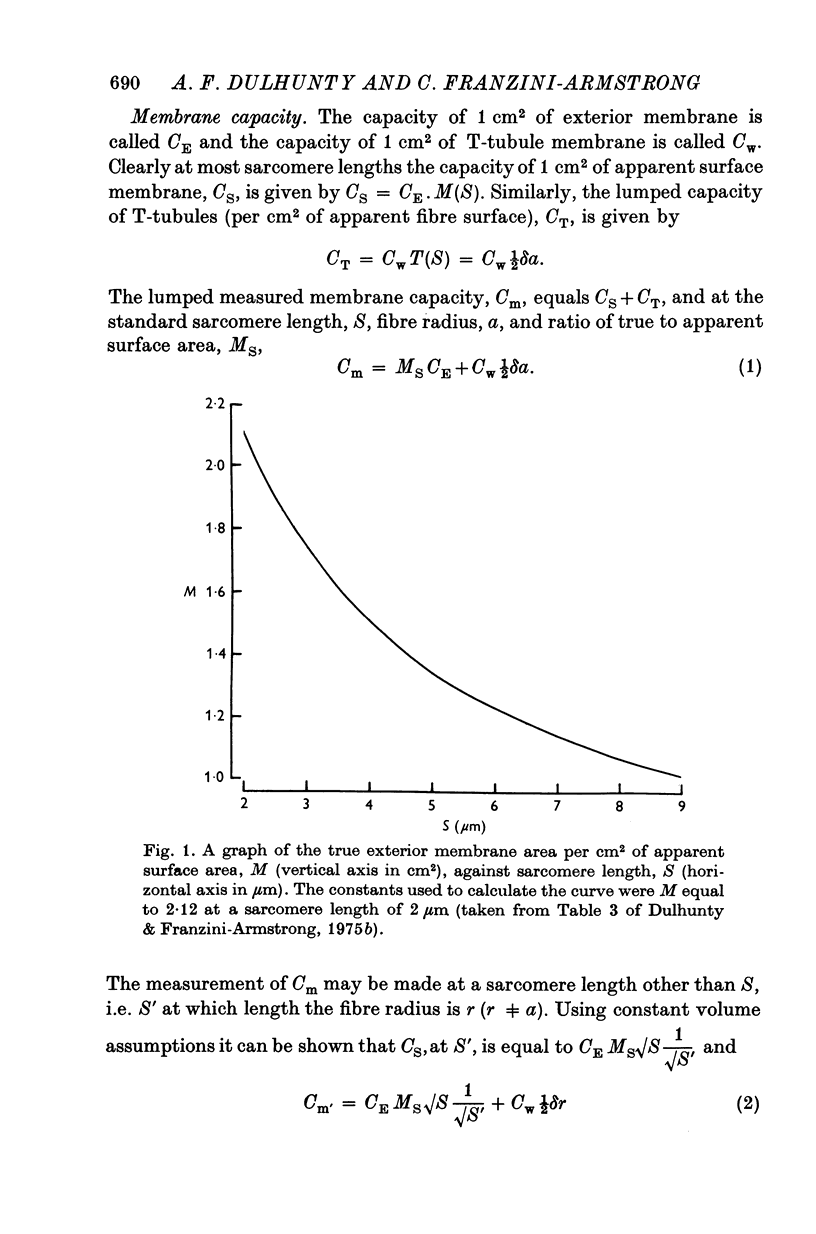
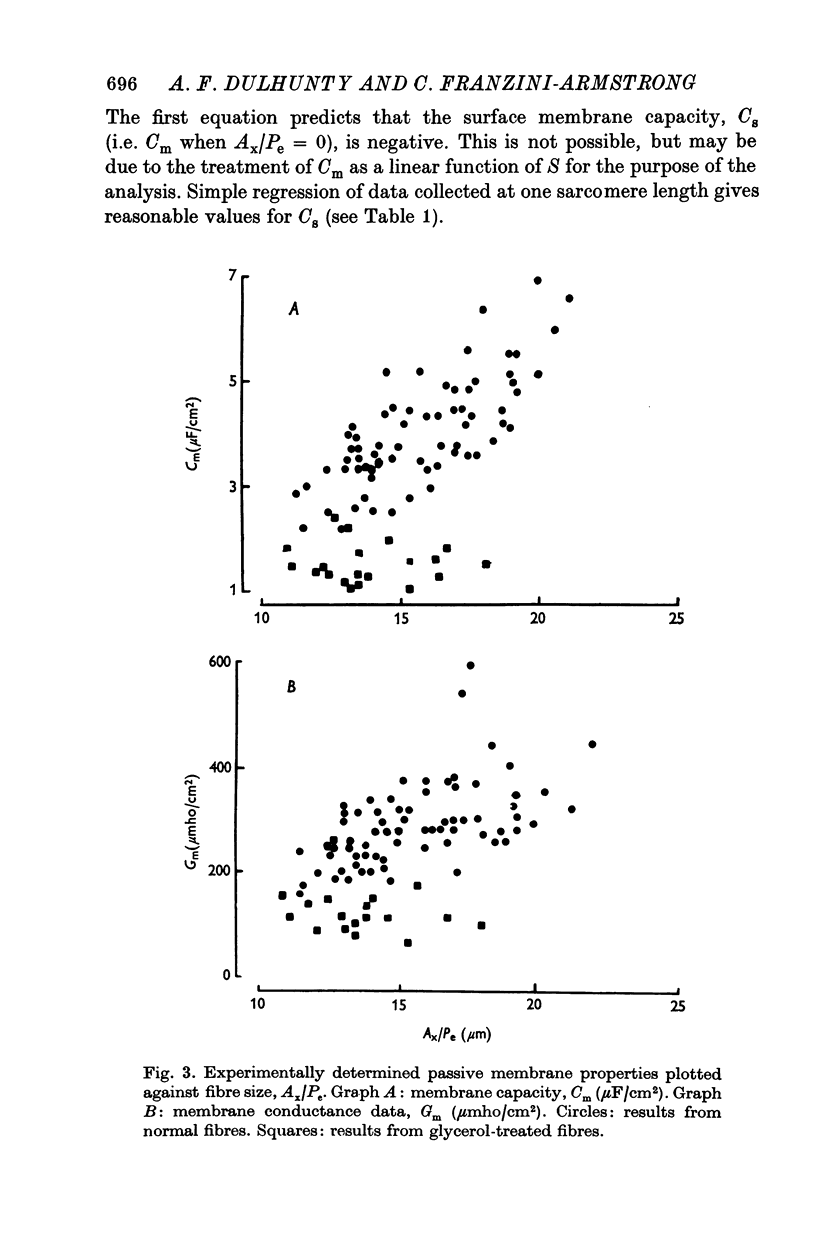
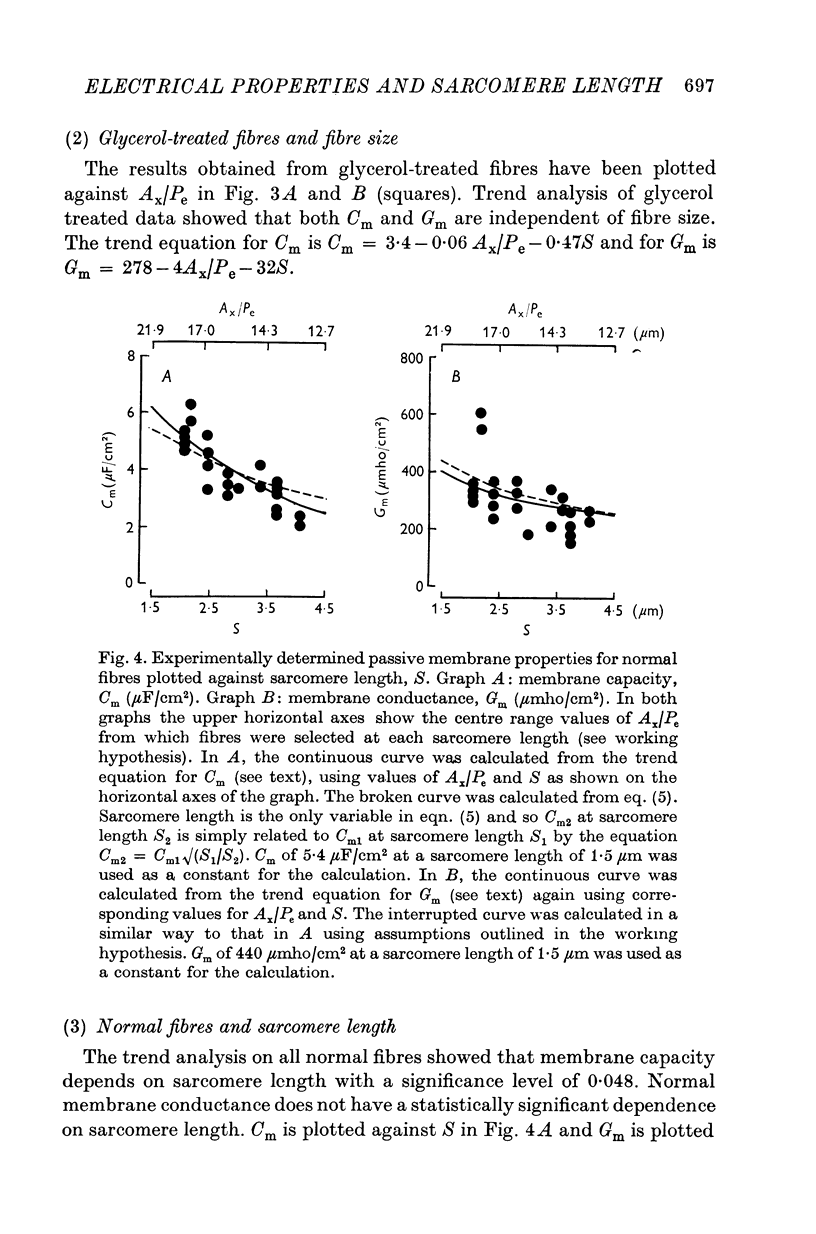
Abstract
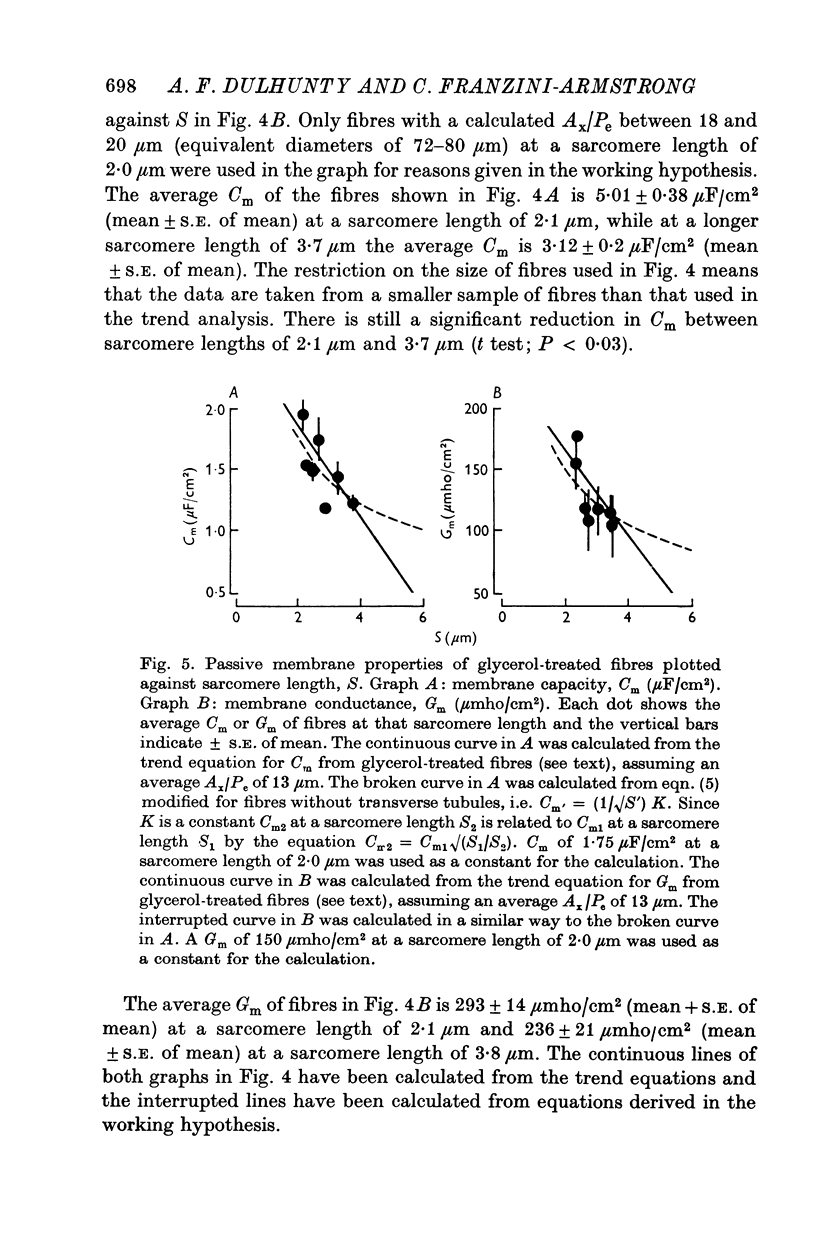
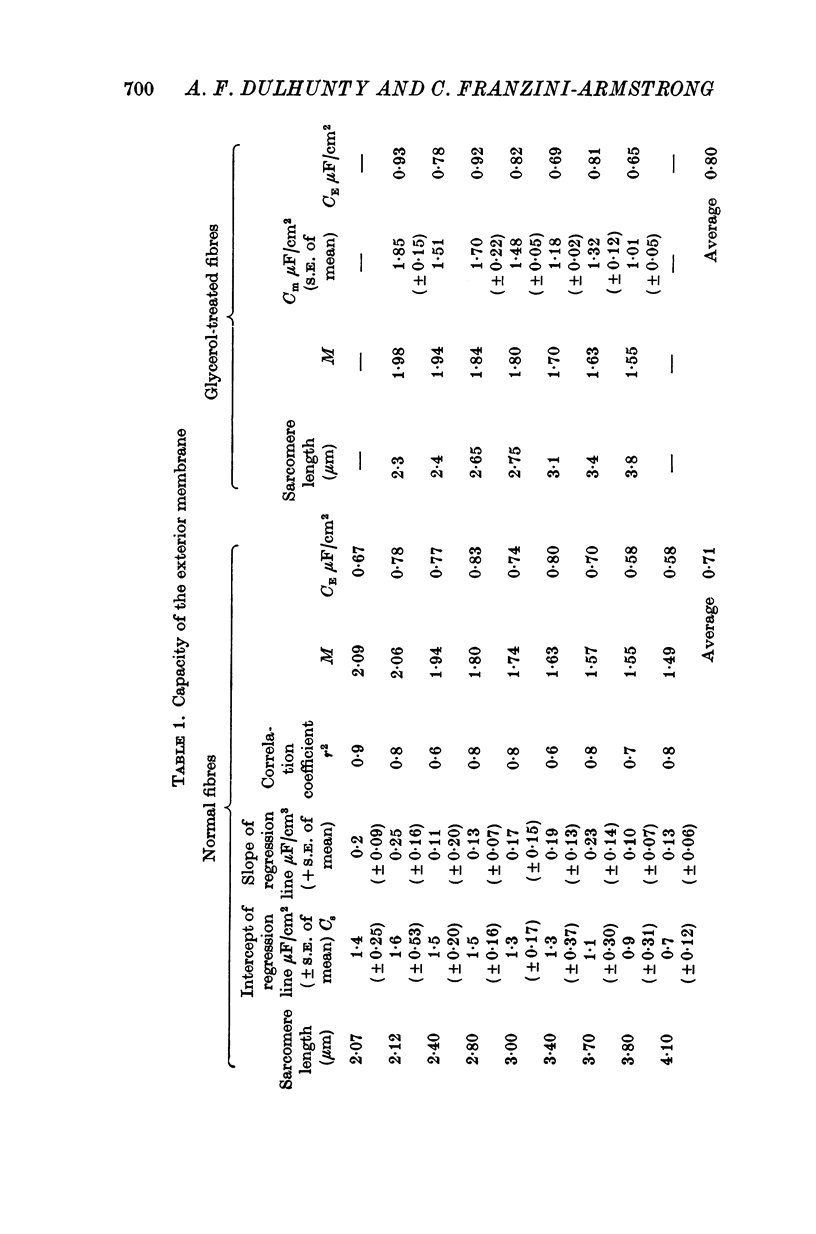
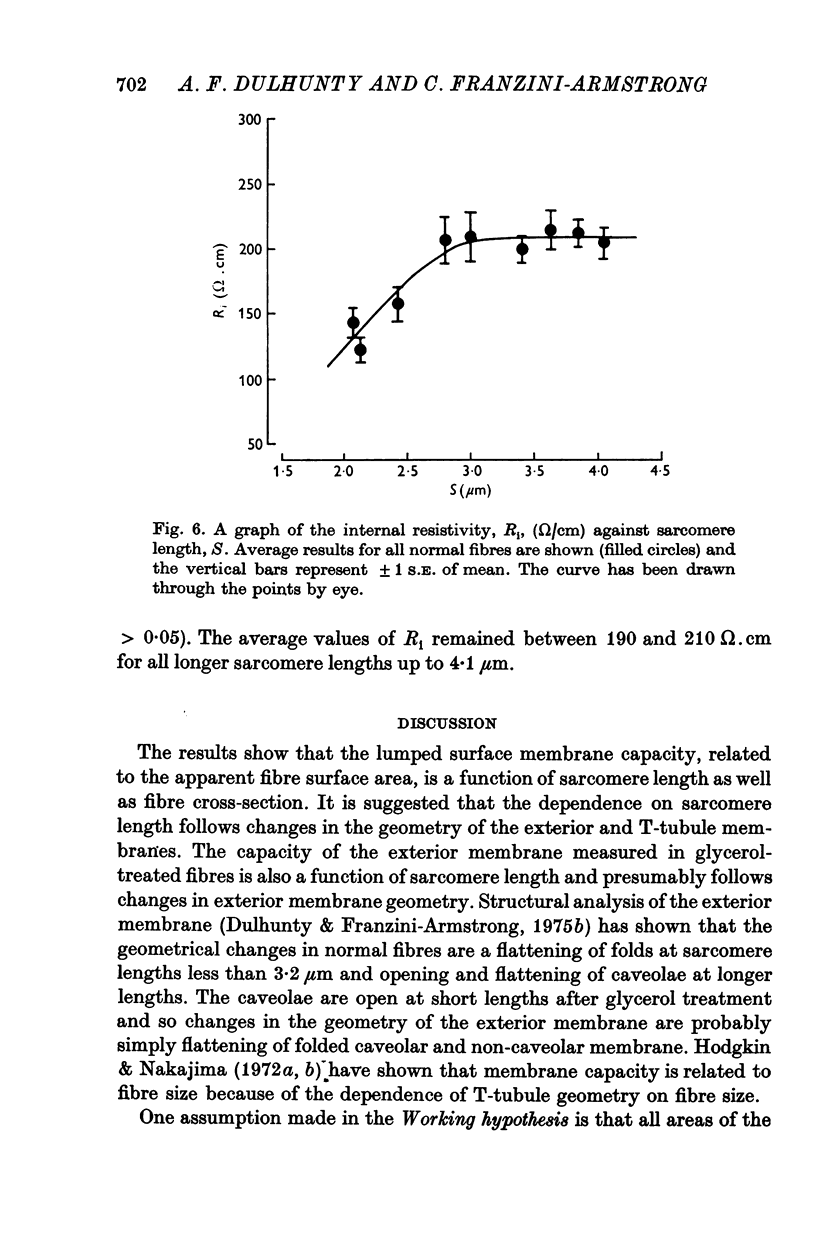
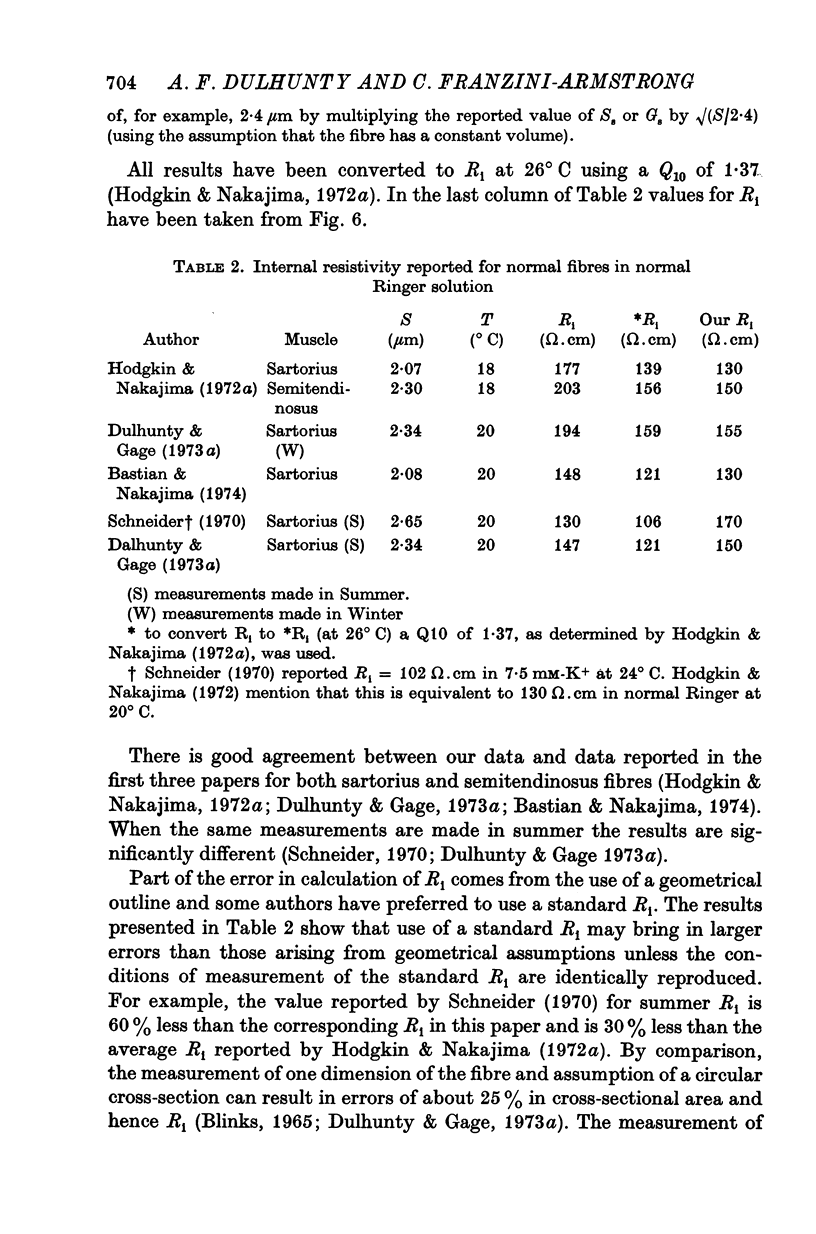
1. The passive electrical properties of frog skeletal muscle fibres have been measured at a number of different sarcomere lengths (from 2-1 to 4-0 micron). The geometrical outline of each fibre was determined from optical cross-sections and sarcomere length was measured by laser beam diffraction. 2. When fibres were stretched to long sarcomere lengths the membrane capacity, Cm, of both normal and detubulated (glycerol-treated) fibres was significantly less than the Cm of fibres at rest length. A significant reduction in membrane conductance of fibres held at long sarcomere lengths was only seen with detubulated fibres. 