Abstract
1. Intracellular responses to flashes and steps of light have been recorded from the outer segment and the cell body of rods in the retina of the Bufo marinus. The identification of the origin of recorded responses has been confirmed by intracellular marking.
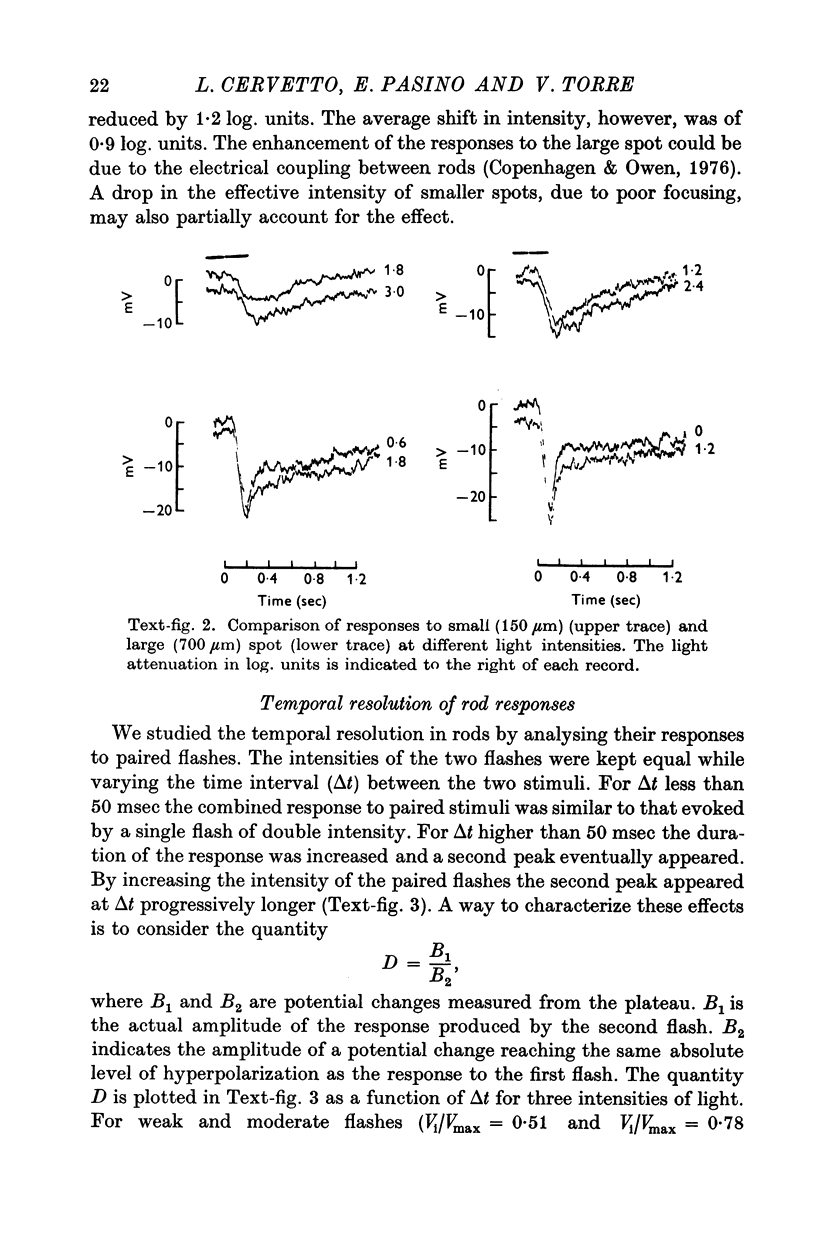
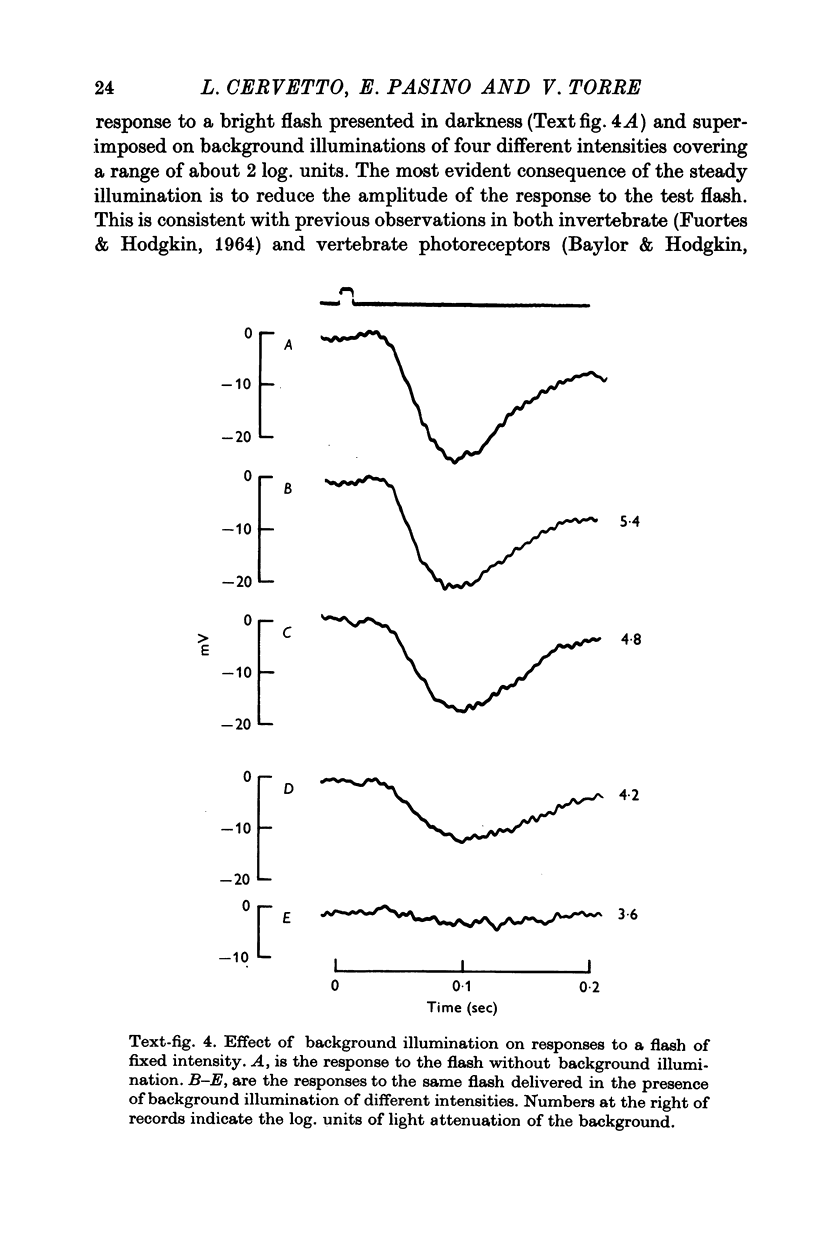
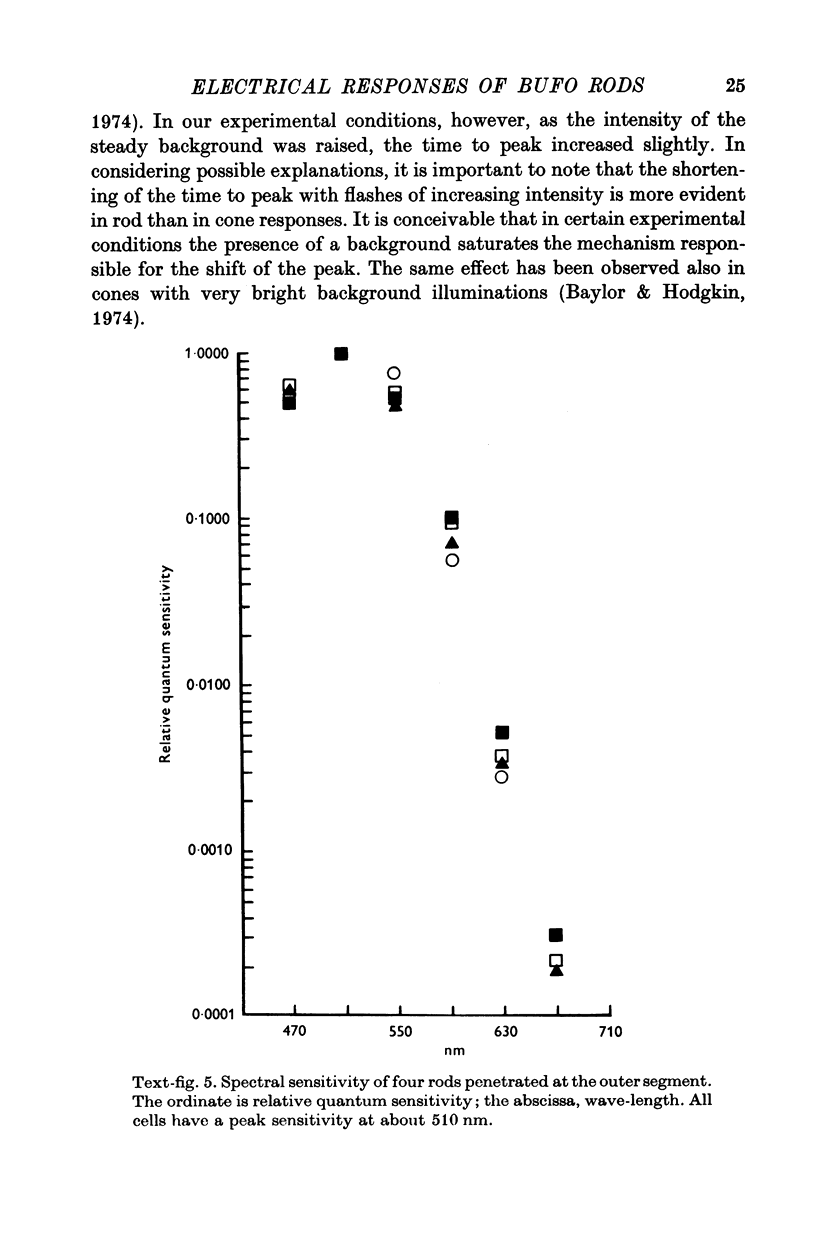
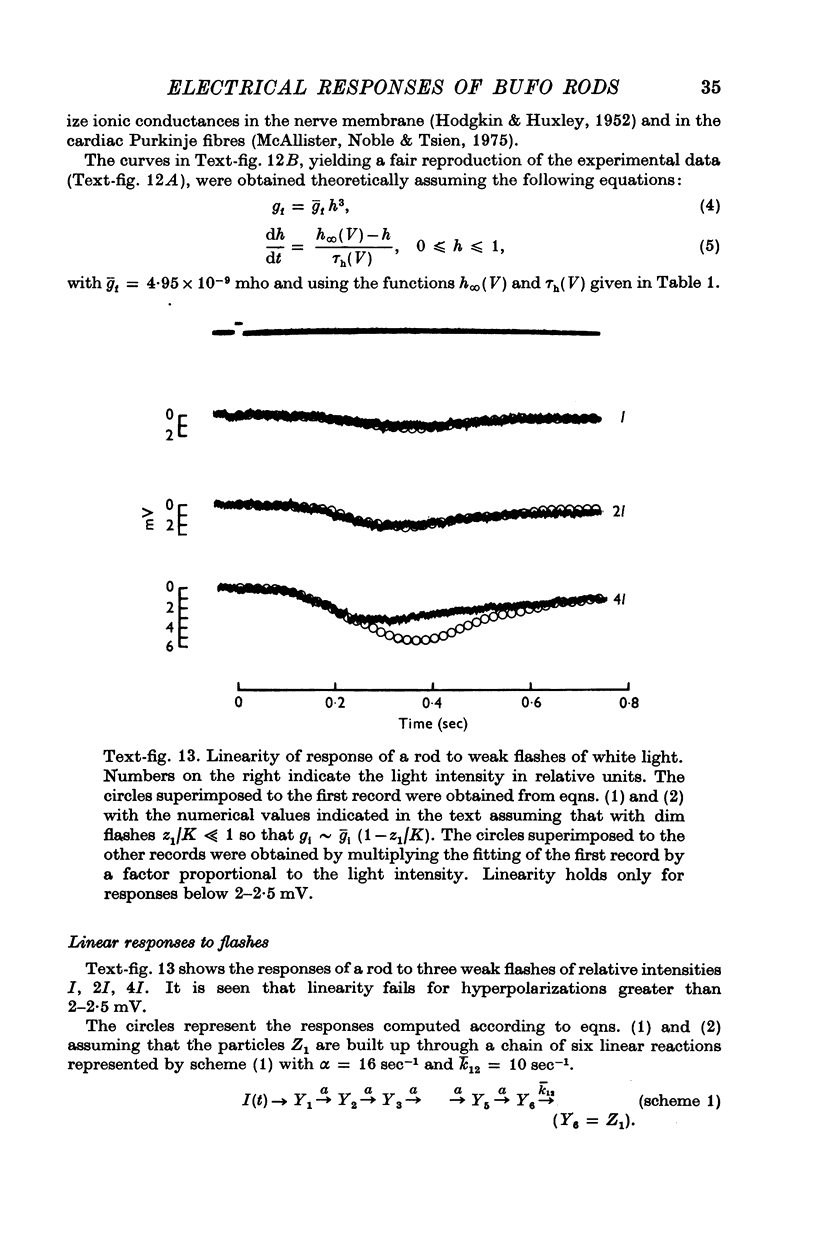
2. Responses to flashes delivered in darkness or