973.225.5.1165. [DOI] [PubMed] [Google Scholar]
- Schurek H. J., Brecht J. P., Lohfert H., Hierholzer K. The basic requirements for the function of the isolated cell free perfused rat kidney. Pflugers Arch. 1975;354(4):349–365. doi: 10.1007/BF00587852. [DOI] [PubMed] [Google Scholar]
- Spector A. A. Fatty acid binding to plasma albumin. J Lipid Res. 1975 May;16(3):165–179. [PubMed] [Google Scholar]
- Spiro R. G. Studies on the renal glomerular basement membrane. Nature of the carbohydrate units and their attachment to the peptide portion. J Biol Chem. 1967 Apr 25;242(8):1923–1932. [PubMed] [Google Scholar]
- Trimble M. E., Bowman R. H. Renal Na+ and K+ transport: effects of glucose, palmitate, and alpha-bromopalmitate. Am J Physiol. 1973 Nov;225(5):1057–1062. doi: 10.1152/ajplegacy.1973.225.5.1057. [DOI] [PubMed] [Google Scholar]
- WEISS C., PASSOW H., ROTHSTEIN A. Autoregulation of flow in isolated rat kidney in the absence of red cells. Am J Physiol. 1959 May;196(5):1115–1118. doi: 10.1152/ajplegacy.1959.196.5.1115. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann M. J., Hems D. A., Krebs H. A. Effects of added nucleotides on renal carbohydrate metabolism. Biochem J. 1969 Oct;115(1):1–10. doi: 10.1042/bj1150001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann M. J., Krebs H. A. The fuel of respiration of rat kidney cortex. Biochem J. 1969 Apr;112(2):149–166. doi: 10.1042/bj1120149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. F., Stubbs M., Veech R. L., Erecińska M., Krebs H. A. Equilibrium relations between the oxidation-reduction reactions and the adenosine triphosphate synthesis in suspensions of isolated liver cells. Biochem J. 1974 Apr;140(1):57–64. doi: 10.1042/bj1400057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamlauski M. J., Cohen J. J. The effects of aortic infusion of ethylene oxide on renal function in the rat. Toxicol Appl Pharmacol. 1976 Nov;38(2):283–295. doi: 10.1016/0041-008x(76)90135-6. [DOI] [PubMed] [Google Scholar]