3. Membrane capacity and membrane conductance have a significant dependence on the cross-sectional area of normal fibres but are independent of cross-sectional area after detubulation. 4. It has been shown that membrane geometry depends on the sarcomere length of the fibre and it is suggested that the passive membrane properties are related to sarcomere length because they depend on membrane geometry. 5. The specific membrane capacity, calculated from the data from detubulated fibres, is 0-8 micronF/cm2. 6. The internal resistivity, Ri, of normal fibres, also depends on sarcomere length between 2-1 and 3-0 micron. At a sarcomere length of 2-1 micron the average Ri is 122 +/- 3 omega. cm (mean +/- S.E. of mean) and at a sarcomere length of 3-0 micron the average Ri is 210 +/- 17 omega. cm (mean +/- S.E. of mean). No further increase in Ri was observed with further increases in sarcomere length.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Almers W. Membrane capacity measurements on frog skeletal muscle in media of low ion content. J Physiol. 1974 Mar;237(3):573–605. doi: 10.1113/jphysiol.1974.sp010499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W. Potassium conductance changes in skeletal muscle and the potassium concentration in the transverse tubules. J Physiol. 1972 Aug;225(1):33–56. doi: 10.1113/jphysiol.1972.sp009928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLINKS J. R. INFLUENCE OF OSMOTIC STRENGTH ON CROSS-SECTION AND VOLUME OF ISOLATED SINGLE MUSCLE FIBRES. J Physiol. 1965 Mar;177:42–57. doi: 10.1113/jphysiol.1965.sp007574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian J., Nakajima S. Action potential in the transverse tubules and its role in the activation of skeletal muscle. J Gen Physiol. 1974 Feb;63(2):257–278. doi: 10.1085/jgp.63.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Horowicz P. Fluorescence intensity changes associated with contractile activation in frog muscle stained with Nile Blue A. J Physiol. 