superimposed on a background were analysed. Responses recorded from outer segments conform to the principle of `spectral univariance'. The shape of the response is not affected by enlarging the spot diameter from 150 to 1000 μm.
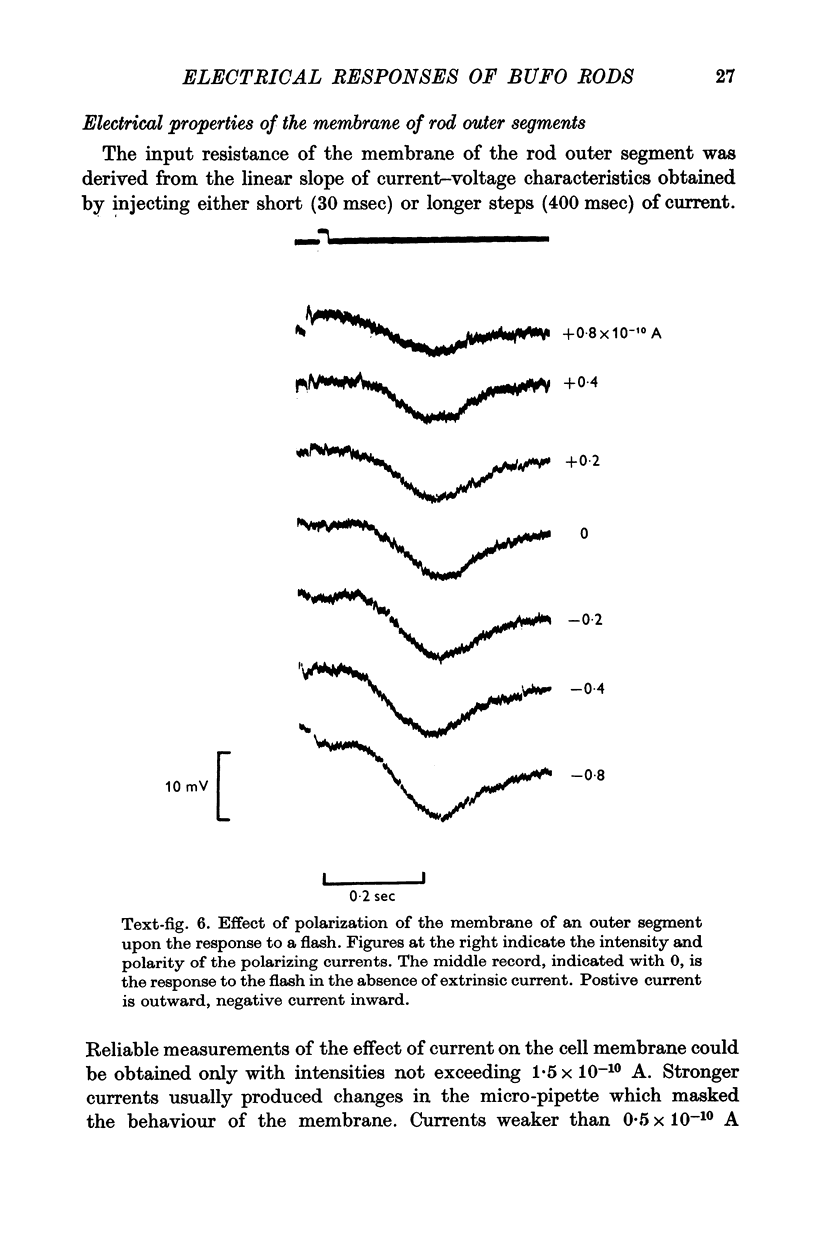
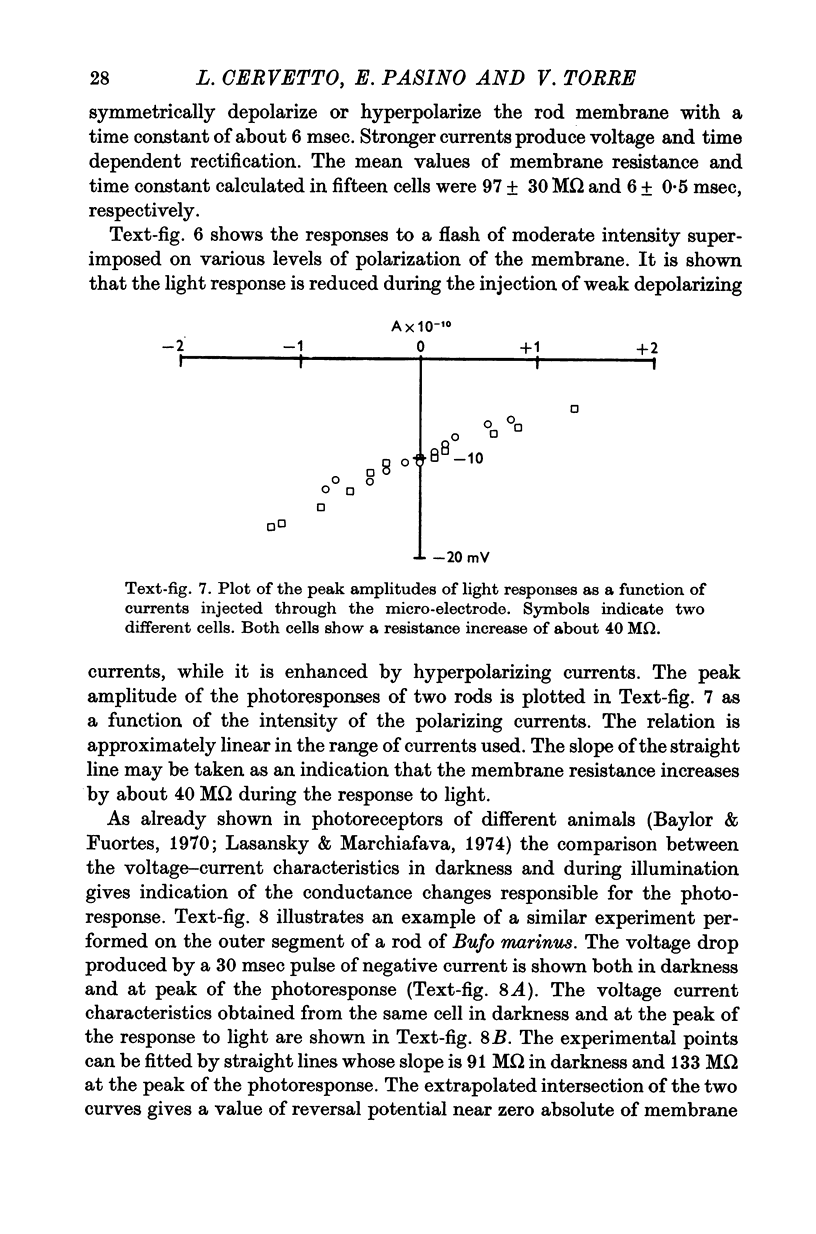
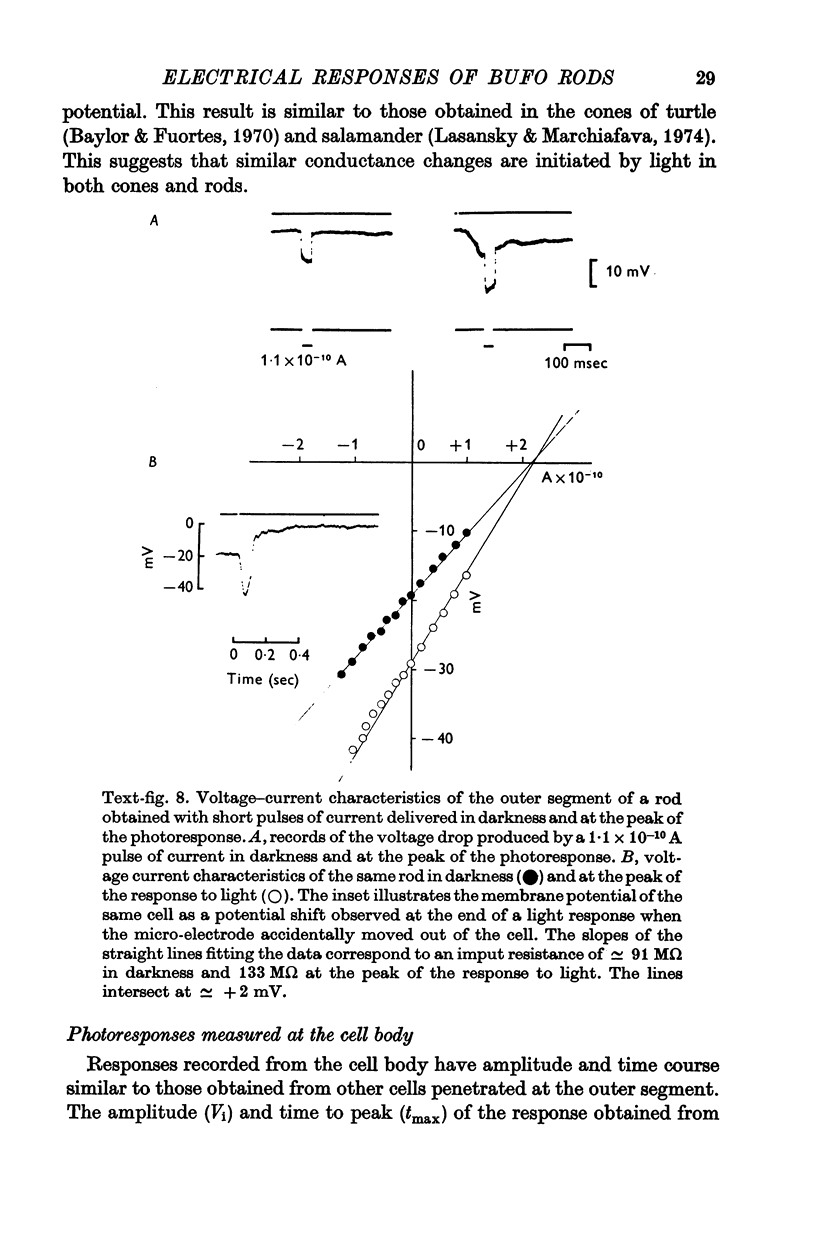
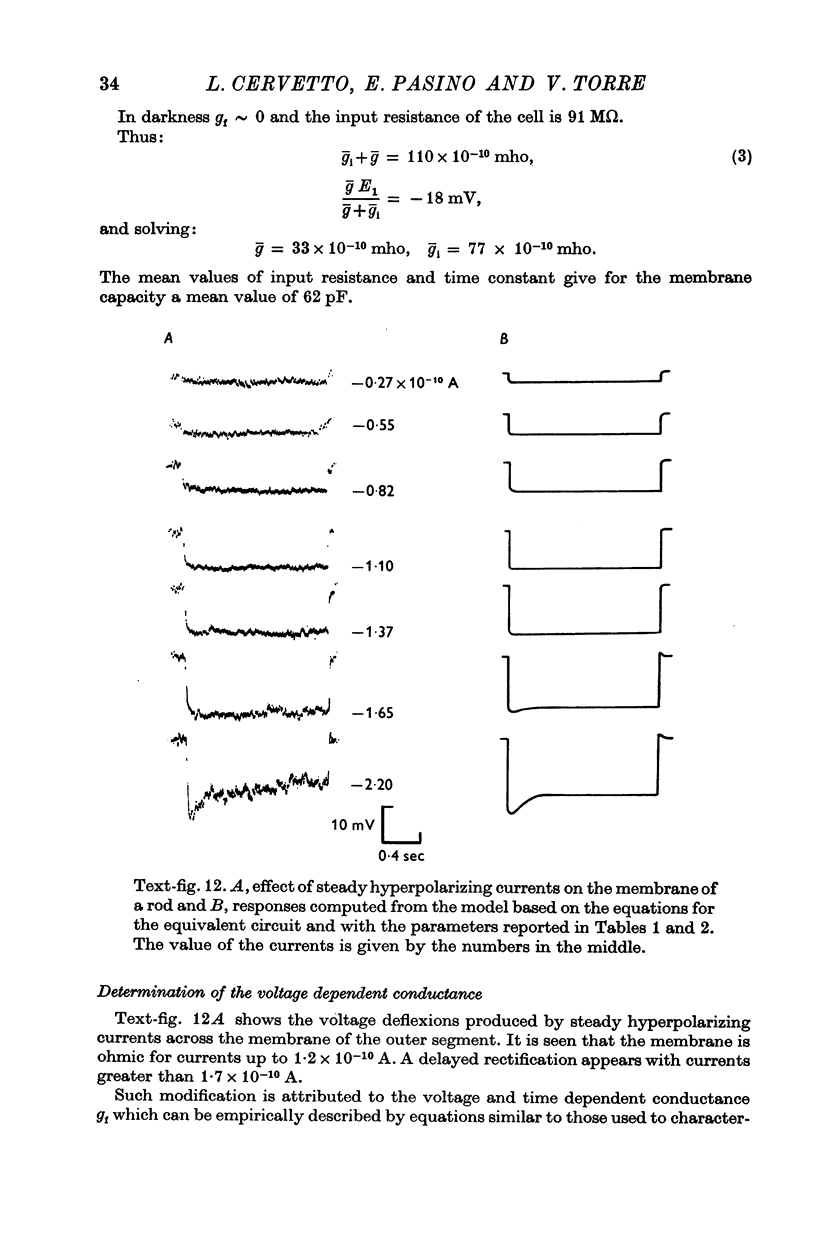
3. The membrane potential measured in darkness at the outer segments varied from -15 to -25 mV. Injection of steady hyperpolarizing currents increases the size of the response to light; depolarizing currents reduce the response. The mean value of the input resistance is 97 ± 30 MΩ in darkness and increases by 20-30% during illumination.
