Abstract
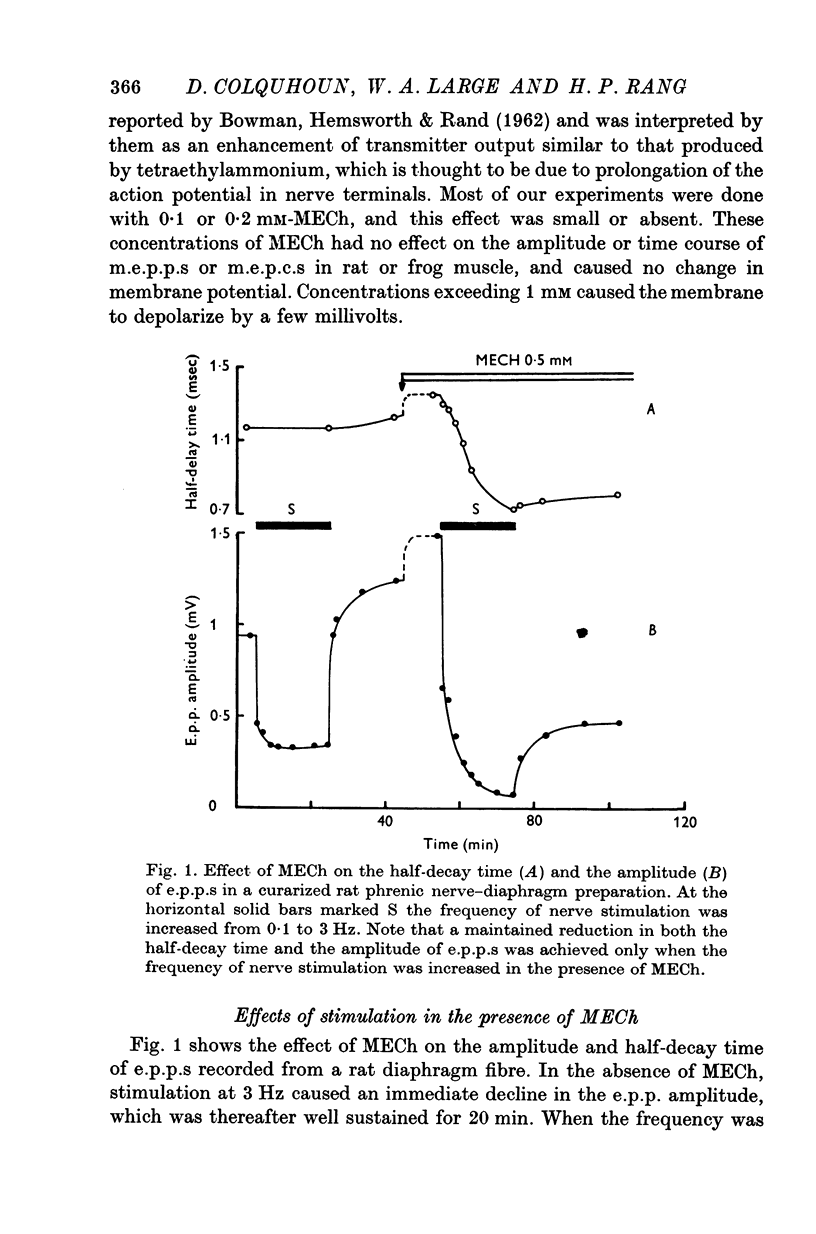
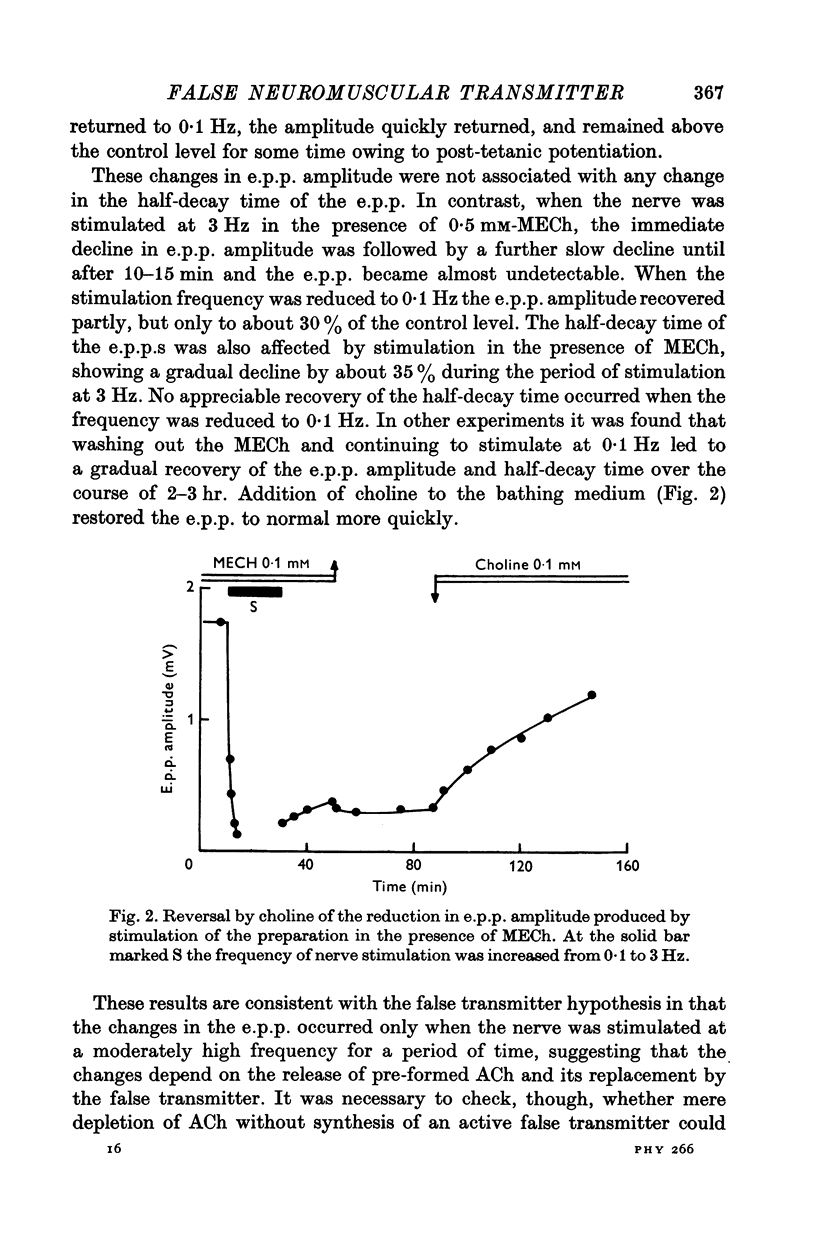
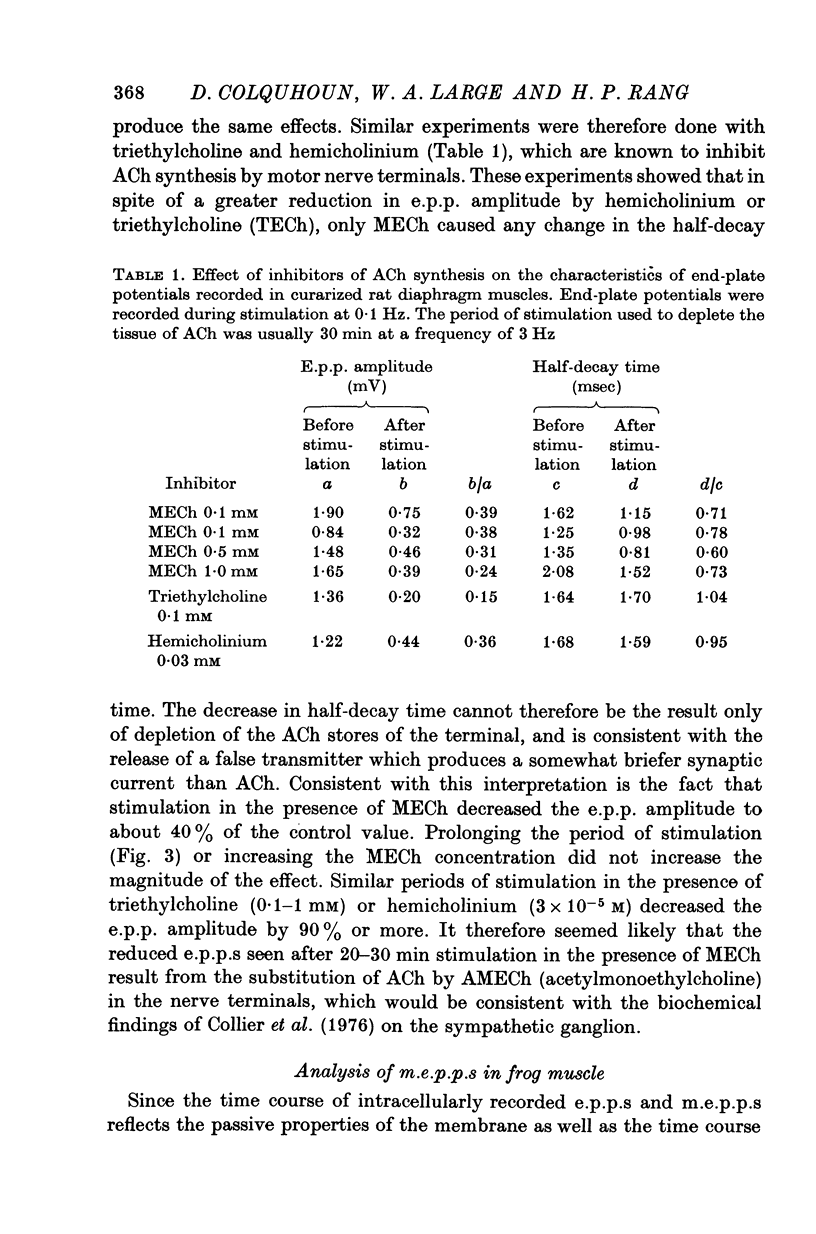
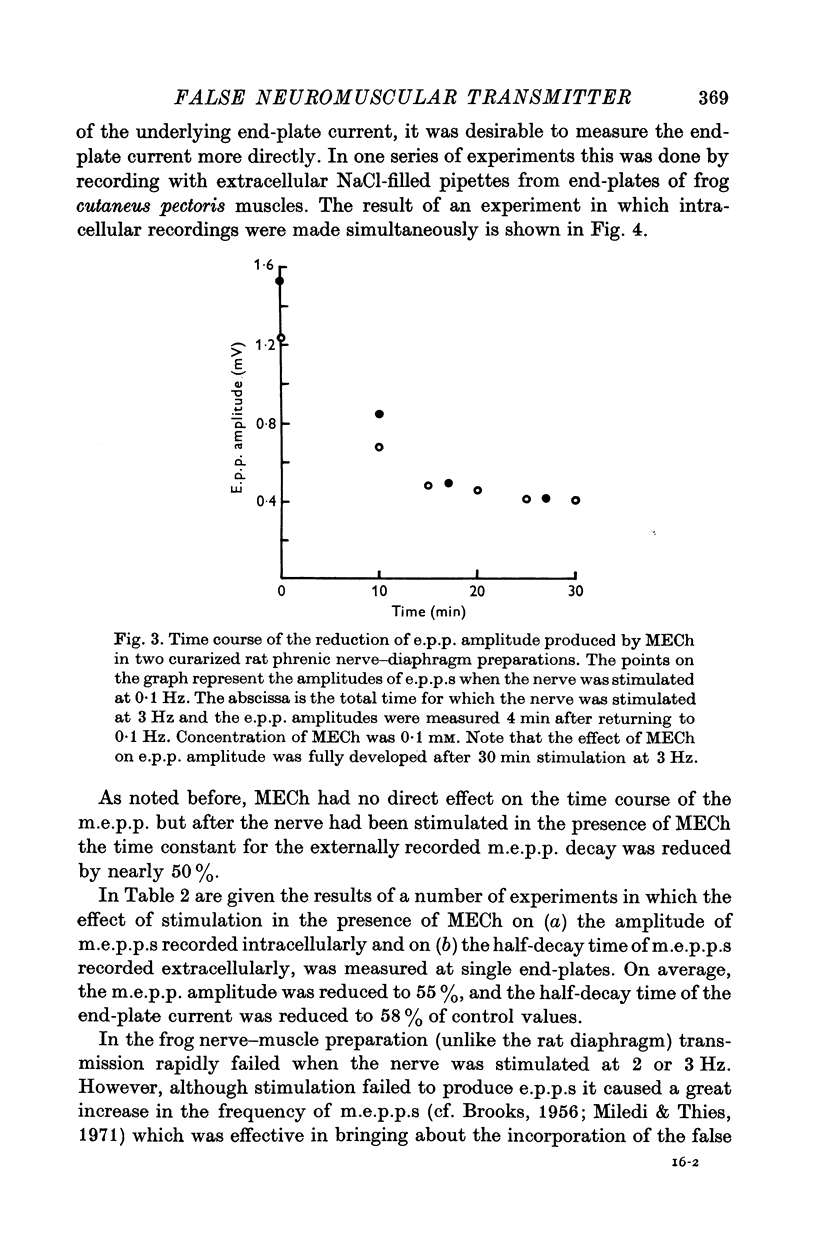
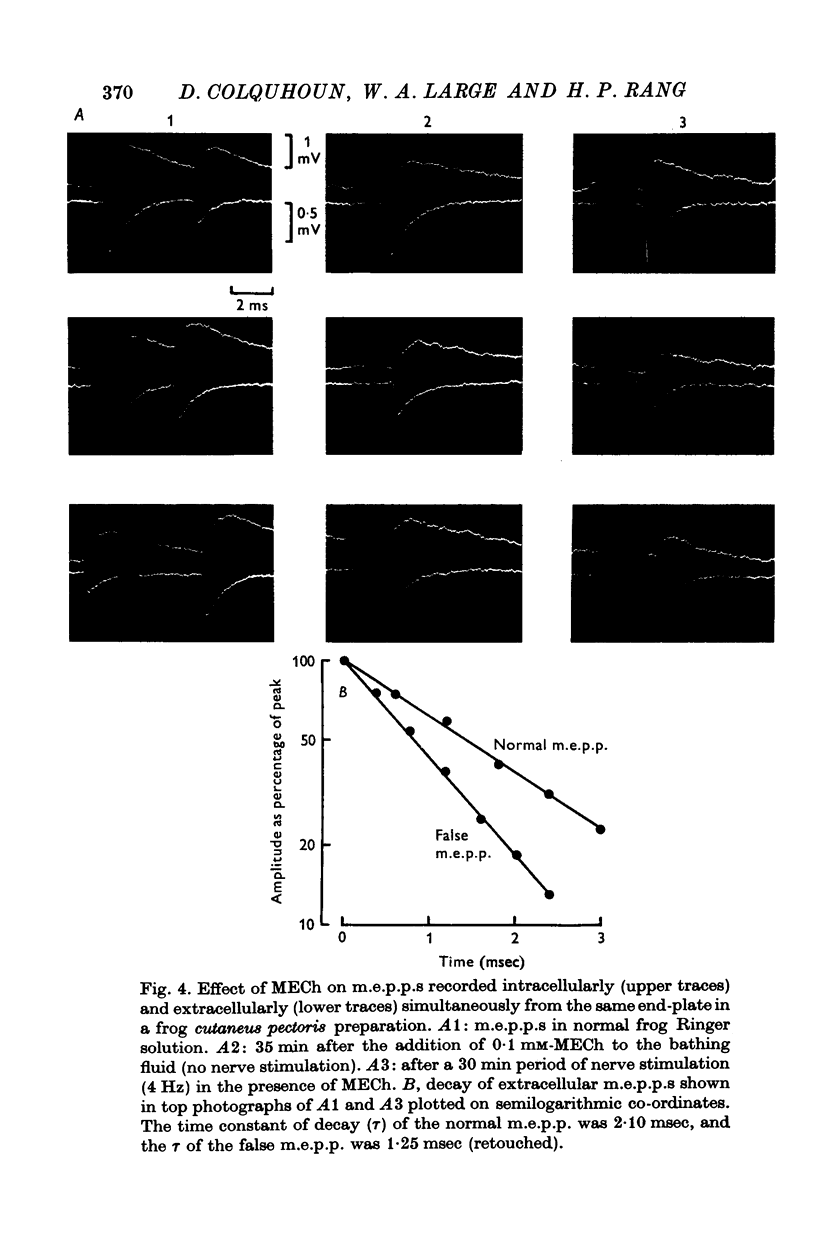
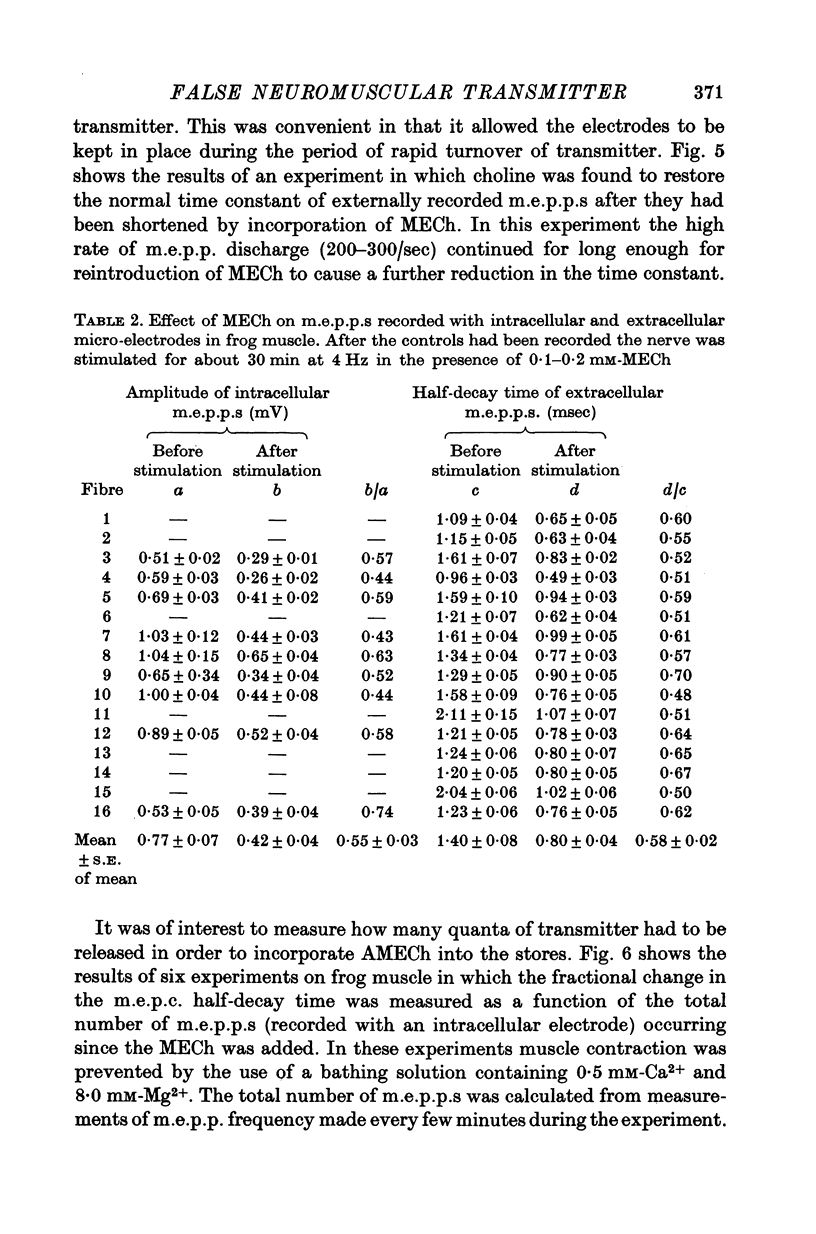
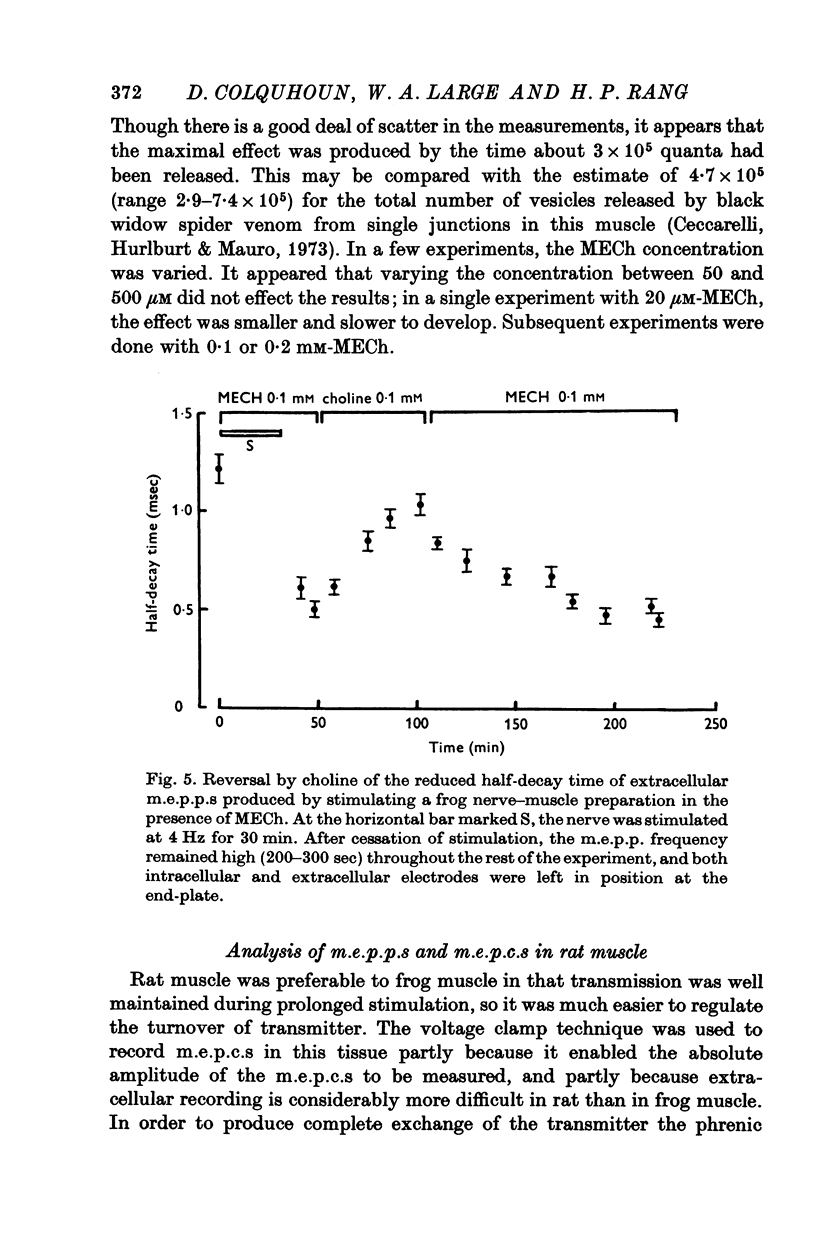
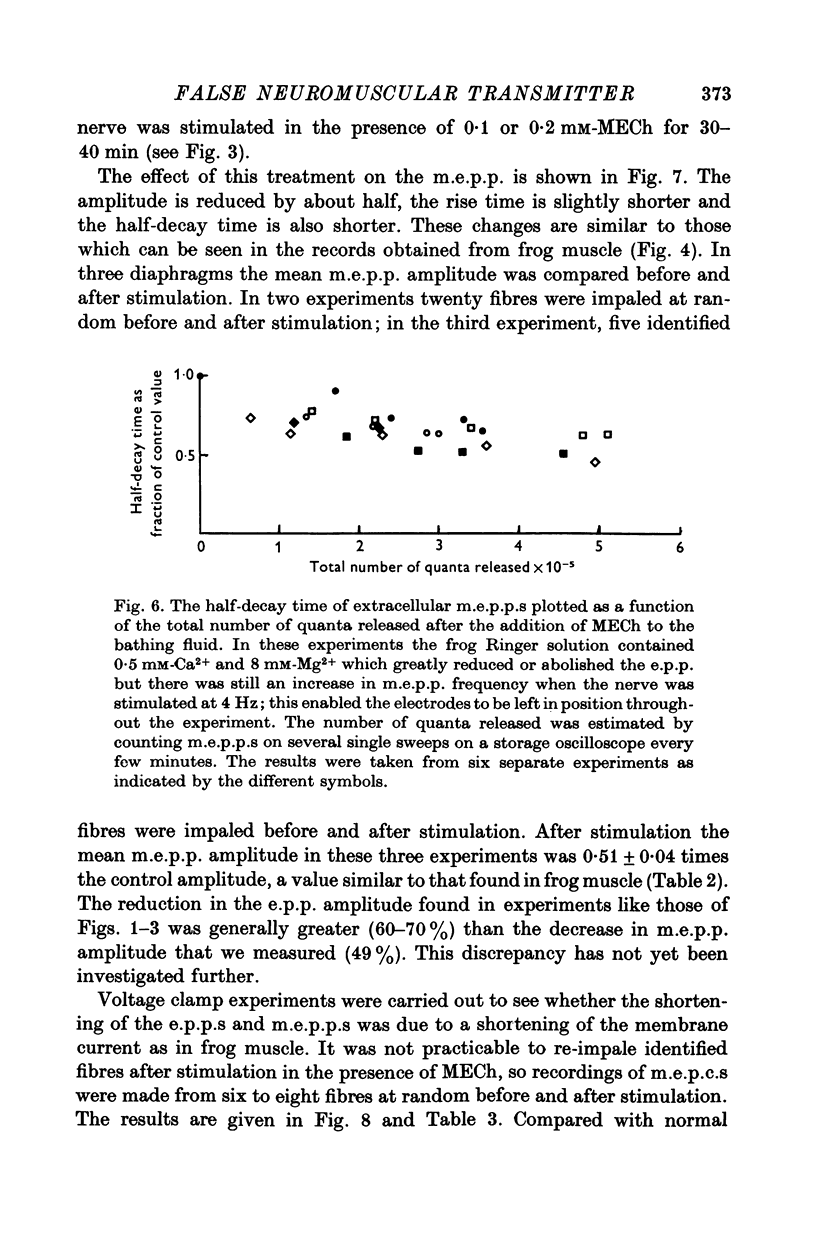
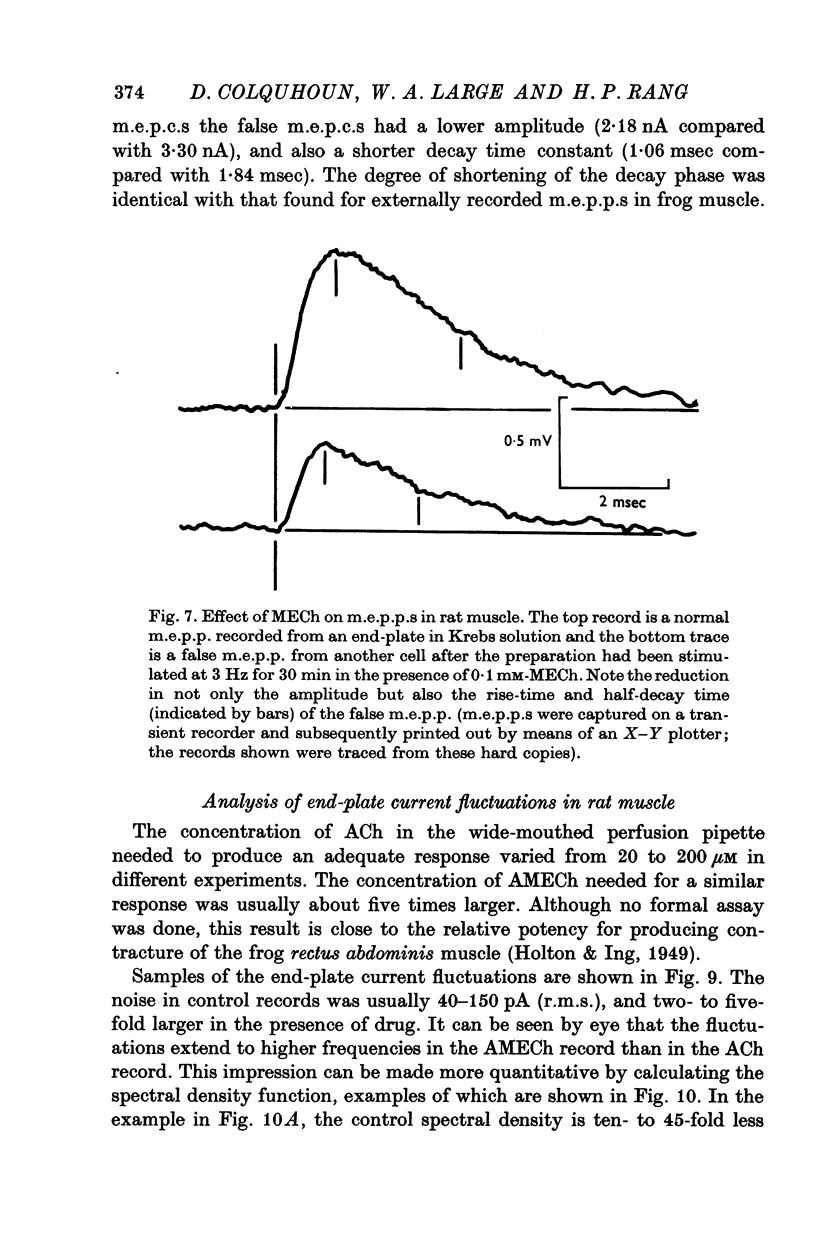
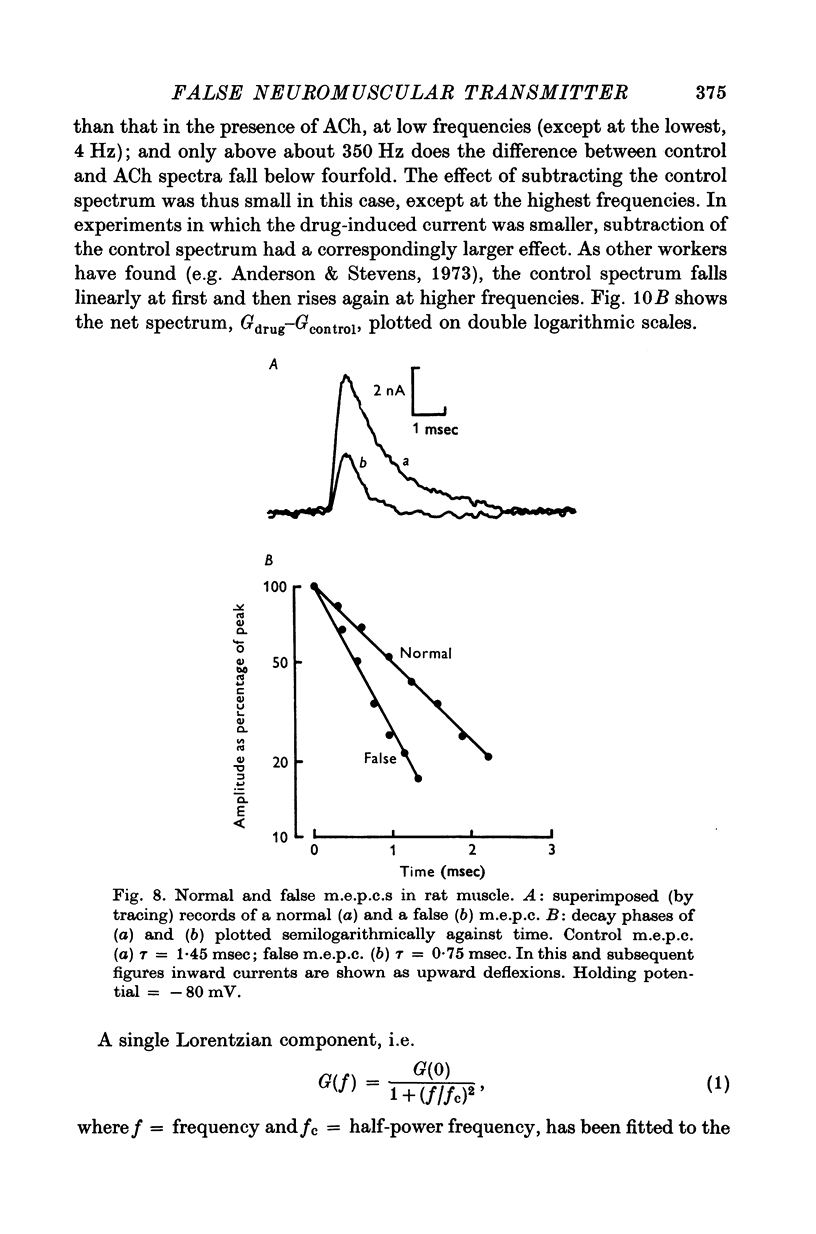
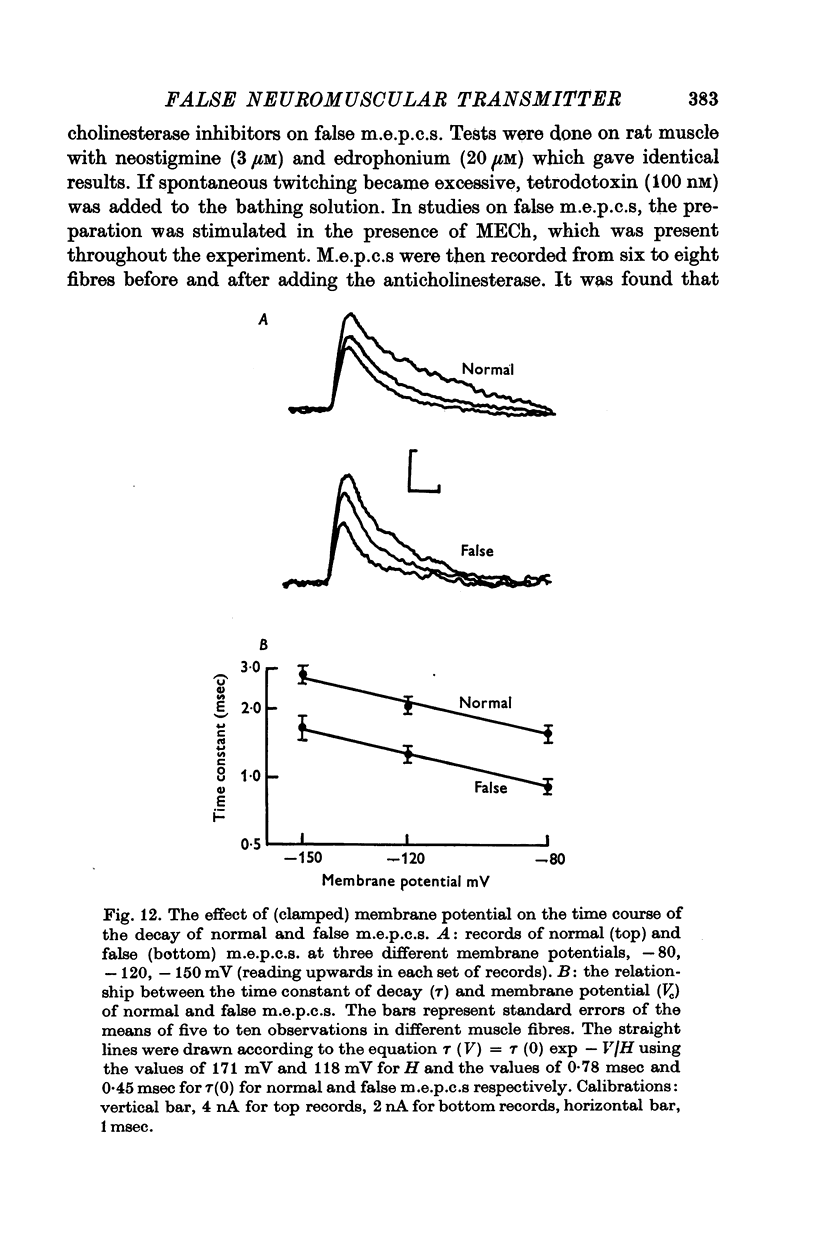
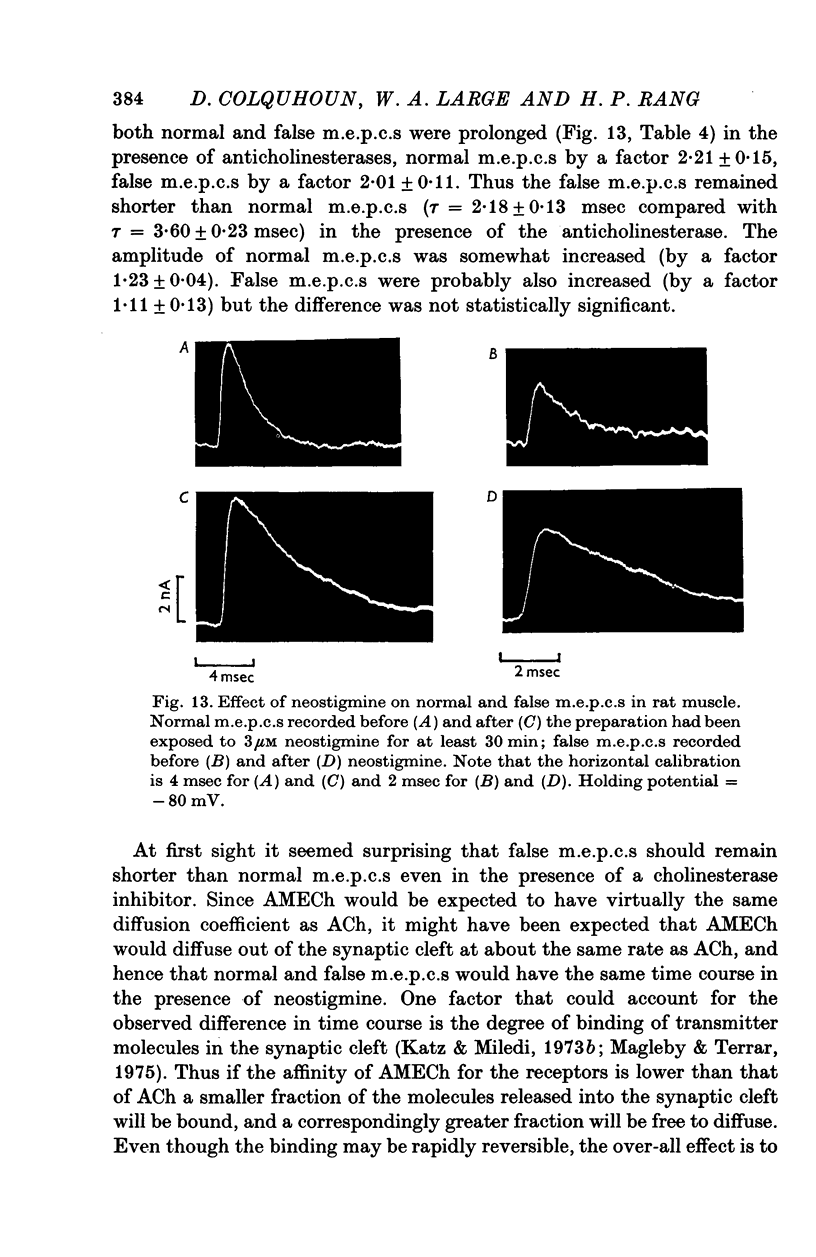
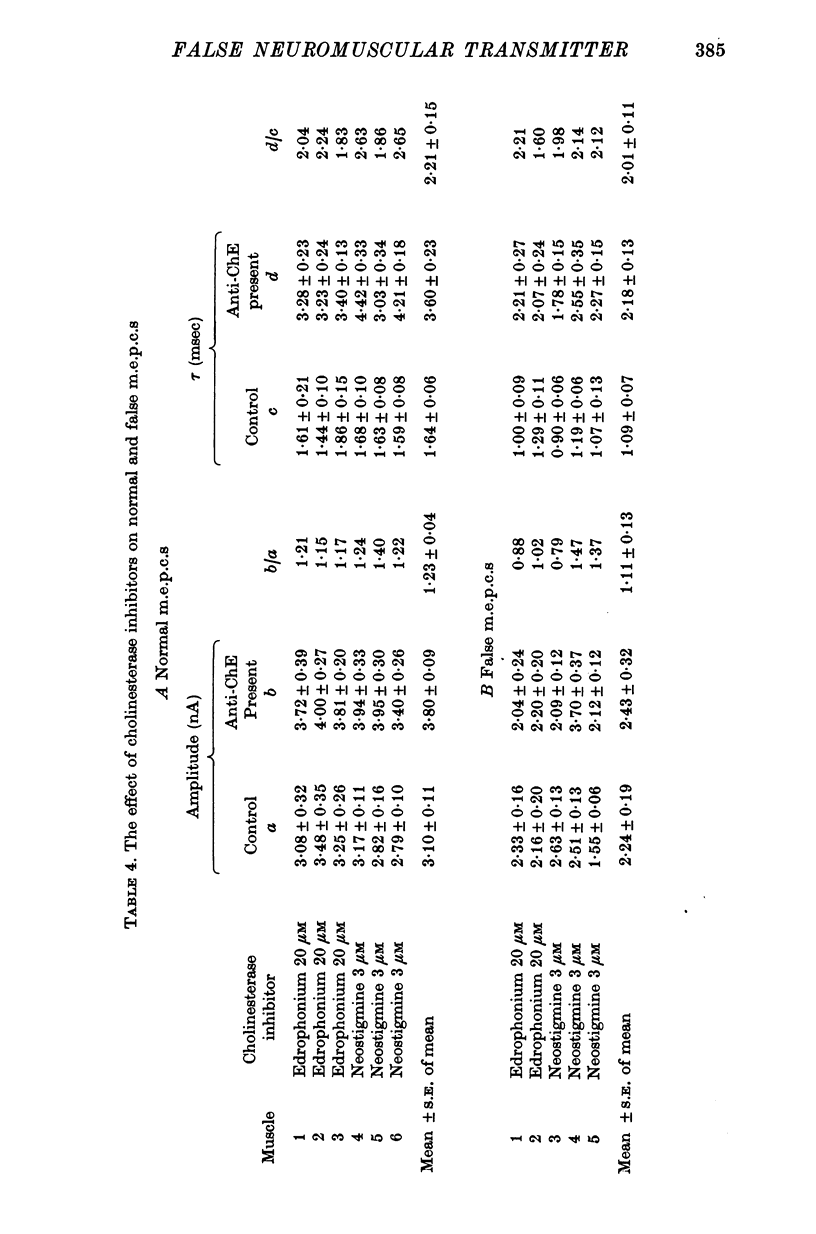
