Abstract
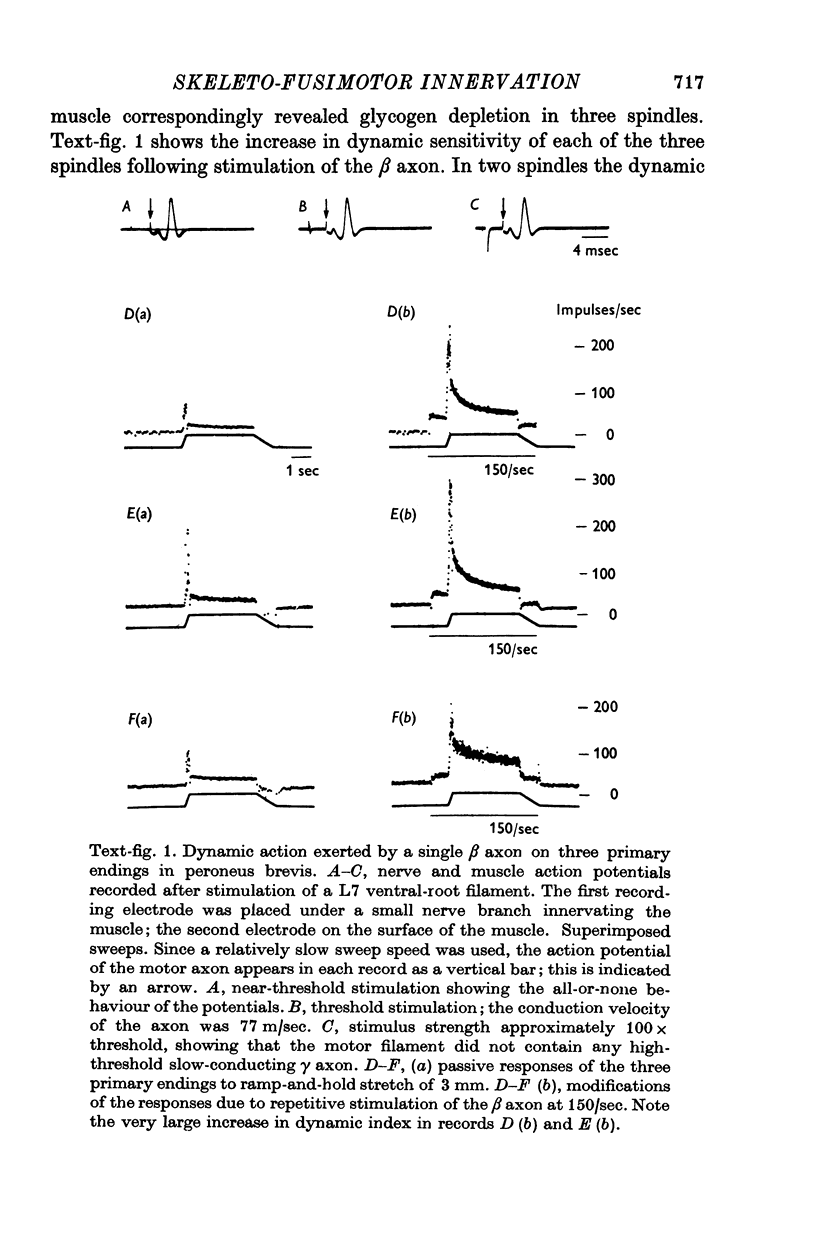
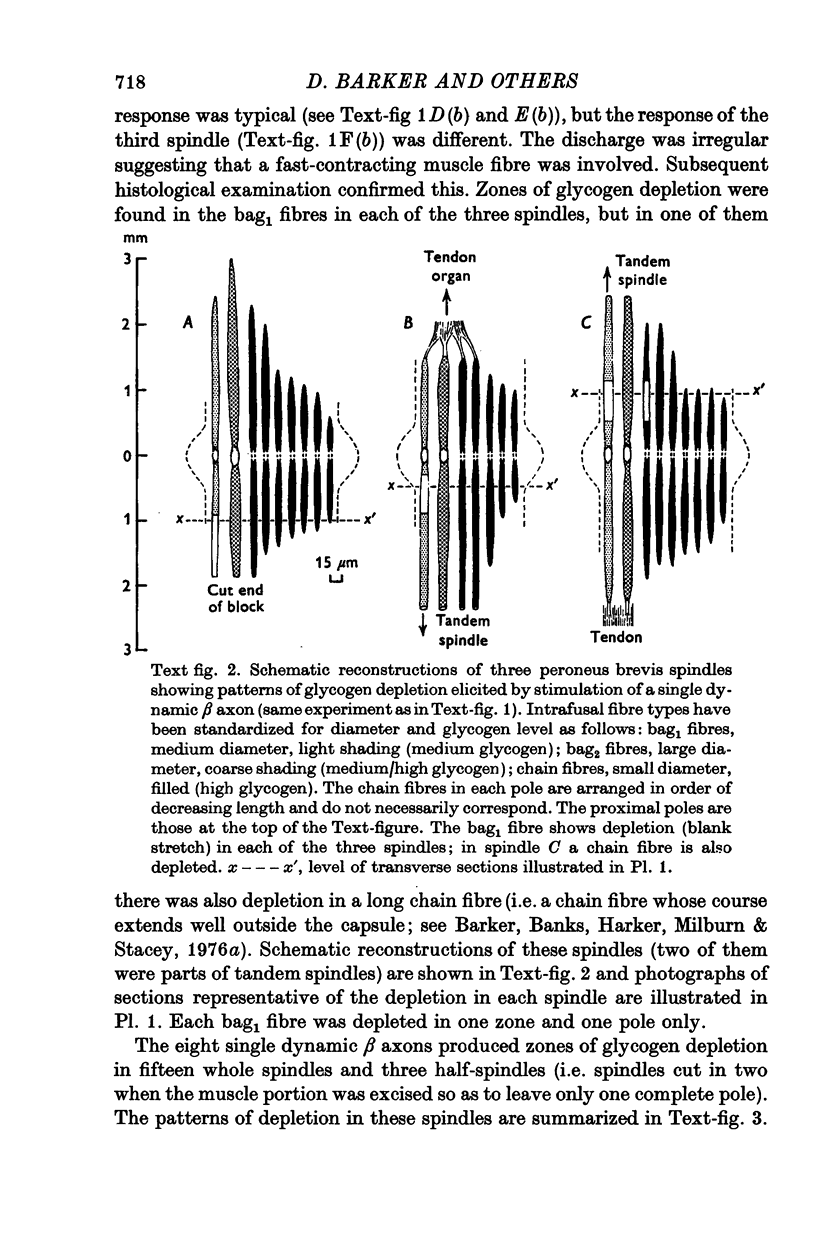
1. The types of intra- and extrafusal muscle fibre innervated by dynamic skeleto-fusimotor (beta) axons were determined by using a modification of the glycogen-depletion method of Edström & Kugelberg (1968) combined with histochemical tests for various enzyme reactions. A single beta axon was prepared in each of the experiments, which were carried out on six peroneus brevis and two tenuissimus muscles. 2. The intrafusal distribution of dynamic beta axons is almost exclusively restricted to bag1 fibres. The bags fibre was depleted in each of twenty-four beta-innervated spindle poles; the only fibres of a different type depleted intrafusally were a bag2 fibre in one pole and a long chain in another. 3. Depletion in the bag1 fibres was usually restricted to one zone in one pole, generally in a mid-polar location. 4. The extrafusal muscle fibres depleted by dynamic beta axons belong to the slow oxidative type as defined by Ariano, Armstrong & Edgerton (1973). The number of such fibres in each motor unit could not be accurately determined, but is almost certainly small. 5. The slow oxidative muscle fibres innervated by dynamic beta axons were not depleted over their entire length. Since there is no reason to assume that they are not twitch fibres, it would seem that the localized depletions result from the conditions required to obtain glycogen depletion, i.e. long periods of motor stimulation applied during the occlusion of the muscle's blood supply. Under similar experimental conditions depletion of glycogen was also restricted to portions of fibres in fast oxidative-glycolytic motor units, but extended over most of the length of the fibres in fast glycolytic units.