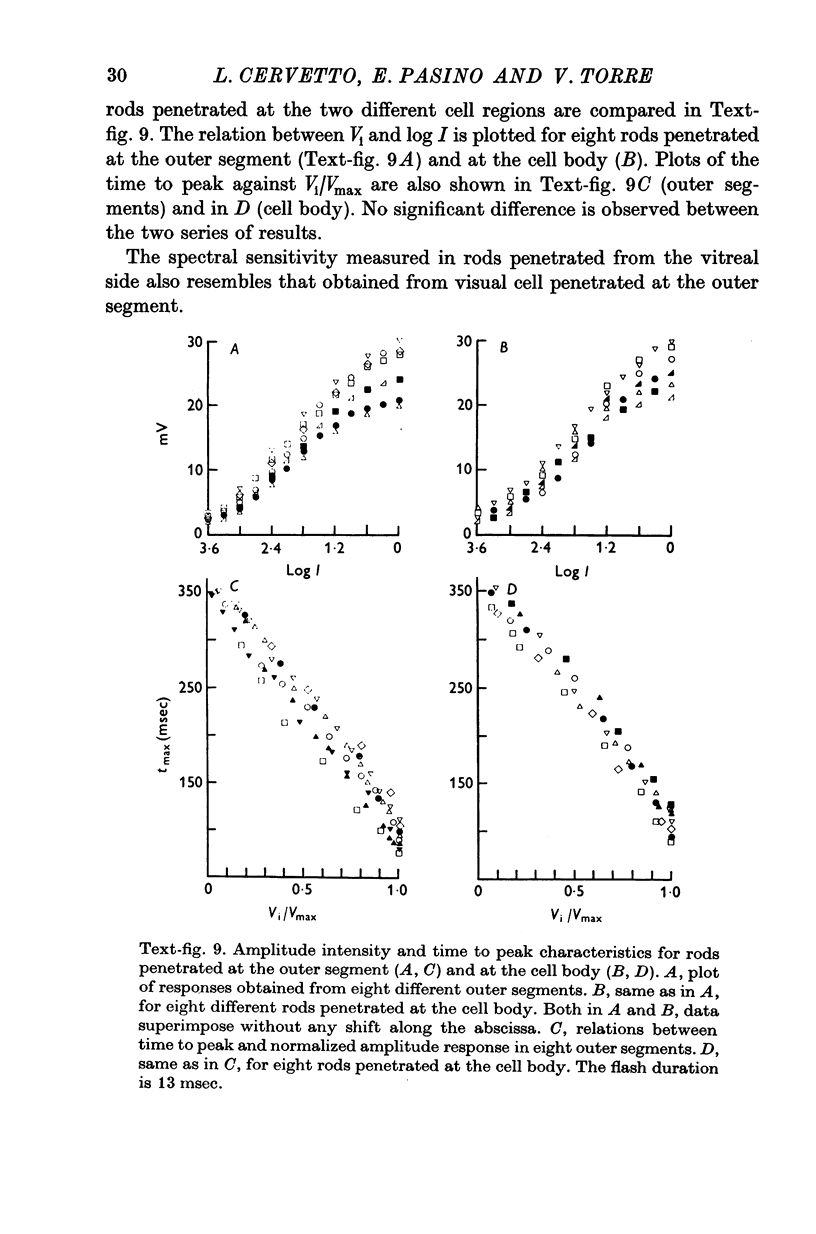
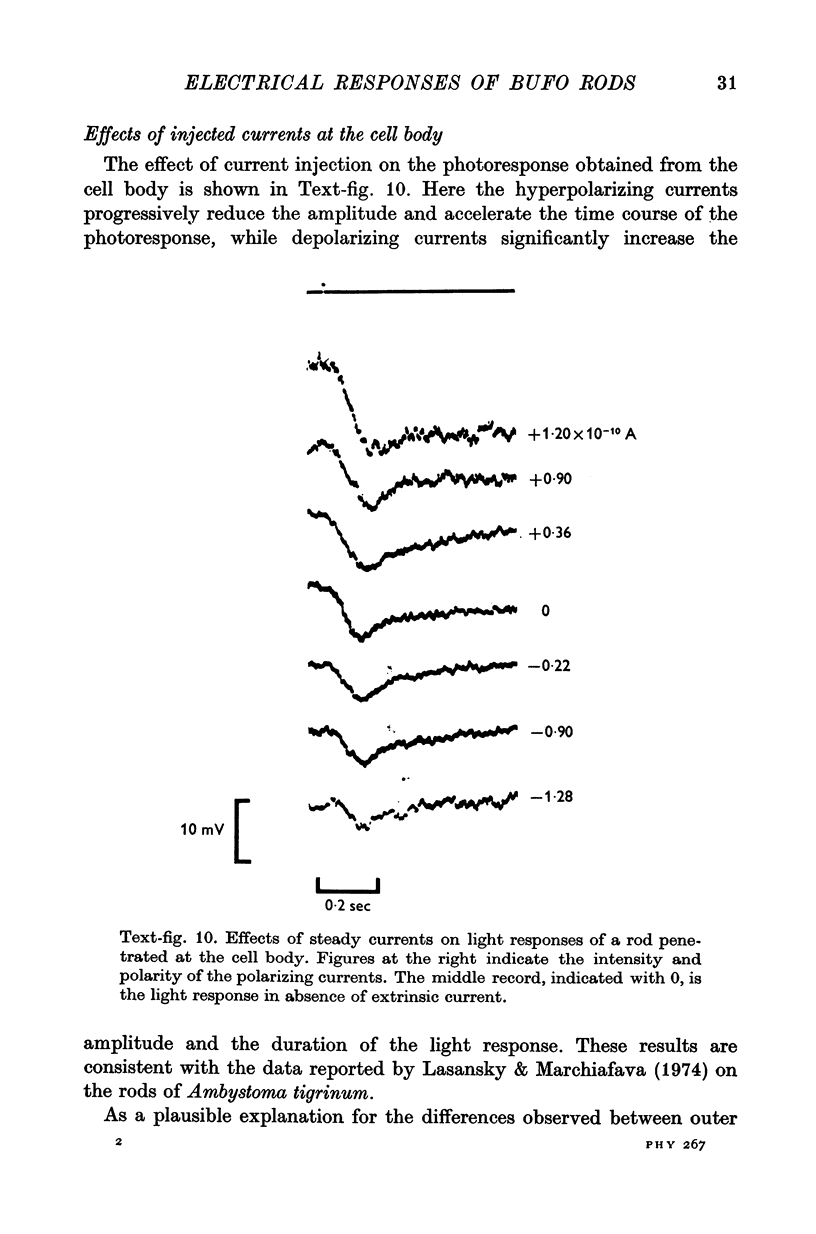
4. The responses obtained from the cell body of rods have the same shape, time course and spectral sensitivity of those recorded at the outer segment. Injection of steady current at the cell body produces different effects than at the outer segment: hyperpolarizing currents reduce the amplitude of the response to light; depolarizing currents increase the response.