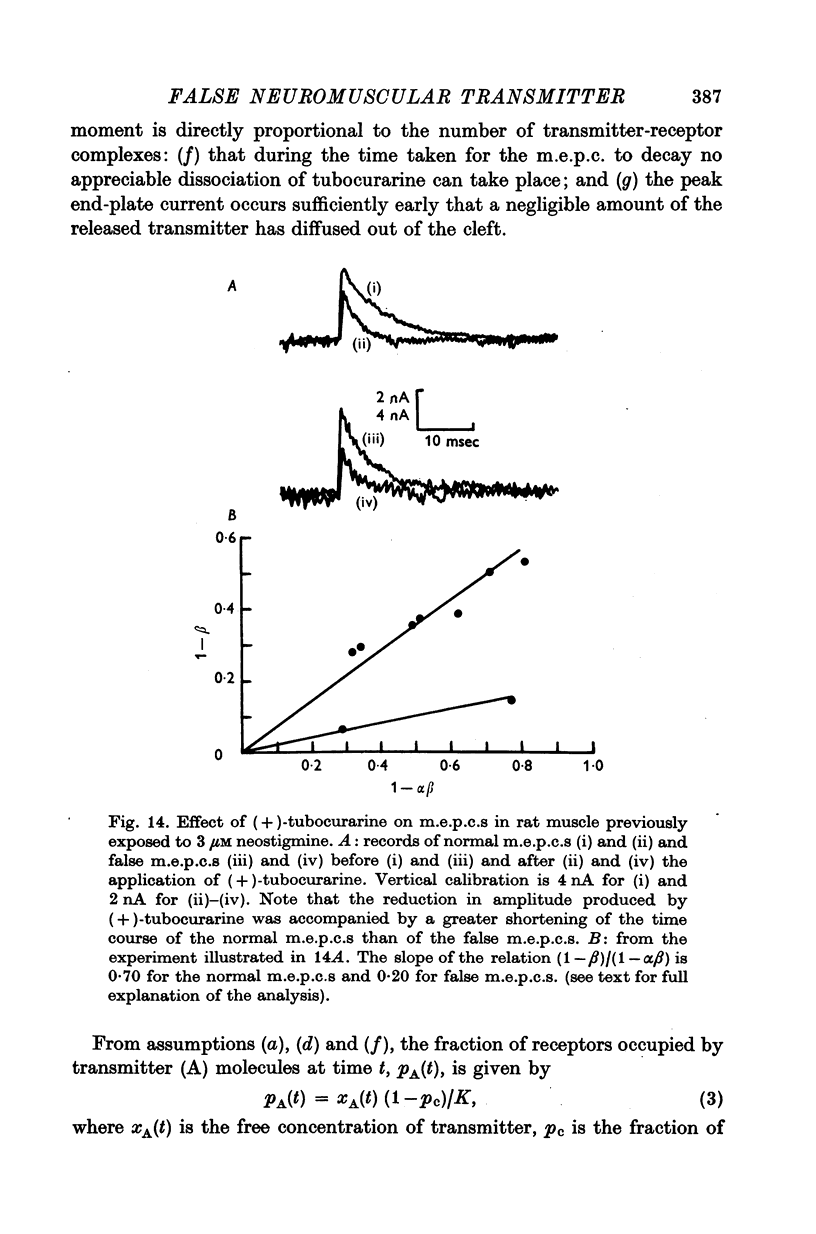
1. The action of monoethylcholine (MECh) on neuromuscular transmission has been studied by electrophysiological methods. 2. End-plate potentials (e.p.p.s.) in curarized rat muscle were unaffected or slightly increased in amplitude by MECh (0-1-1 mM). Stimulation at 3 Hz for about 30 min in the presence of MECh caused a progressive decline in e.p.p. amplitude, and a shortening of the e.p.p. time course. These changes were reversed by addition of choline to the medium. Similar changes in amplitude, but