Full text
PDF




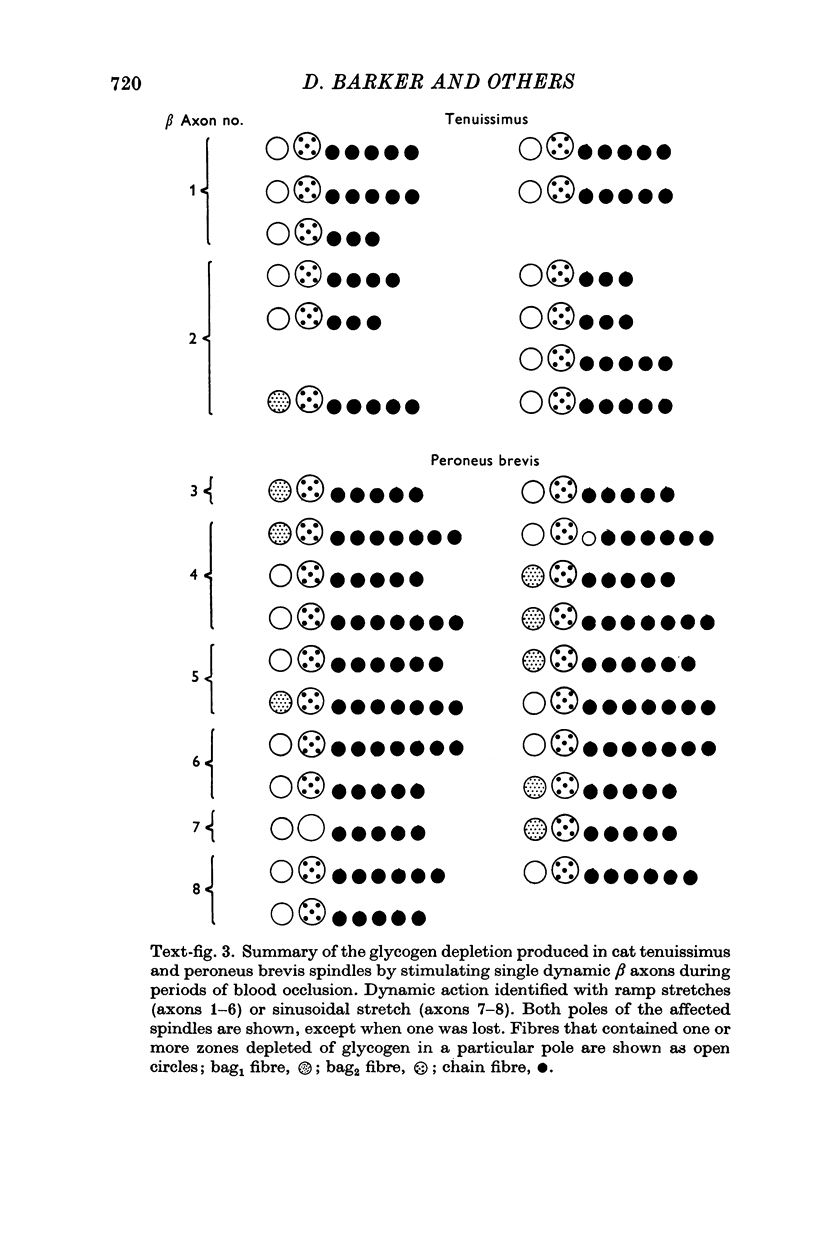







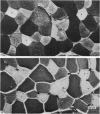
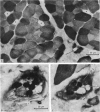
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAL M. N., BARKER D. INTRAMUSCULAR BRANCHING OF FUSIMOTOR FIBRES. J Physiol. 1965 Mar;177:288–299. doi: 10.1113/jphysiol.1965.sp007592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian E. D. The spread of activity in the tenuissimus muscle of the cat and in other complex muscles. J Physiol. 1925 Sep 4;60(4):301–315. doi: 10.1113/jphysiol.1925.sp002249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariano M. A., Armstrong R. B., Edgerton V. R. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem. 1973 Jan;21(1):51–55. doi: 10.1177/21.1.51. [DOI] [PubMed] [Google Scholar]
- Barker D., Banks R. W., Harker D. W., Milburn A., Stacey M. J. Studies of the histochemistry, ultrastructure, motor innervation, and regeneration of mammalian intrafusal muscle fibres. Prog Brain Res. 1976;44:67–88. doi: 10.1016/s0079-6123(08)60724-4. [DOI] [PubMed] [Google Scholar]
- Barker D., Emonet-Dénand F., Harker D. W., Jami L., Laporte Y. Distribution of fusimotor axons to intrafusal muscle fibres in cat tenuissimus spindles as determined by the glycogen-depletion method. J Physiol. 1976 Sep;261(1):49–69. doi: 10.1113/jphysiol.1976.sp011548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrios P., Haase J., Heinrich W. Fusimotorische Alpha-reflexe an prätibialen Flexorenspindeln der Katze. Pflugers Arch Gesamte Physiol Menschen Tiere. 1967;296(1):49–69. [PubMed] [Google Scholar]
- Bessou P., Emonet-Dénand F., Laporte Y. Motor fibres innervating extrafusal and intrafusal muscle fibres in the cat. J Physiol. 1965 Oct;180(3):649–672. doi: 10.1113/jphysiol.1965.sp007722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessou P., Pagés B. Cinematographic analysis of contractile events produced in intrafusal muscle fibres by stimulation of static and dynamic fusimotor axons. J Physiol. 1975 Nov;252(2):397–427. doi: 10.1113/jphysiol.1975.sp011150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd I. A., Gladden M. H., McWilliam P. N., Ward J. Proceedings: "Static" and "dynamic" nuclear bag fibres in isolated cat muscle spindles. J Physiol. 1975 Aug;250(1):11P–12P. [PMC free article] [PubMed] [Google Scholar]
