Abstract
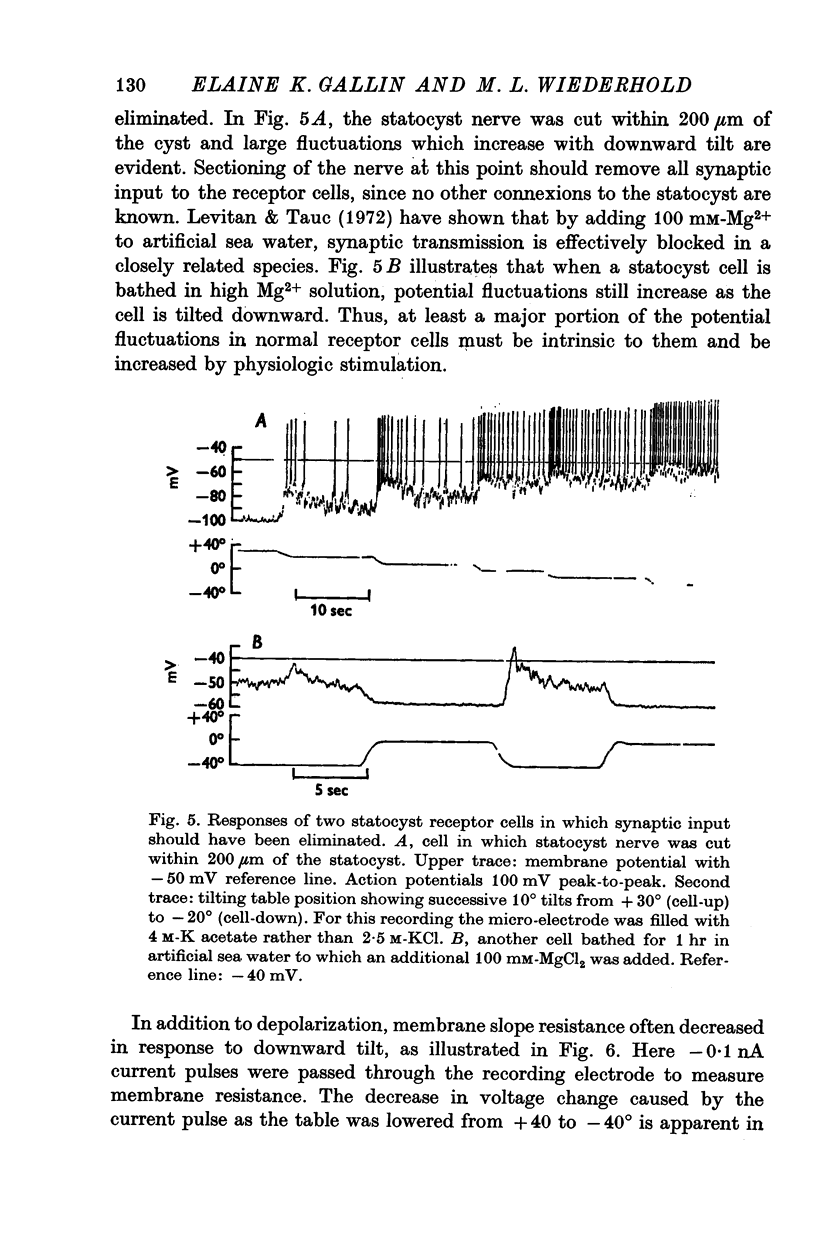
1. The electrical responses of Aplysia statocyst receptor cells were investigated using intracellular micro-electrodes. These ciliated mechanoreceptor cells were stimulated by downward tilting about a horizontal axis. 2. Tilting so that the receptor cell was excited produced a depolarizing receptor potential which, if large enough, could generate action potentials. 3. Large fluctuations in membrane potential were evident during depolarizing receptor potentials and were reduced or sometimes absent when a cell was tilted upward. Power-density spectra of the noise voltage revealed that most of the energy added by downward tilt is contained in frequency components below 3 Hz. 4. Removing synaptic input to the receptor cells by cutting the statocyst nerve or adding excess Mg2+ to the bath did not abolish the increase in fluctuations caused by downward, excitatory tilts. 5. The depolarizing receptor potential was often associated with a decrease in membrane resistance as measured with constant current pulses using a bridge circuit. 6. Replacing most of the Na+ in the bath with either Tris or Mg2+ abolished both potential and resistance changes caused by downward tilt. These results indicate that an increased permeability to Na+ underlies the receptor potential.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkon D. L., Bak A. Hair cell generator potentials. J Gen Physiol. 1973 May;61(5):619–637. doi: 10.1085/jgp.61.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwyl R., Usherwood P. N. Ionic permeability changes occurring at excitatory receptor membranes of chemical synapses. Nature. 1975 Oct 2;257(5525):410–412. doi: 10.1038/257410a0. [DOI] [PubMed] [Google Scholar]
- Blankenship J. E., Wachtel H., Kandel E. R. Ionic mechanisms of excitatory, inhibitory, and dual synaptic actions mediated by an identified interneuron in abdominal ganglion of Aplysia. J Neurophysiol. 1971 Jan;34(1):76–92. doi: 10.1152/jn.1971.34.1.76. [DOI] [PubMed] [Google Scholar]
- Bowen R. E. Movement of the So-Called Hairs in the Ampullar Organs of Fish Ears. Proc Natl Acad Sci U S A. 1931 Apr;17(4):192–194. doi: 10.1073/pnas.17.4.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter D. O., Hovey M. M., Bak A. F. Measurements of intracellular conductivity in Aplysia neurons: evidence for organization of water and ions. Ann N Y Acad Sci. 1973 Mar 30;204:502–533. doi: 10.1111/j.1749-6632.1973.tb30801.x. [DOI] [PubMed] [Google Scholar]
