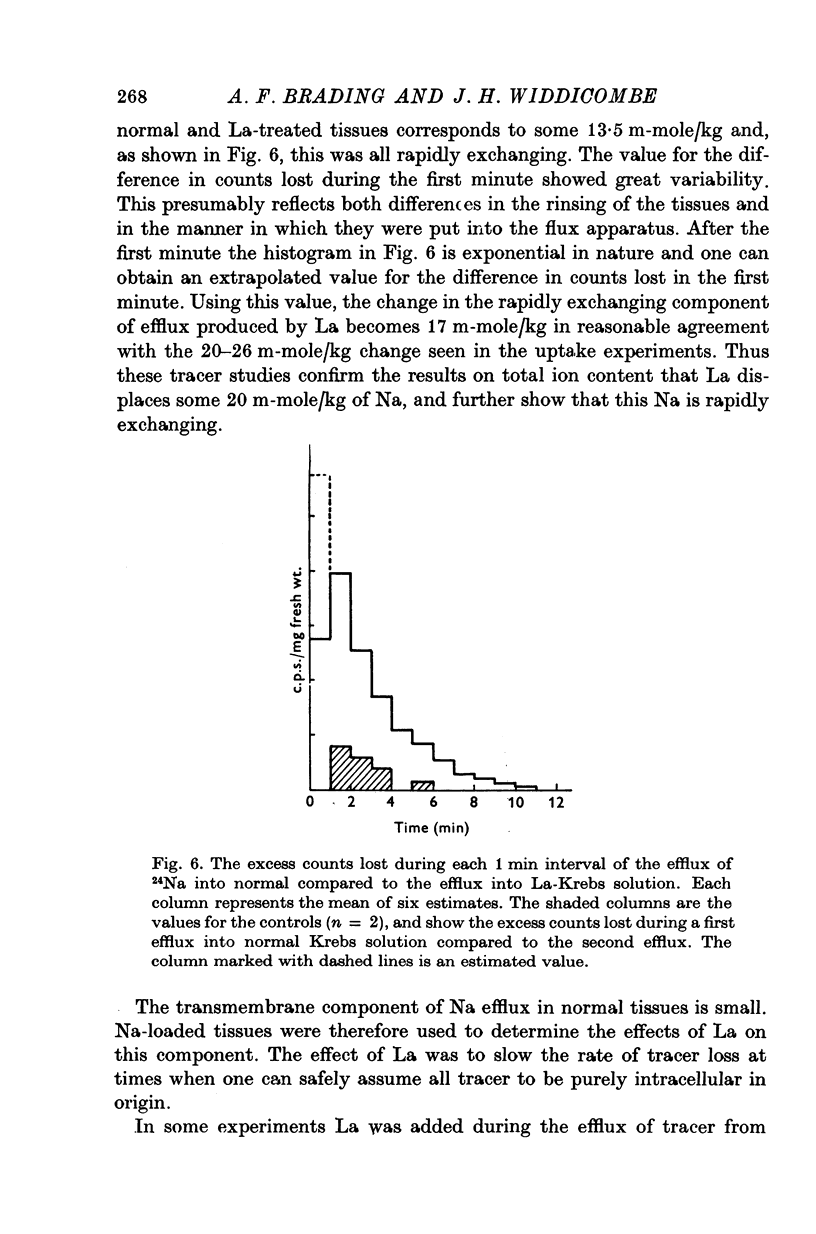
Abstract
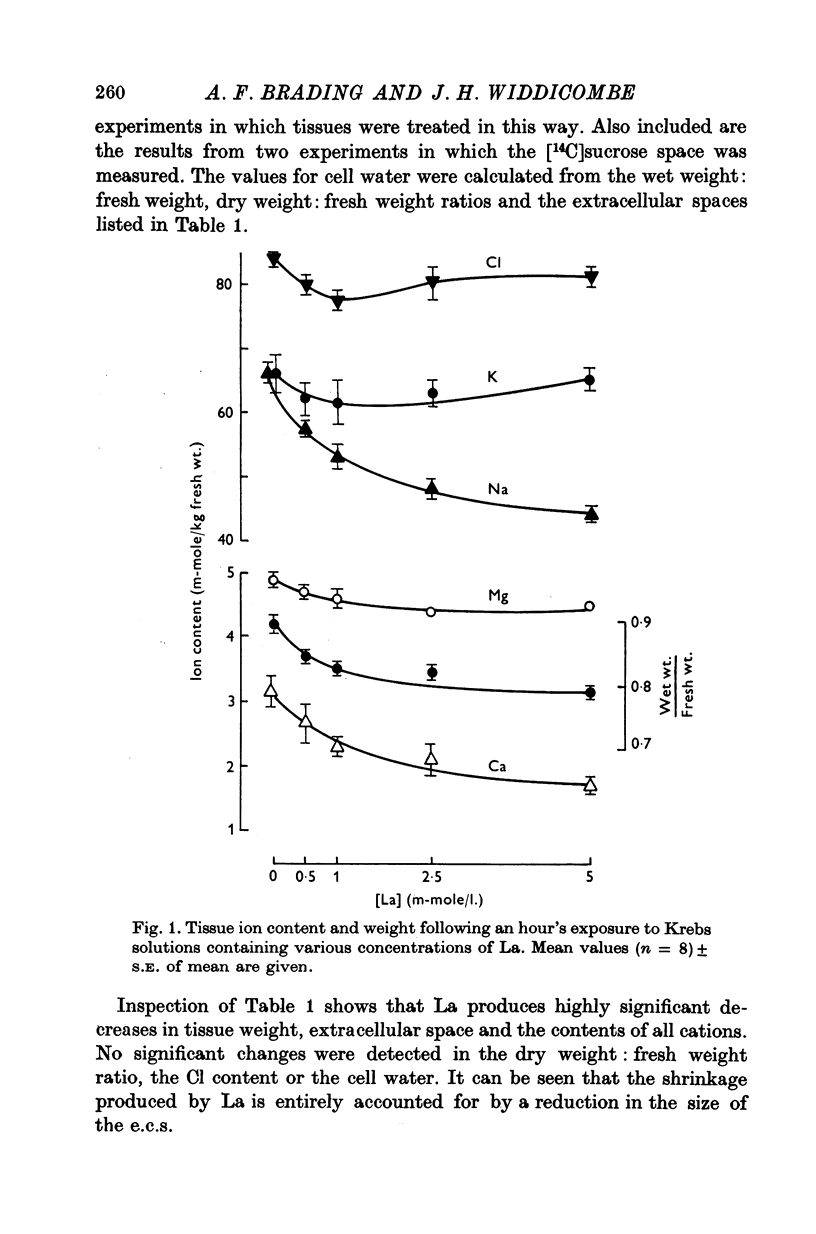
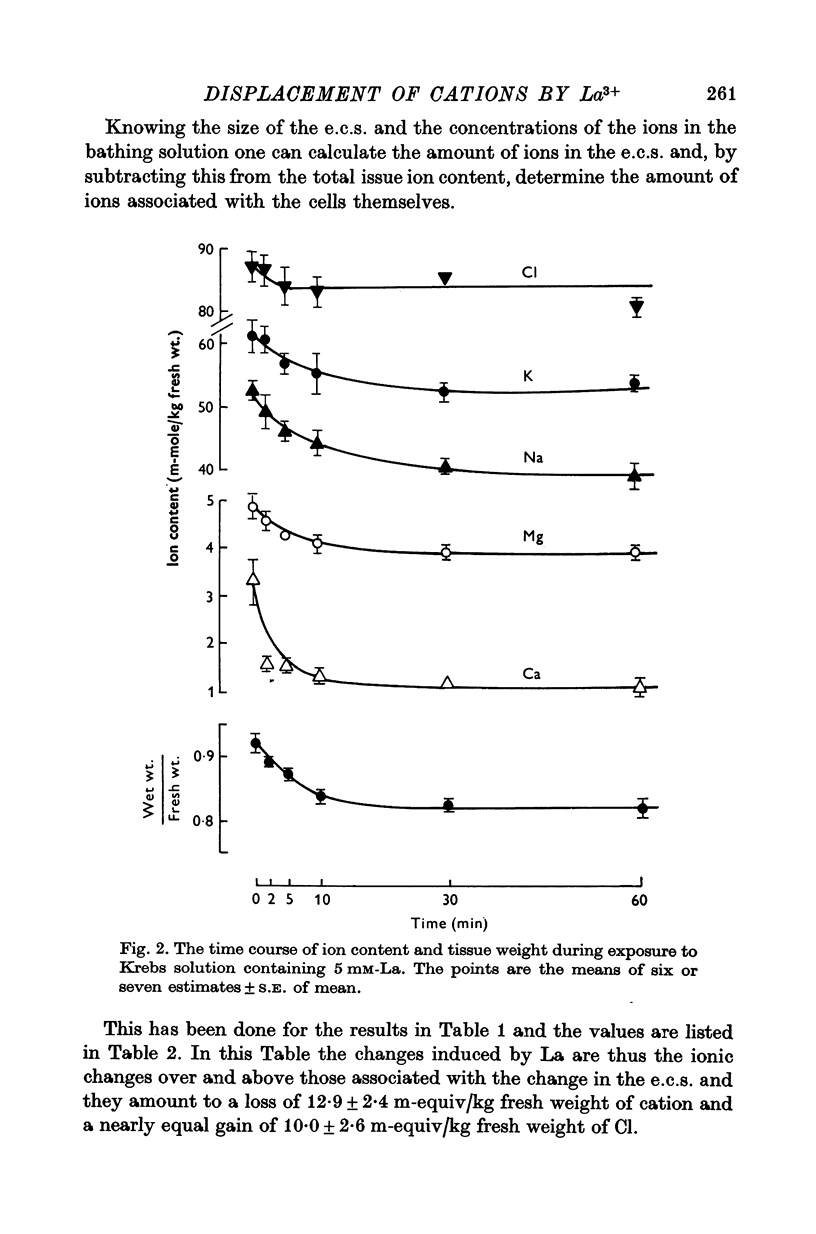
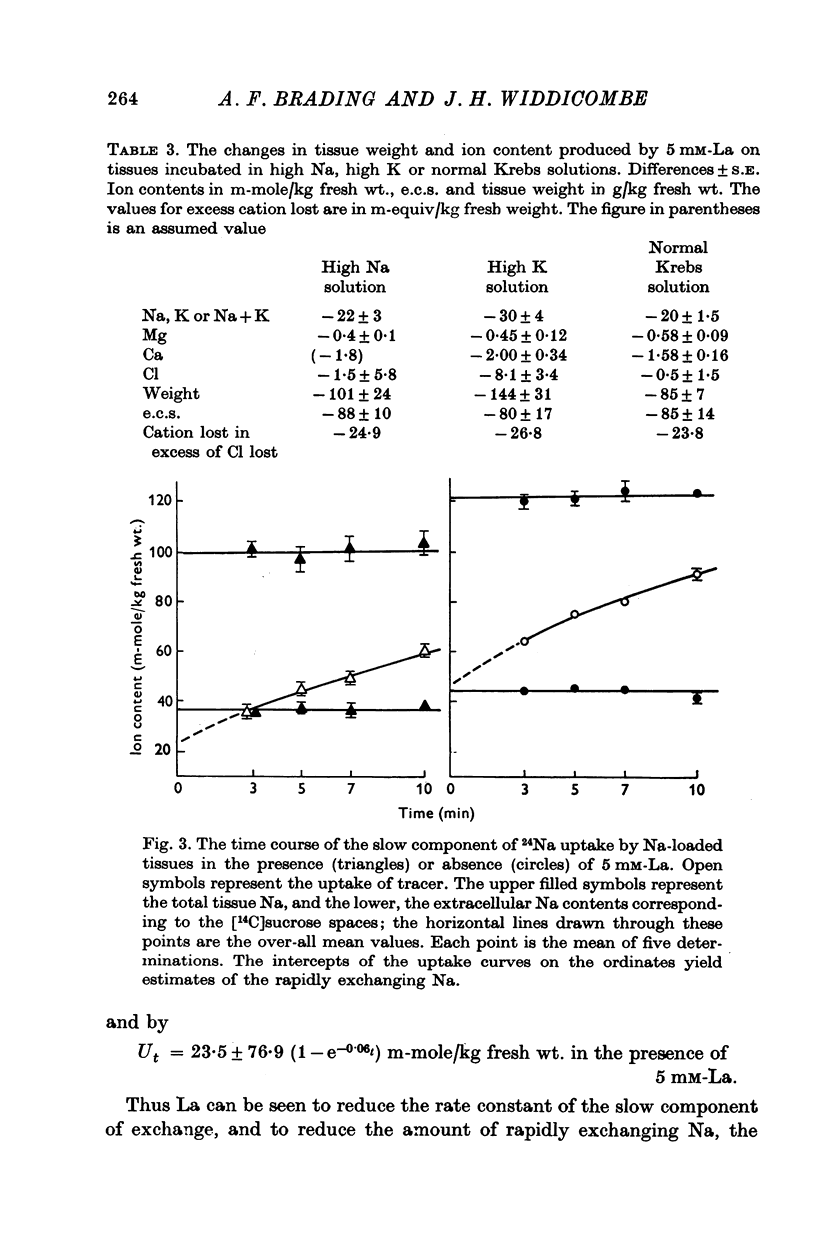
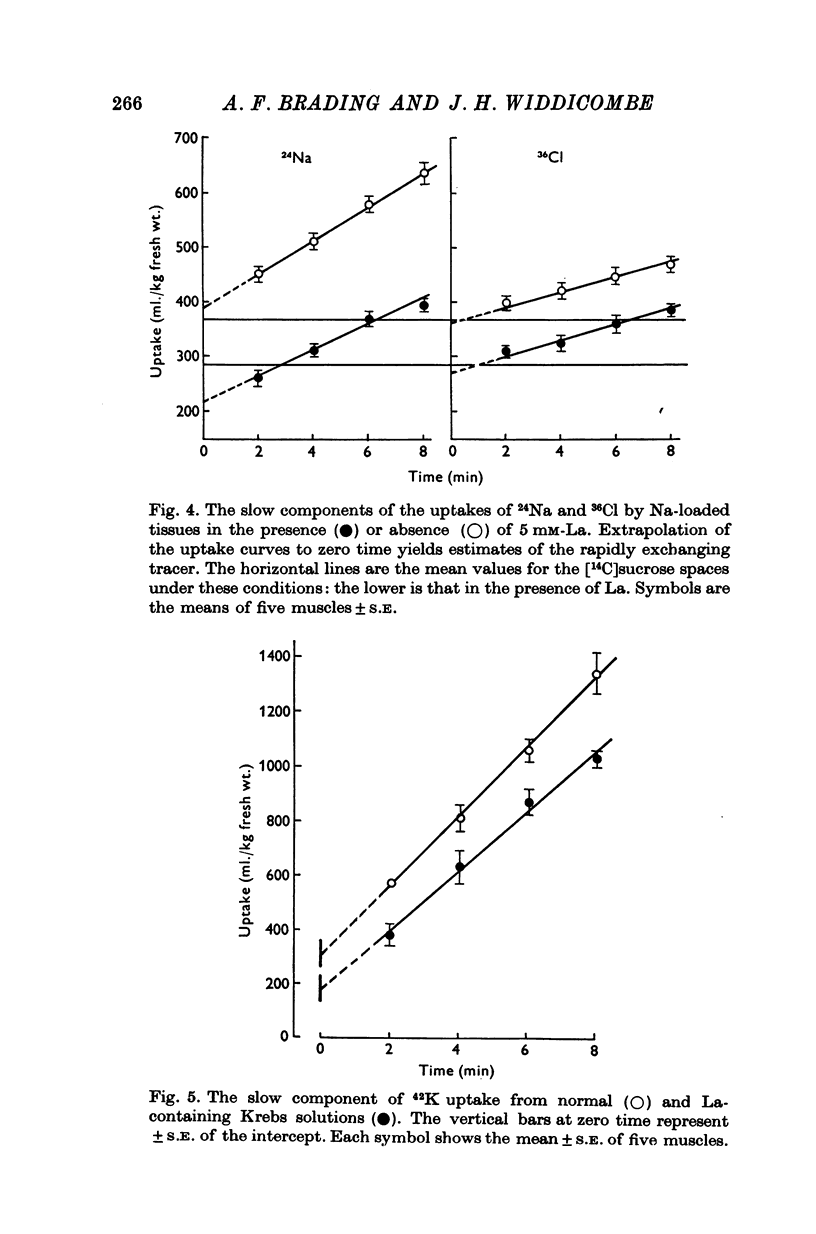
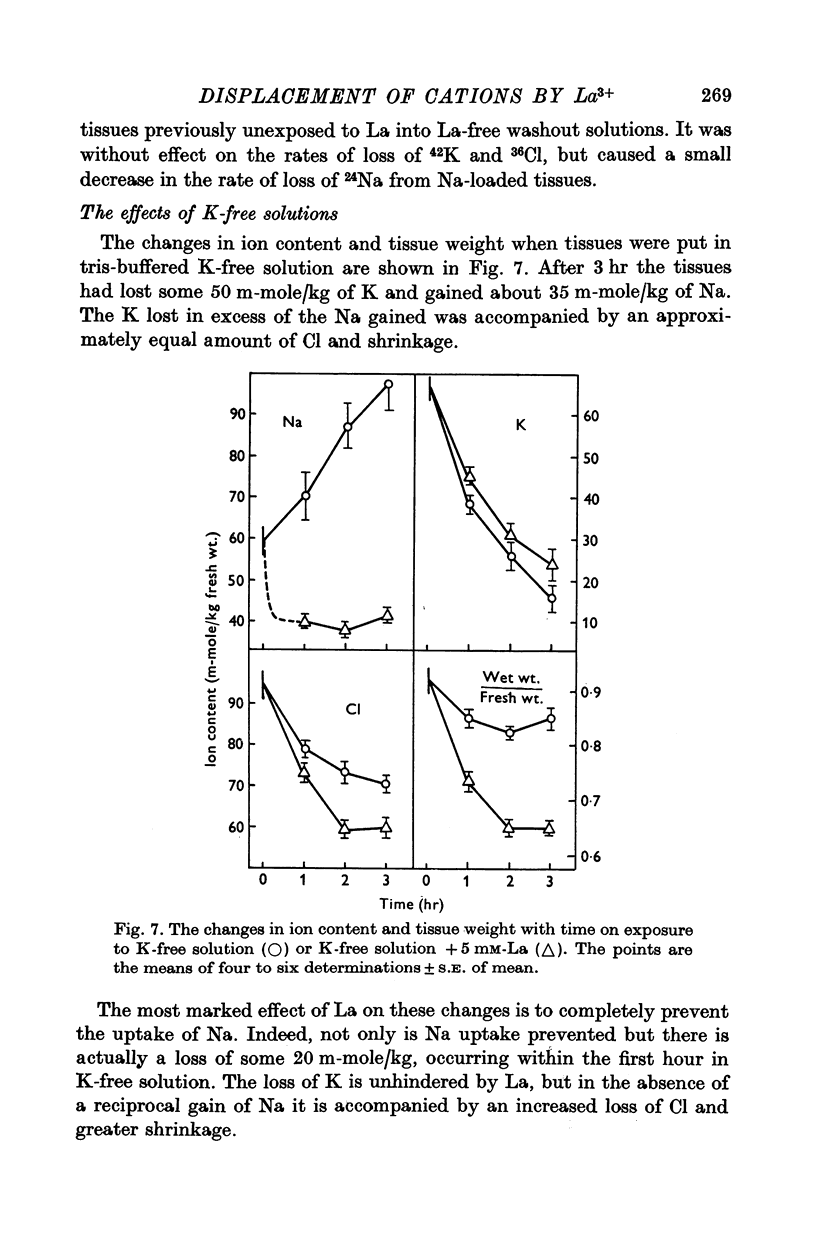
1. Tissues were allowed to equilibrate in a Tris-buffered Krebs solution and were then exposed to similar solutions containing up to 5 mM-La. La caused shrinkage and significant losses of tissue K, Na, Mg and Ca. The shrinkage was exactly accountable for by a reduction in the extracellular space (e.c.s.) as measured by [14C]sucrose. No significant change was seen in tissue Cl content. Exposure for 1 