1975 Apr;246(3):709–735. doi: 10.1113/jphysiol.1975.sp010912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coster H. G., Simons R. Anomalous dielectric dispersion in bimolecular lipid membranes. Biochim Biophys Acta. 1970 Mar 17;203(1):17–27. doi: 10.1016/0005-2736(70)90031-3. [DOI] [PubMed] [Google Scholar]
- Dulhunty A. F., Franzini-Armstrong C. The relative contributions of the folds and caveolae to the surface membrane of frog skeletal muscle fibres at different sarcomere lengths. J Physiol. 1975 Sep;250(3):513–539. doi: 10.1113/jphysiol.1975.sp011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulhunty A. F., Gage P. W. Electrical properties of toad sartorius muscle fibres in summer and winter. J Physiol. 1973 May;230(3):619–641. doi: 10.1113/jphysiol.1973.sp010208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg B., Eisenberg R. S. Selective disruption of the sarcotubular system in frog sartorius muscle. A quantitative study with exogenous peroxidase as a marker. J Cell Biol. 1968 Nov;39(2):451–467. doi: 10.1083/jcb.39.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. S., Gage P. W. Ionic conductances of the surface and transverse tubular membranes of frog sartorius fibers. J Gen Physiol. 1969 Mar;53(3):279–297. doi: 10.1085/jgp.53.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Eisenberg R. S. Capacitance of the surface and transverse tubular membrane of frog sartorius muscle fibers. J Gen Physiol. 1969 Mar;53(3):265–278. doi: 10.1085/jgp.53.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. Tension development in highly stretched vertebrate muscle fibres. J Physiol. 1966 May;184(1):143–169. doi: 10.1113/jphysiol.1966.sp007908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L. A note on conduction velocity. J Physiol. 