- Detwiler P. B., Alkon D. L. Hair cell interactions in the statocyst of Hermissenda. J Gen Physiol. 1973 Nov;62(5):618–642. doi: 10.1085/jgp.62.5.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detwiler P. B., Fuortes M. G. Responses of hair cells in the statocyst of Hermissenda. J Physiol. 1975 Sep;251(1):107–129. doi: 10.1113/jphysiol.1975.sp011083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flock A., Jorgensen J. M., Russell I. J. Passive electrical properties of hair cells and supporting cells in the lateral line canal organ. Acta Otolaryngol. 1973 Aug-Sep;76(2):190–198. doi: 10.3109/00016487309121499. [DOI] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan H., Tauc L. Acetylcholine receptors: topographic distribution and pharmacological properties of two receptor types on a single molluscan neurone. J Physiol. 1972 May;222(3):537–558. doi: 10.1113/jphysiol.1972.sp009813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmor M. F. The membrane of giant molluscan neurons: electrophysiologic properties and the origin of the resting potential. Prog Neurobiol. 1975;5(2):167–195. doi: 10.1016/0301-0082(75)90018-0. [DOI] [PubMed] [Google Scholar]
- McKee A. E., Wiederhold M. L. Aplysia statocyst receptor cells: fine structure. Brain Res. 1974 Dec 6;81(2):310–313. doi: 10.1016/0006-8993(74)90944-5. [DOI] [PubMed] [Google Scholar]
- Naitoh Y. Reversal response elicited in nonbeating cilia of paramecium by membrane depolarizatin. Science. 1966 Nov 4;154(3749):660–662. doi: 10.1126/science.154.3749.660. [DOI] [PubMed] [Google Scholar]
- Sjodin R. A., Beaugé L. A. An analysis of the leakages of sodium ions into and potassium ions out of striated muscle cells. J Gen Physiol. 1973 Feb;61(2):222–250. doi: 10.1085/jgp.61.2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickholm A. Reduction of response time for potential in salt bridge reference electrodes for electrophysiology. Nature. 1968 Jan 6;217(5123):80–81. doi: 10.1038/217080a0. [DOI] [PubMed] [Google Scholar]
- WERSALL J. Studies on the structure and innervation of the sensory epithelium of the cristae ampulares in the guinea pig; a light and electron microscopic investigation. Acta Otolaryngol Suppl. 1956;126:1–85. [PubMed] [Google Scholar]
- Wiederhold M. L. Aplysia statocyst receptor cells: intracellular responses to physiological stimuli. Brain Res. 1974 Oct 4;78(3):490–494. doi: 10.1016/0006-8993(74)90931-7. [DOI] [PubMed] [Google Scholar]
- Wiederhold M. L. Mechanosensory transduction in "sensory" and "motile" cilia. Annu Rev Biophys Bioeng. 1976;5:39–62. doi: 10.1146/annurev.bb.05.060176.000351. [DOI] [PubMed] [Google Scholar]
- Wiederhold M. L. Rectification in Aplysia statocyst receptor cells. J Physiol. 1977 Mar;266(1):139–156. doi: 10.1113/jphysiol.1977.sp011760. [DOI] [PMC free article] [PubMed] [Google Scholar]