no change in time course, occurred when the preparation was stimulated in the presence of hemicholinium or triethylcholine. 3. Extracellular recordings of miniature end-plate potentials in frog muscle showed that stimulation in the presence of MECh caused the time constant of the exponential decay of the m.e.p.p.s. to decrease by 42%. The amplitude of intracellular m.e.p.p.s. was reduced by 45%. These changes were maximal by the time about 3 X 10(5) quanta had been released. 4. Voltage clamp experiments in rat muscle in which miniature end-plate currents (m.e.p.c.s) were recorded showed that stimulation in the presence of MECh reduced the amplitude (by 33%) and the decay time constant (by 42%). 5. Analysis of end-plate current flucutations produced by local application of acetylcholine (ACh) and acetylmonoethycholine (AMECh) to voltage clamped rat end-plates showed that the amplitude of the elementary current events was the same for both compounds whereas the average channel lifetime was 44% shorter for AMECh than for ACh. 6. The voltage-sensitivity of the channel lifetime (measured from end-plate current fluctuations) was the same for ACh and AMECh. The voltage-sensitivity of the m.e.p.c. decay time constant was the same as that found from noise measurements. The shortened m.e.p.c.s. (false m.e.p.c.s.) occurring after stimulation in the presence of MECh also showed the same voltage-sensitivity. 7. Both normal and false m.e.p.c.s. were prolonged by neostigmine by almost the same factor; false m.e.p.c.s. were thus shorter than normal m.e.p.c.s. even when cholinesterase was inactivated. Experiments with progressive curarization of neostigmine-treated end-plates suggested that the fraction of transmitter molecules bound is smaller for false than for normal m.e.p.c.s. The difference implies that the false transmitter has one quarter of the affinity of ACh for the receptors. 8. It is concluded that stimulation in the presence of MECh gives rise to a false transmitter, presumably AMECh, which has a lower affinity for receptors than ACh, and gives rise to ionic channels with a shorter average lifetime than those activated by ACh.
