Abstract
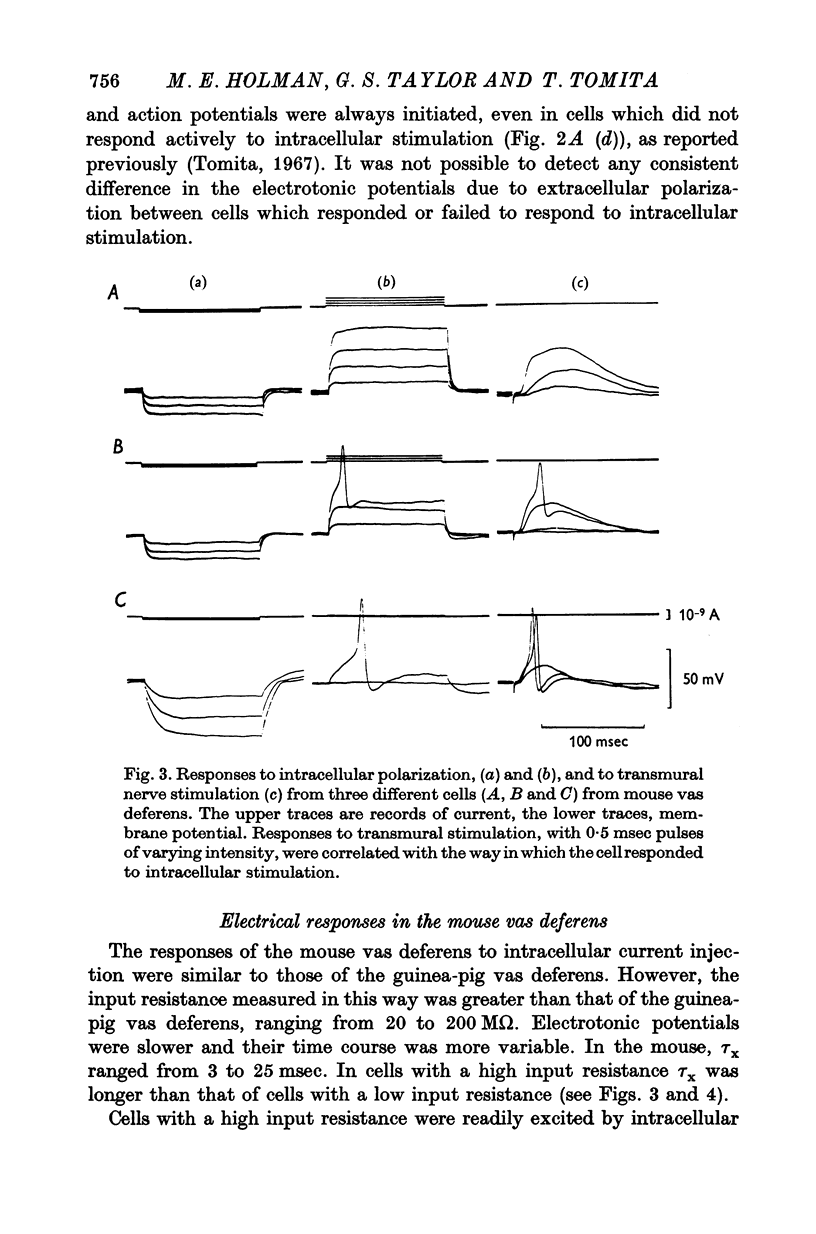
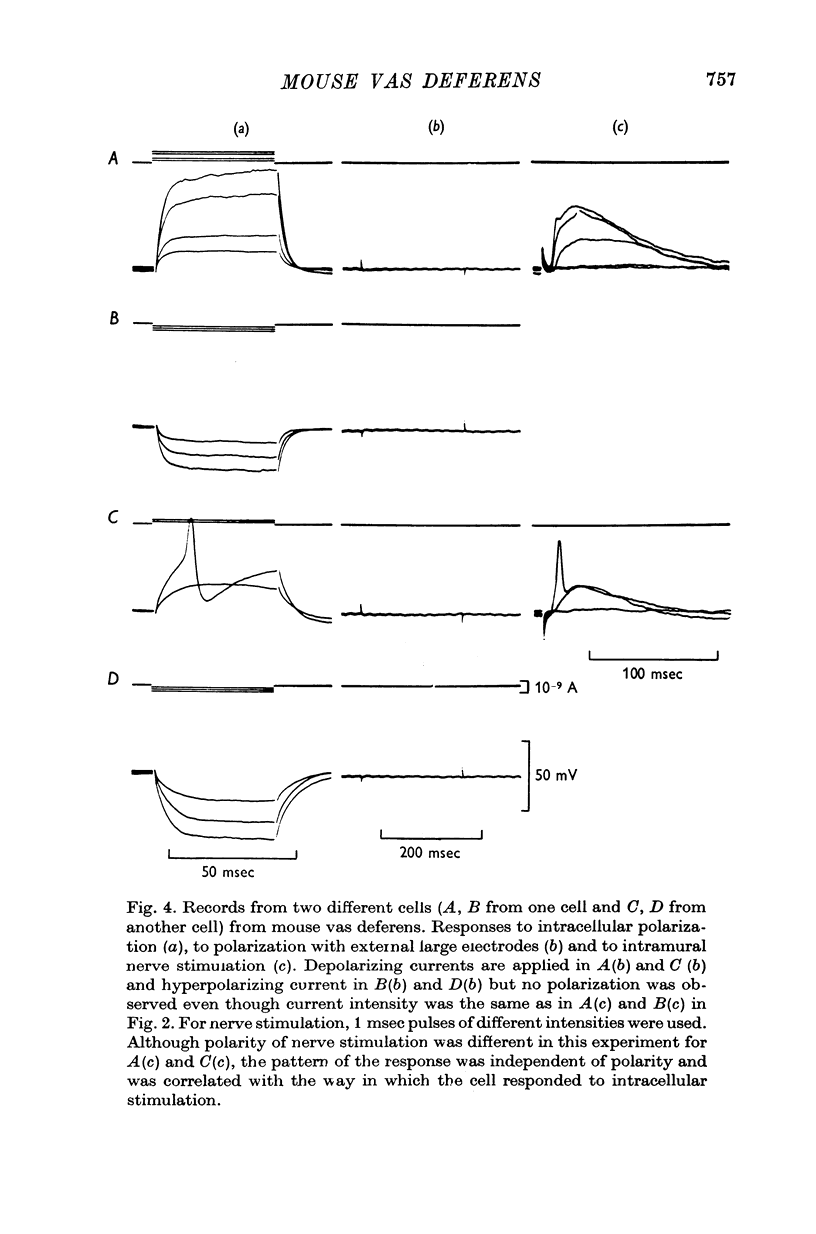
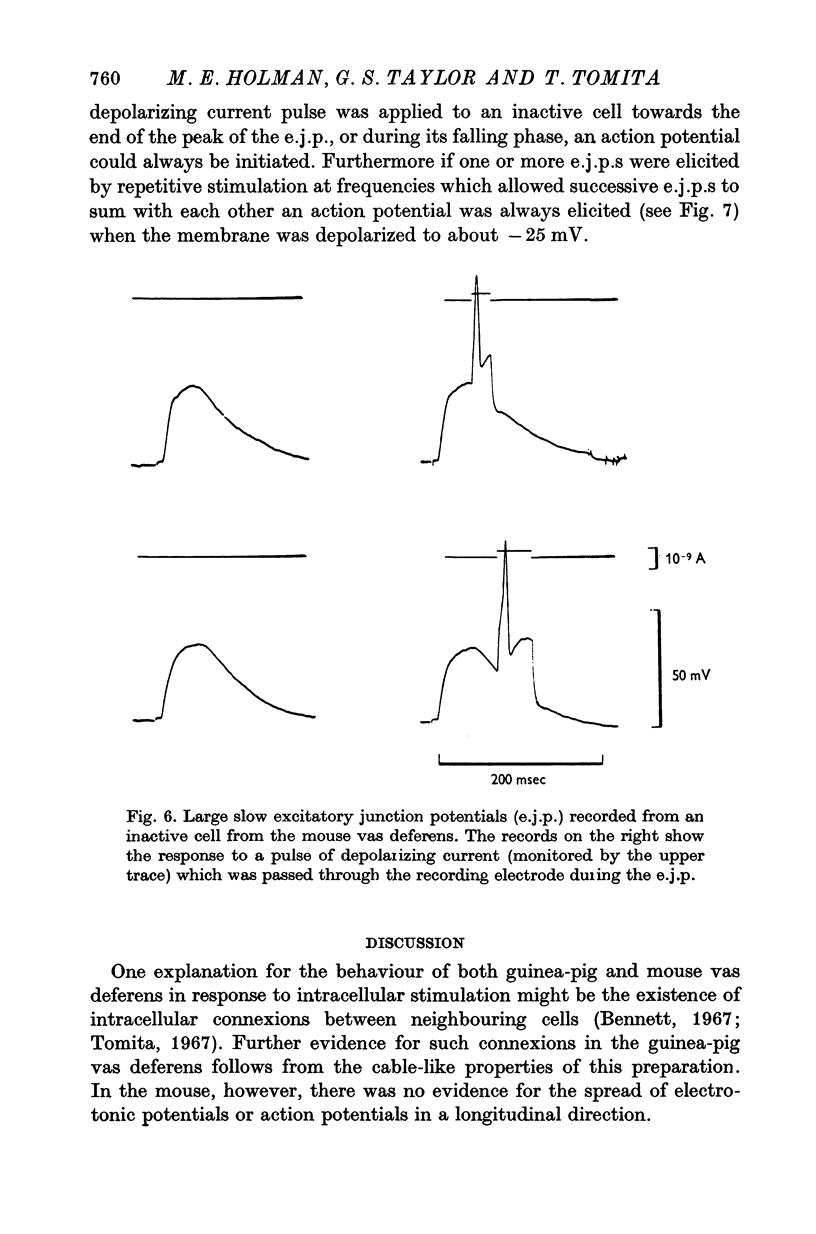
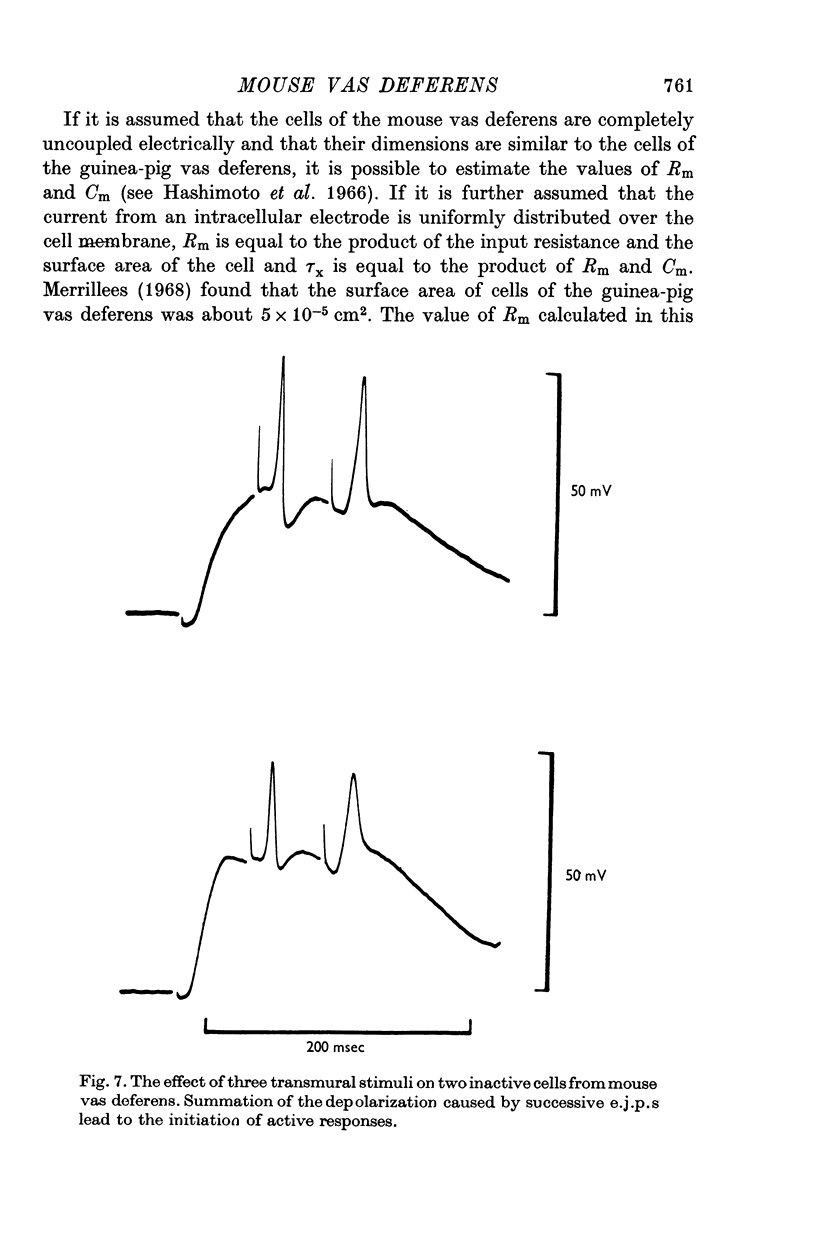
1. Contractions of the mouse vas deferens in response to electrical stimulation differ form those recorded form the guinea-pig vas deferens in that they are abolished by tetrodotoxin. 2. Changes in membrane potentials were recorded form the smooth muscle of both preparations in response to stimulation with current pulses applied by an intracellular electrode and by alrge extracellular plate electrodes. 3. Both preparations behaved similarly in response to intracellular stimulation. Electrotonic potentials in response to extracellular current pulses spread in a longitudinal direction in the guinea-pig vas deferens in accordance with the cable-like properties of this preparation. In contrast, no longitudinal spread of eletrotonus was observed in the mouse vas deferens. 4. Responses to nerve stimulation differed in the two preparations. In the guinea-pig, single stimuli caused excitatory junction potentials (e.j.p.s) which gave rise to action potentials. Some cells from the mouse vas deferens showed similar e.j.p.s and action potentials, although the threshold for the initiation of action potentials was lower and more variable. 