1954 Jul 28;125(1):221–224. doi: 10.1113/jphysiol.1954.sp005152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., Nakajima S. Analysis of the membrane capacity in frog muscle. J Physiol. 1972 Feb;221(1):121–136. doi: 10.1113/jphysiol.1972.sp009743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., Nakajima S. The effect of diameter on the electrical constants of frog skeletal muscle fibres. J Physiol. 1972 Feb;221(1):105–120. doi: 10.1113/jphysiol.1972.sp009742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell J. N. A lesion of the transverse tubules of skeletal muscle. J Physiol. 1969 May;201(3):515–533. doi: 10.1113/jphysiol.1969.sp008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R. The effect of change in length on conduction velocity in muscle. J Physiol. 1954 Jul 28;125(1):215–220. doi: 10.1113/jphysiol.1954.sp005151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley B. A., Leung J., Eisenberg R. S. Longitudinal impedance of single frog muscle fibers. J Gen Physiol. 1975 Jan;65(1):97–113. doi: 10.1085/jgp.65.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley B. A., Leung J., Eisenberg R. S. Longitudinal impedance of skinned frog muscle fibers. J Gen Physiol. 1974 May;63(5):625–637. doi: 10.1085/jgp.63.5.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S., Nakajima Y., Peachey L. D. Speed of repolarization and morphology of glygerol-treated frog muscle fibres. J Physiol. 1973 Oct;234(2):465–480. doi: 10.1113/jphysiol.1973.sp010355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. F. Linear electrical properties of the transverse tubules and surface membrane of skeletal muscle fibers. J Gen Physiol. 1970 Nov;56(5):640–671. doi: 10.1085/jgp.56.5.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdiosera R., Clausen C., Eisenberg R. S. Impedance of frog skeletal muscle fibers in various solutions. J Gen Physiol. 1974 Apr;63(4):460–491. doi: 10.1085/jgp.63.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]