hr to 5 mM-La led to a total cation loss of 24-3 +/- 1-6 m-equiv/kg or, correcting for the small change in Cl content, a loss of positive charge of 23-8 +/- 2-2 m-equiv/kg fresh wt. 2. Using the radioisotope 140La it was shown that this loss of cation was balanced by an uptake of La3+. 3. Subtraction of the ions in the measured [14C]sucrose space from the total tissue ion contents led to estimates of the "cellular" ion contents. The effects of 1 hr exposure to 5 mM-La on these were a loss of 12-9 +/- 2-4 m-equiv/kg of cation and a gain of 10-0 +/- 2-6 m-equiv/kg of Cl. 4. Similar changes in ion content were produced by La on "Na-loaded" and "K-loaded" tissues, these being tissues which by exposure to K-free or Na-free (high K) solutions had replaced all their K with Na or vice versa. 5. The uptakes of 24Na and 36Cl by Na-loaded tissues were both describable as the sum of two exponentail processes: a fast component (t 1/2 congruent to 1/2 min), which was presumed to be extracellular and a slower, presumed transmembrane, component. La reduced the rapid component of uptake of 24Na by an amount greater than that predicted by the reduction in the e.c.s., the extra amount lost being some 10--15 m-equiv/kg. La also reduced the amount of rapidly exchanging 36Cl, bwt this reduction was entirely accounted for by the change in the e.c.s. La reduced the rate constant of the slow component of 24Na uptake. 6. La reduced the rapidly exchanging component of 42K uptake by normal tissues by an amount equivalent to about 0-5 m-mole/kg fresh wt. of K in excess of the change in the extracellular space. 7. La had little effect on the effluxes of 36Cl and 42K from normal tissues. However, it reduced the size of the fastest component of exchange of 42K efflux from K-loaded tissues by an amount equal to some 10-15 m-equiv/kg in excess of the reduction in the e.c.s. A similar reduction in the rapidly exchanging component of 24Na efflux from normal tissues was also seen. La slowed the efflux of 24Na from Na-loaded tissues at times when the tracer lost could safely be regarded as intracellular. 8. The taenia coli when exposed to K-free solutions gains Na and loses K. In the presence of La the gain in Na was completely blocked. K was still lost, however, being accompanied by Cl and increased shrinkage. La also prevented the uptake of Na from high Na media by ion-depleted tissues (produced by exposure to sucrose media), while having little effect on the uptake of K from high K media by such tissues. 9 Tedia by such tissues. 9. The cation displaced by La in excess of that lost due to the reduction of the e.c.s...