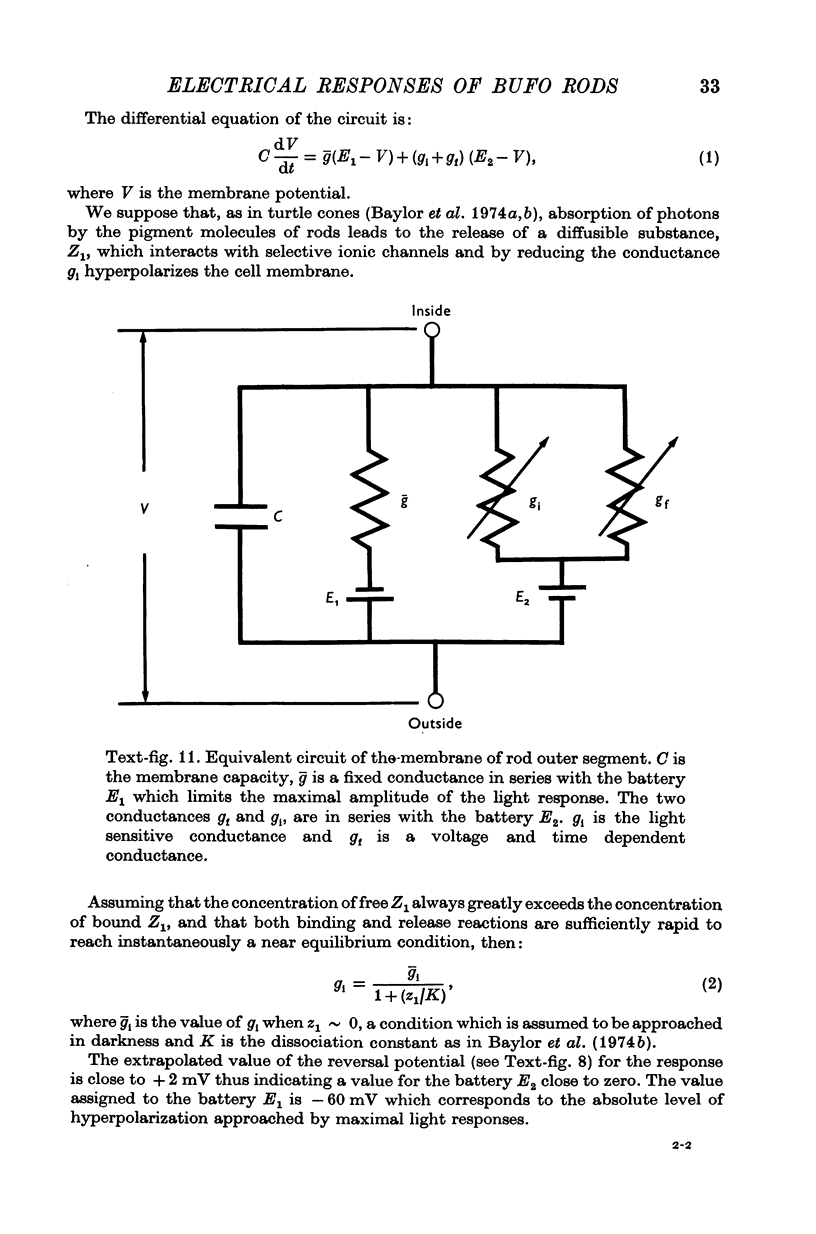
5. The experimental data are fitted according to a model similar to that used to describe the responses of turtle cones (Baylor & Hodgkin, 1974; Baylor, Hodgkin & Lamb, 1974a, b).