5. The majority of cells in the mouse vas deferens failed to show action potentials in response to a single stimuli even though the amplitude of e.j.p.s was from 35 to 40 mV. This was probably due to the large resting membrane potentials of these cells, as all-or-nothing action potentials could be evoked if successive e.j.p.s were allowed to sum with each other or if a depolarizing current pulse was applied at the peak of an e.j.p. 6. The nature of the response to nerve stimulation recorded from differnt cells in the mouse vas deferens could be correlated with the amplitude and time course of the response of the same cell to intracellular stimulation. 7. It is concluded that individual smooth muscle cells in both preparations are probably coupled electrically but that there are few, if any, low resistance pathways in the longitudinal direction in the mouse vas deferens.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., HOLMAN M. E. The transmission of excitation from autonomic nerve to smooth muscle. J Physiol. 1961 Jan;155:115–133. doi: 10.1113/jphysiol.1961.sp006617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R. The effect of intracellular current pulses in smooth muscle cells of the guinea pig vas deferens at rest and during transmission. J Gen Physiol. 1967 Nov;50(10):2459–2475. doi: 10.1085/jgp.50.10.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Properties of the inhibitory potential of smooth muscle as observed in the response to field stimulation of the guinea-pig taenia coli. J Physiol. 1967 Apr;189(2):299–315. doi: 10.1113/jphysiol.1967.sp008169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness J. B., Burnstock G. A comparative study of spike potentials in response to nerve stimulation in the vas deferens of the mouse, rat and guinea-pig. Comp Biochem Physiol. 1969 Oct 15;31(2):337–345. doi: 10.1016/0010-406x(69)91658-2. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Holman M. E. Effect of manganese ions on the electrical activity of mouse vas deferens. Aust J Exp Biol Med Sci. 1967 Oct;45(5):533–539. doi: 10.1038/icb.1967.52. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Holman M. E., McLean A. J. Effect of tetrodotoxin on the electrical activity of the smooth muscle of the vas deferens. Nature. 1967 Jul 22;215(5099):430–432. doi: 10.1038/215430a0. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Holman M. E., Tille J. Electrical properties of the smooth muscle membrane of the guinea-pig vas deferens. J Physiol. 