- Boyd I. A., Ward J. Motor control of nuclear bag and nuclear chain intrafusal fibres in isolated living muscle spindles from the cat. J Physiol. 1975 Jan;244(1):83–112. doi: 10.1113/jphysiol.1975.sp010785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Butler R. G. Studies on the site of termination of static and dynamic fusimotor fibres within muscle spindles of the tenuissimus muscle of the cat. J Physiol. 1973 Sep;233(3):553–573. doi: 10.1113/jphysiol.1973.sp010323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Tsairis P., Zajac F. E., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973 Nov;234(3):723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972 Jan;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- ERANKO O., PALKAMA A. Improved localization of phosphorylase by the use of polyvinyl pyrrolidone and high substrate concentration. J Histochem Cytochem. 1961 Sep;9:585–585. doi: 10.1177/9.5.585. [DOI] [PubMed] [Google Scholar]
- Edström L., Kugelberg E. Histochemical composition, distribution of fibres and fatiguability of single motor units. Anterior tibial muscle of the rat. J Neurol Neurosurg Psychiatry. 1968 Oct;31(5):424–433. doi: 10.1136/jnnp.31.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H., Emonet-Denand F., Joffroy M., Laporte Y. Lack of exclusively fusimotor -axons in flexor and extensor leg muscles of the cat. J Neurophysiol. 1972 Jan;35(1):149–153. doi: 10.1152/jn.1972.35.1.149. [DOI] [PubMed] [Google Scholar]
- Emonet-Dénand F., Jami L., Laporte Y. Skeleto-fusimotor axons in the hind-limb muscles of the cat. J Physiol. 1975 Jul;249(1):153–166. doi: 10.1113/jphysiol.1975.sp011008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emonet-Dénand F., Laporte Y. Proportion of muscles spindles supplied by skeletofusimotor axons (beta-axons) in peroneus brevis muscle of the cat. J Neurophysiol. 1975 Nov;38(6):1390–1394. doi: 10.1152/jn.1975.38.6.1390. [DOI] [PubMed] [Google Scholar]
- Guth L., Samaha F. J. Procedure for the histochemical demonstration of actomyosin ATPase. Exp Neurol. 1970 Aug;28(2):365–367. [PubMed] [Google Scholar]
- Jami L., Petit J. Correlation between axonal conduction velocity and tetanic tension of motor units in four muscles of the cat hind limb. Brain Res. 1975 Oct 10;96(1):114–118. doi: 10.1016/0006-8993(75)90581-8. [DOI] [PubMed] [Google Scholar]
- NACHLAS M. M., TSOU K. C., DE SOUZA E., CHENG C. S., SELIGMAN A. M. Cytochemical demonstration of succinic dehydrogenase by the use of a new p-nitrophenyl substituted ditetrazole. J Histochem Cytochem. 1957 Jul;5(4):420–436. doi: 10.1177/5.4.420. [DOI] [PubMed] [Google Scholar]
- Ovalle W. K., Smith R. S. Histochemical identification of three types of intrafusal muscle fibers in the cat and monkey based on the myosin ATPase reaction. Can J Physiol Pharmacol. 1972 Mar;50(3):195–202. doi: 10.1139/y72-030. [DOI] [PubMed] [Google Scholar]