Full text
PDF




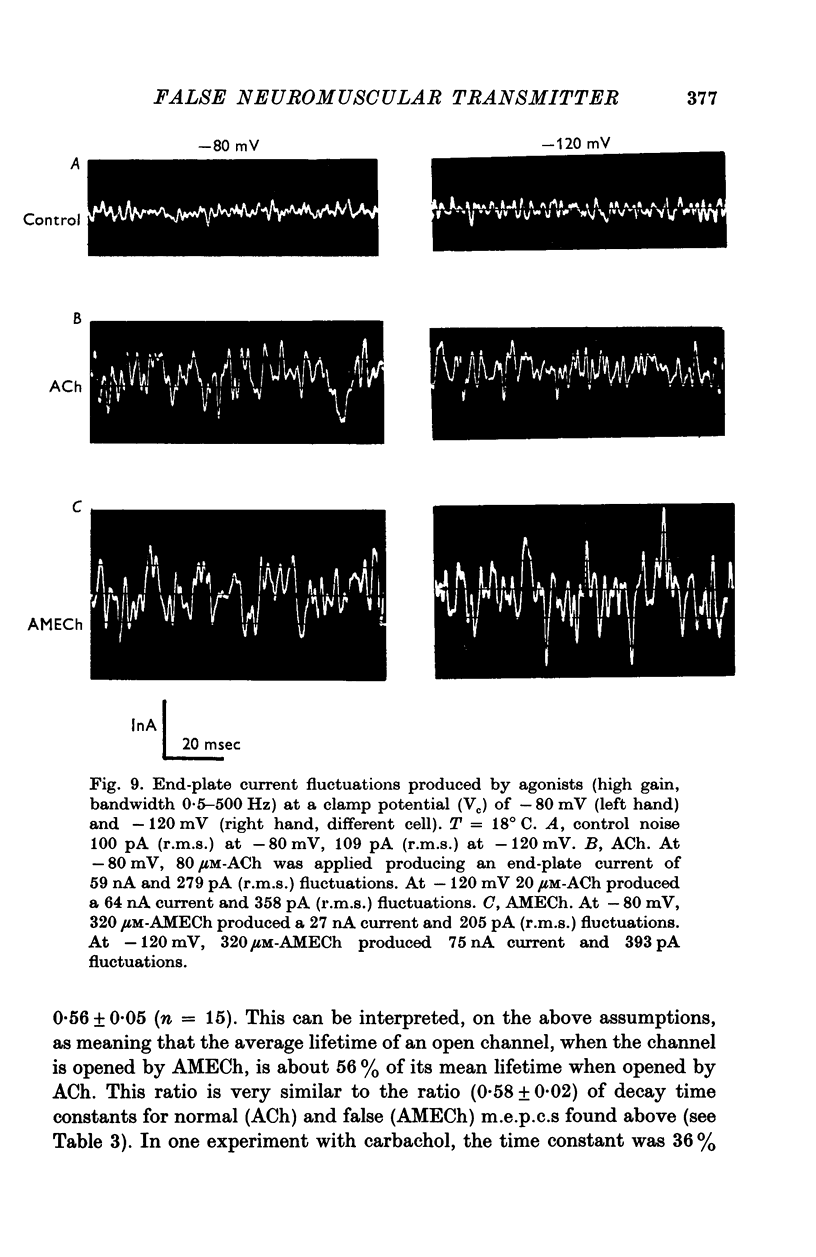
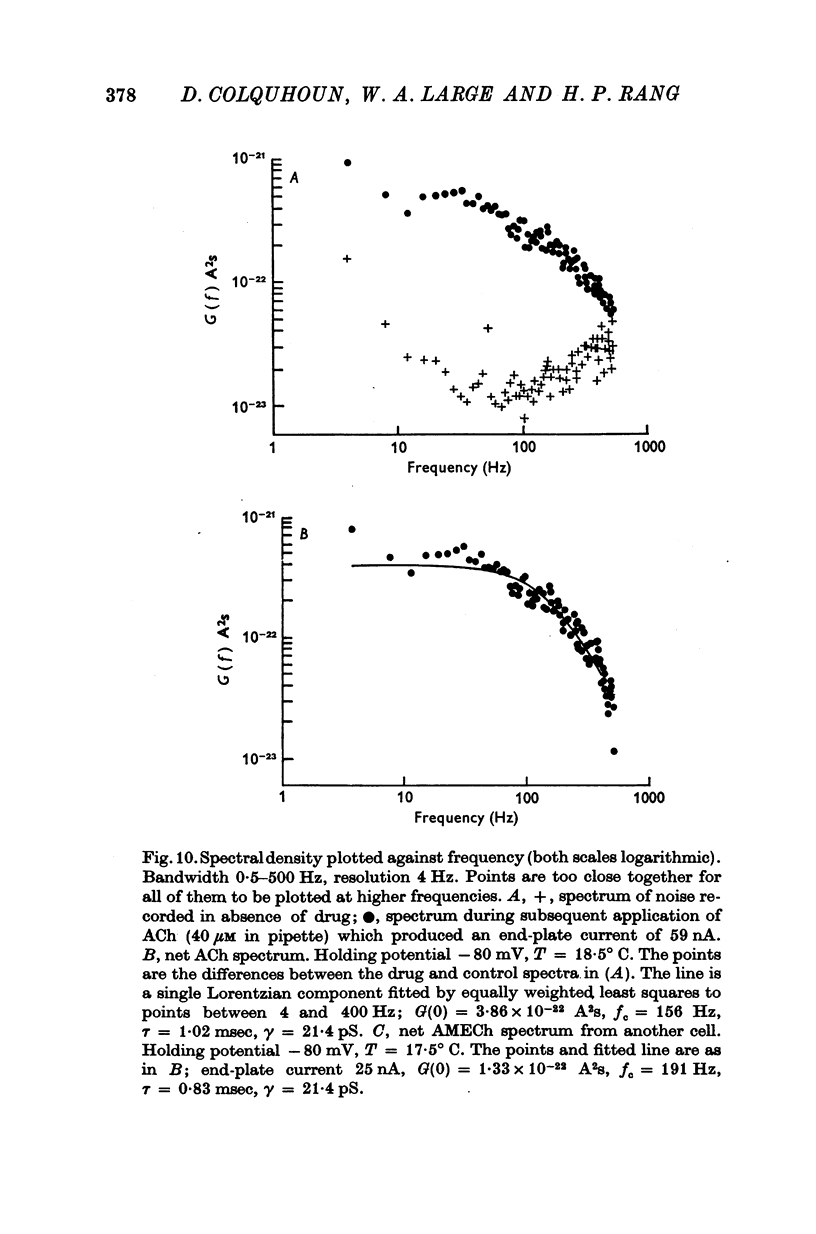
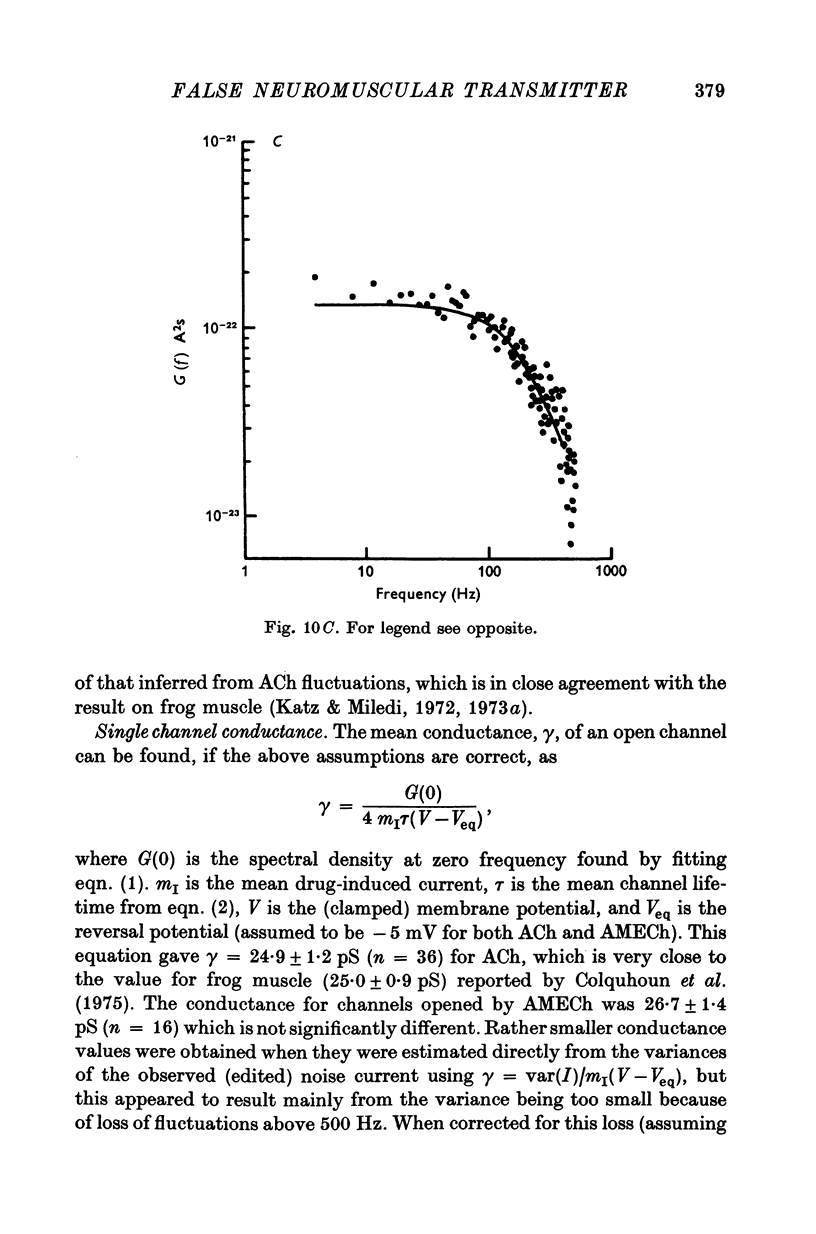


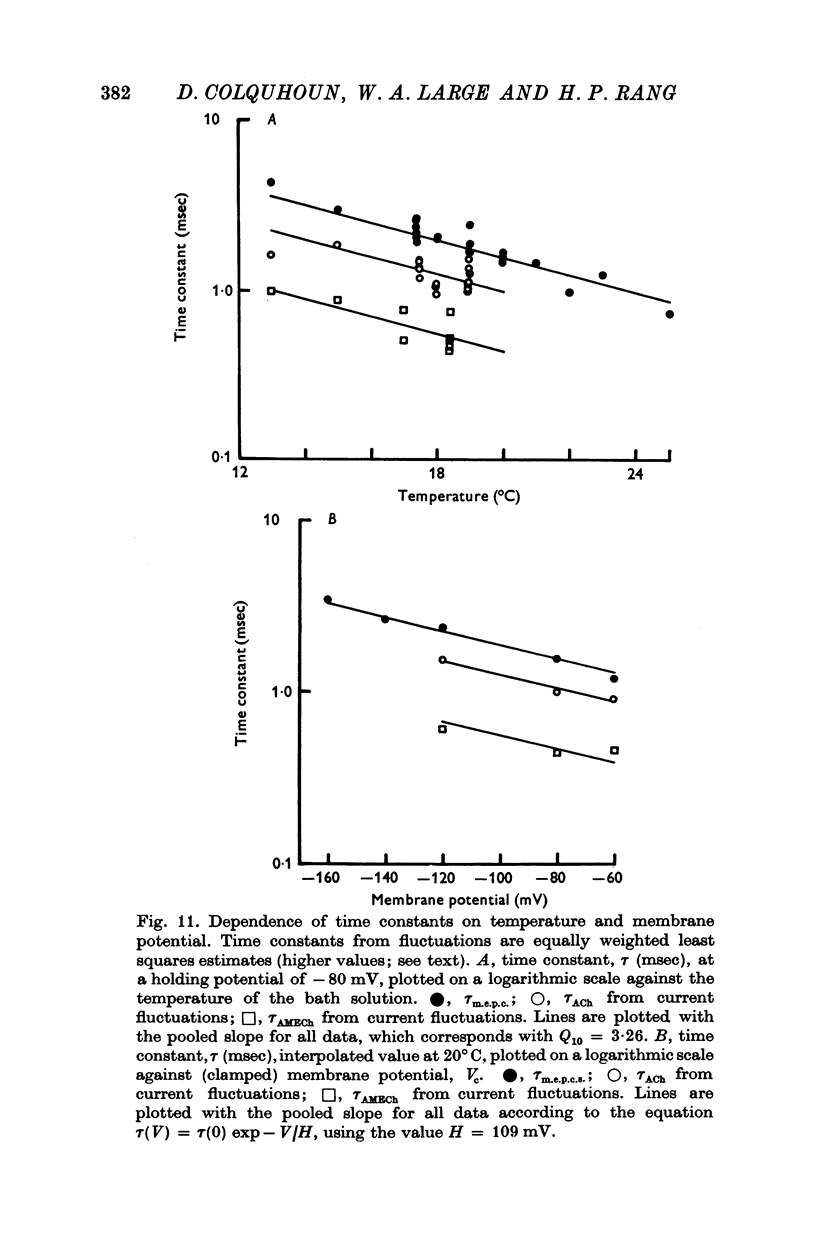









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOWMAN W. C., HEMSWORTH B. A., RAND M. J. Triethylcholine compared with other substances affecting neuromuscular transmission. Br J Pharmacol Chemother. 1962 Aug;19:198–218. doi: 10.1111/j.1476-5381.1962.tb01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROOKS V. B. An intracellular study of the action of repetitive nerve volleys and of botulinum toxin on miniature end-plate potentials. J Physiol. 1956 Nov 28;134(2):264–277. doi: 10.1113/jphysiol.1956.sp005642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker L. A., Mittag T. W. Comparative studies of substrates and inhibitors of choline transport and choline acetyltransferase. J Pharmacol Exp Ther. 1975 Jan;192(1):86–94. [PubMed] [Google Scholar]
- Ceccarelli B., Hurlbut W. P., Mauro A. Turnover of transmitter and synaptic vesicles at the frog neuromuscular junction. J Cell Biol. 1973 May;57(2):499–524. doi: 10.1083/jcb.57.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier B., Barker L. A., Mittag T. W. The release of acetylated choline analogues by a sympathetic ganglion. Mol Pharmacol. 1976 Mar;12(2):340–344. [PubMed] [Google Scholar]
- Colquhoun D., Dionne V. E., Steinbach J. H., Stevens C. F. Conductance of channels opened by acetylcholine-like drugs in muscle end-plate. Nature. 1975 Jan 17;253(5488):204–206. doi: 10.1038/253204a0. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Henderson R., Ritchie J. M. The binding of labelled tetrodotoxin to non-myelinated nerve fibres. J Physiol. 1972 Dec;227(1):95–126. doi: 10.1113/jphysiol.1972.sp010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Ritchie J. M. The interaction at equilibrium between tetrodotoxin and mammalian non-myelinated nerve fibres. J Physiol. 1972 Mar;221(3):533–553. doi: 10.1113/jphysiol.1972.sp009766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. D., Quastel D. M. Transmitter release by mammalian motor nerve terminals in response to focal polarization. J Physiol. 1973 Jan;228(2):377–405. doi: 10.1113/jphysiol.1973.sp010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne V. E., Stevens C. F. Voltage dependence of agonist effectiveness at the frog neuromuscular junction: resolution of a paradox. J Physiol. 1975 Oct;251(2):245–270. doi: 10.1113/jphysiol.1975.sp011090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELMQVIST D., QUASTEL D. M. PRESYNAPTIC ACTION OF HEMICHOLINIUM AT THE NEUROMUSCULAR JUNCTION. J Physiol. 