1966 Sep;186(1):27–41. doi: 10.1113/jphysiol.1966.sp008018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURIYAMA H. ELECTROPHYSIOLOGICAL OBSERVATIONS ON THE MOTOR INNERVATION OF THE SMOOTH MUSCLE CELLS IN THE GUINEA-PIG VAS DEFERENS. J Physiol. 1963 Nov;169:213–228. doi: 10.1113/jphysiol.1963.sp007251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Osa T., Toida N. Effect of tetrodotoxin on smooth muscle cells of the guinea-pig taenia coli. Br J Pharmacol Chemother. 1966 Aug;27(2):366–376. doi: 10.1111/j.1476-5381.1966.tb01668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekata F. Electrophysiological studies of the smooth muscle cell membrane of the rabbit common carotid artery. J Gen Physiol. 1971 Jun;57(6):738–751. doi: 10.1085/jgp.57.6.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D. Applications of Hodgkin-Huxley equations to excitable tissues. Physiol Rev. 1966 Jan;46(1):1–50. doi: 10.1152/physrev.1966.46.1.1. [DOI] [PubMed] [Google Scholar]
- Tomita T. Current spread in the smooth muscle of the guinea-pig vas deferens. J Physiol. 1967 Mar;189(1):163–176. doi: 10.1113/jphysiol.1967.sp008161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. Electrophysiology of mammalian smooth muscle. Prog Biophys Mol Biol. 1975;30(2-3):185–203. doi: 10.1016/0079-6107(76)90009-2. [DOI] [PubMed] [Google Scholar]