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brading A. F. A constant flow apparatus for measuring radioactive ion effluxes from guinea-pig taenia coli. J Physiol. 1967 Sep;192(2):15P–16P. [PubMed] [Google Scholar]
- Brading A. F. Analysis of the effluxes of sodium, potassium and chloride ions from smooth muscle in normal and hypertonic solutions. J Physiol. 1971 May;214(3):393–416. doi: 10.1113/jphysiol.1971.sp009440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F. Sodium/sodium exchange in the smooth muscle of the guinea-pig taenia coli. J Physiol. 1975 Sep;251(1):79–105. doi: 10.1113/jphysiol.1975.sp011082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton J., Godfraind T. Sodium-calcium sites in smooth muscle and their accessibility to lanthanum. J Physiol. 1974 Sep;241(2):287–298. doi: 10.1113/jphysiol.1974.sp010656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler D. E., Williams J. A. Pancreatic acinar cells: effects of lanthanum ions on amylase release and calcium ion fluxes. J Physiol. 1974 Dec;243(3):831–846. doi: 10.1113/jphysiol.1974.sp010779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODFORD P. J., HERMANSEN K. Sodium and potassium movements in the unstriated muscle of the guinea-pig taenia coli. J Physiol. 1961 Oct;158:426–448. doi: 10.1113/jphysiol.1961.sp006778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodford P. J. An interaction between potassium and sodium in the smooth muscle of the guinea-pig taenia coli. J Physiol. 1966 Sep;186(1):11–26. doi: 10.1113/jphysiol.1966.sp008017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson B. J., Kidwai A. M., Daniel E. E. Uptake of lanthanum by smooth muscle. Can J Physiol Pharmacol. 1972 Jul;50(7):730–733. doi: 10.1139/y72-107. [DOI] [PubMed] [Google Scholar]
- LETTVIN J. Y., PICKARD W. F., MCCULLOCH W. S., PITTS W. A THEORY OF PASSIVE ION FLUX THROUGH AXON MEMBRANES. Nature. 1964 Jun 27;202:1338–1339. doi: 10.1038/2021338a0. [DOI] [PubMed] [Google Scholar]
- Sparrow M. P. Interaction of 28Mg with Ca and K in the smooth muscle of guinea-pig taenia coli. J Physiol. 1969 Nov;205(1):19–38. doi: 10.1113/jphysiol.1969.sp008948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breemen C., Farinas B. R., Gerba P., McNaughton E. D. Excitation-contraction coupling in rabbit aorta studied by the lanthanum method for measuring cellular calcium influx. Circ Res. 1972 Jan;30(1):44–54. doi: 10.1161/01.res.30.1.44. [DOI] [PubMed] [Google Scholar]
- Van Breemen C., McNaughton E. The separation of cell membrane calcium transport from extracellular calcium exchange in vascular smooth muscle. Biochem Biophys Res Commun. 1970 May 22;39(4):567–574. doi: 10.1016/0006-291x(70)90241-x. [DOI] [PubMed] [Google Scholar]
- Van Breemen C. Permselectivity of a porous phospholipid-cholesterol artificial membrane. Calcium and lanthanum effects. Biochem Biophys Res Commun. 1968 Sep 30;32(6):977–983. doi: 10.1016/0006-291x(68)90124-1. [DOI] [PubMed] [Google Scholar]
- Widdicombe J. H. Proceedings: The effect of lanthanum on ion content and movement in the guinea-pig's taenia coli. J Physiol. 1974 Sep;241(2):106P–107P. [PubMed] [Google Scholar]