1965 Apr;177:463–482. doi: 10.1113/jphysiol.1965.sp007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenberg L., Heimbürger G., Nilsson C., Sörbo B. Biochemical and pharmacological studies on the sulfonium analogues of choline and acetylcholine. Eur J Pharmacol. 1973 Jul;23(1):37–46. doi: 10.1016/0014-2999(73)90242-2. [DOI] [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N. Effects of membrane potential, temperature and neostigmine on the conductance change caused by a quantum or acetylcholine at the toad neuromuscular junction. J Physiol. 1975 Jan;244(2):385–407. doi: 10.1113/jphysiol.1975.sp010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilson D., Collier B. Triethylcholine as a precursor to a cholinergic false transmitter. Nature. 1975 Apr 17;254(5501):618–619. doi: 10.1038/254618a0. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973 Jun;231(3):549–574. doi: 10.1113/jphysiol.1973.sp010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The characteristics of 'end-plate noise' produced by different depolarizing drugs. J Physiol. 1973 May;230(3):707–717. doi: 10.1113/jphysiol.1973.sp010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordas M. An attempt at an analysis of the factors determining the time course of the end-plate current. II. Temperature. J Physiol. 1972 Jul;224(2):333–348. doi: 10.1113/jphysiol.1972.sp009898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large W. A., Rang H. P. Proceedings: A false transmitter at the neuromuscular junction. J Physiol. 1976 Jun;258(2):105P–106P. [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Terrar D. A. Factors affecting the time course of decay of end-plate currents: a possible cooperative action of acetylcholine on receptors at the frog neuromuscular junction. J Physiol. 1975 Jan;244(2):467–495. doi: 10.1113/jphysiol.1975.sp010808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Thies R. Tetanic and post-tetanic rise in frequency of miniature end-plate potentials in low-calcium solutions. J Physiol. 1971 Jan;212(1):245–257. doi: 10.1113/jphysiol.1971.sp009320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976 Apr 29;260(5554):799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- REITZEL N. L., LONG J. P. Hemicholinium antagonism by choline analogues. J Pharmacol Exp Ther. 1959 Sep;127:15–21. [PubMed] [Google Scholar]
- Rang H. P. Acetylcholine receptors. Q Rev Biophys. 1974 Jul;7(3):283–399. doi: 10.1017/s0033583500001463. [DOI] [PubMed] [Google Scholar]
- Rang H. P. The kinetics of action of acetylcholine antagonists in smooth muscle. Proc R Soc Lond B Biol Sci. 1966 Apr 19;164(996):488–510. doi: 10.1098/rspb.1966.0045. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Active phase of frog's end-plate potential. J Neurophysiol. 1959 Jul;22(4):395–411. doi: 10.1152/jn.1959.22.4.395. [DOI] [PubMed] [Google Scholar]