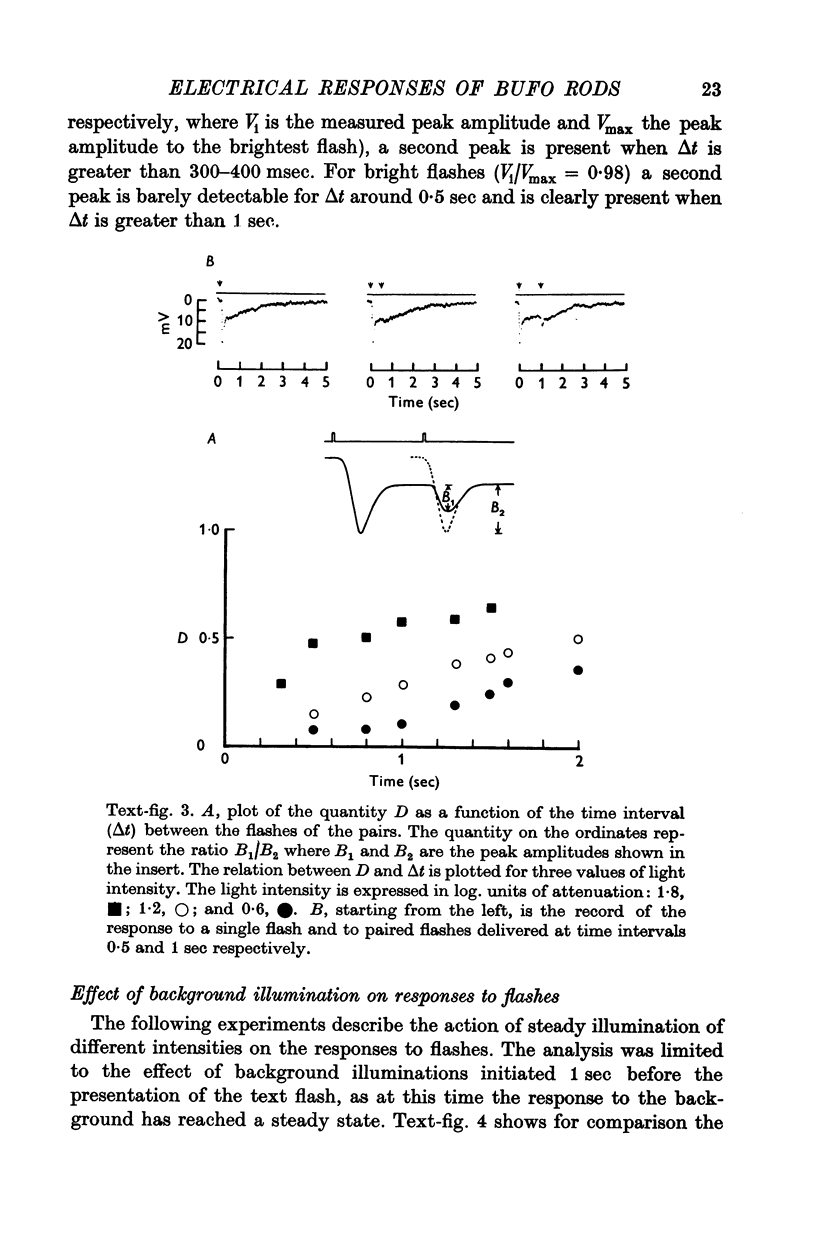
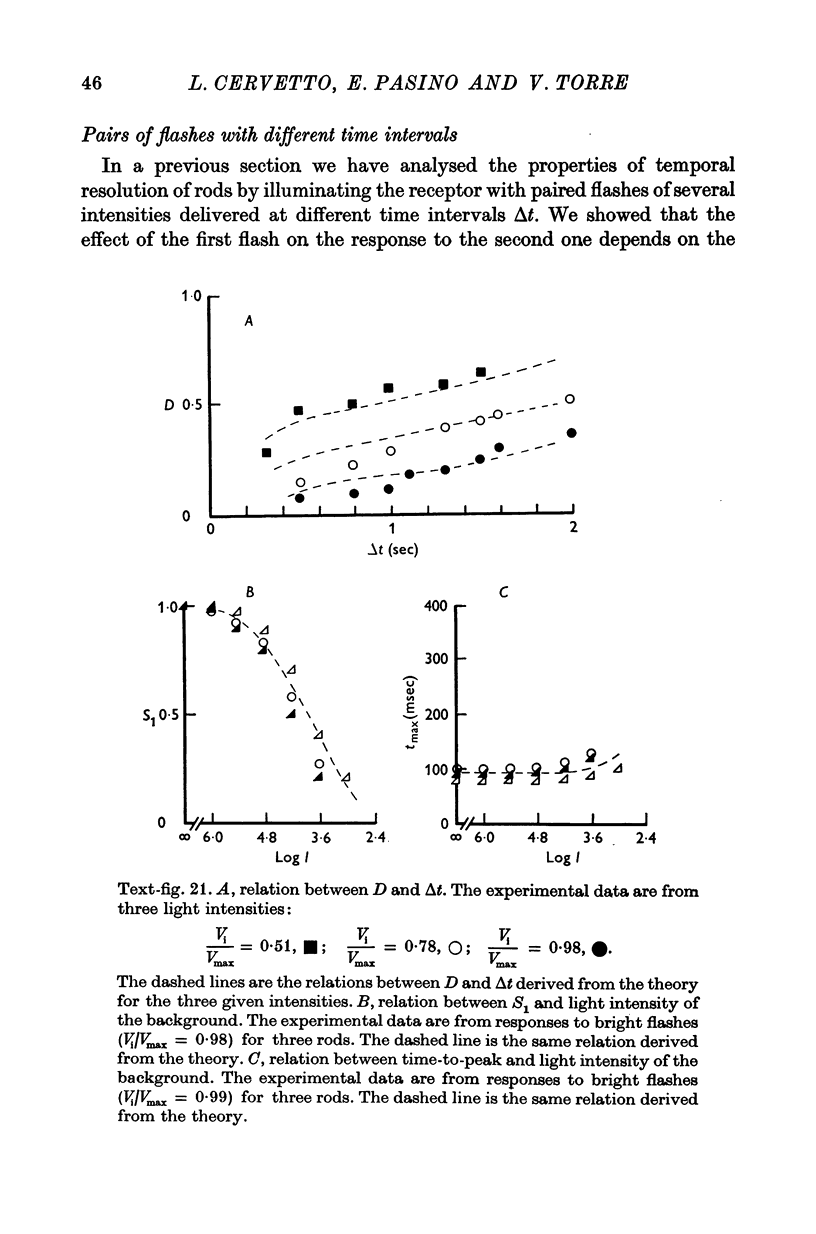
6. The model reproduces the electrical responses of the rod outer segment to a variety of stimuli: (a) brief flashes and steps of light in dark adapted conditions; (b) bright flashes superimposed on background illuminations; (c) pairs of flashes delivered at different time intervals. Responses to hyperpolarizing steps of current are also reproduced by the model.
Full text
PDF






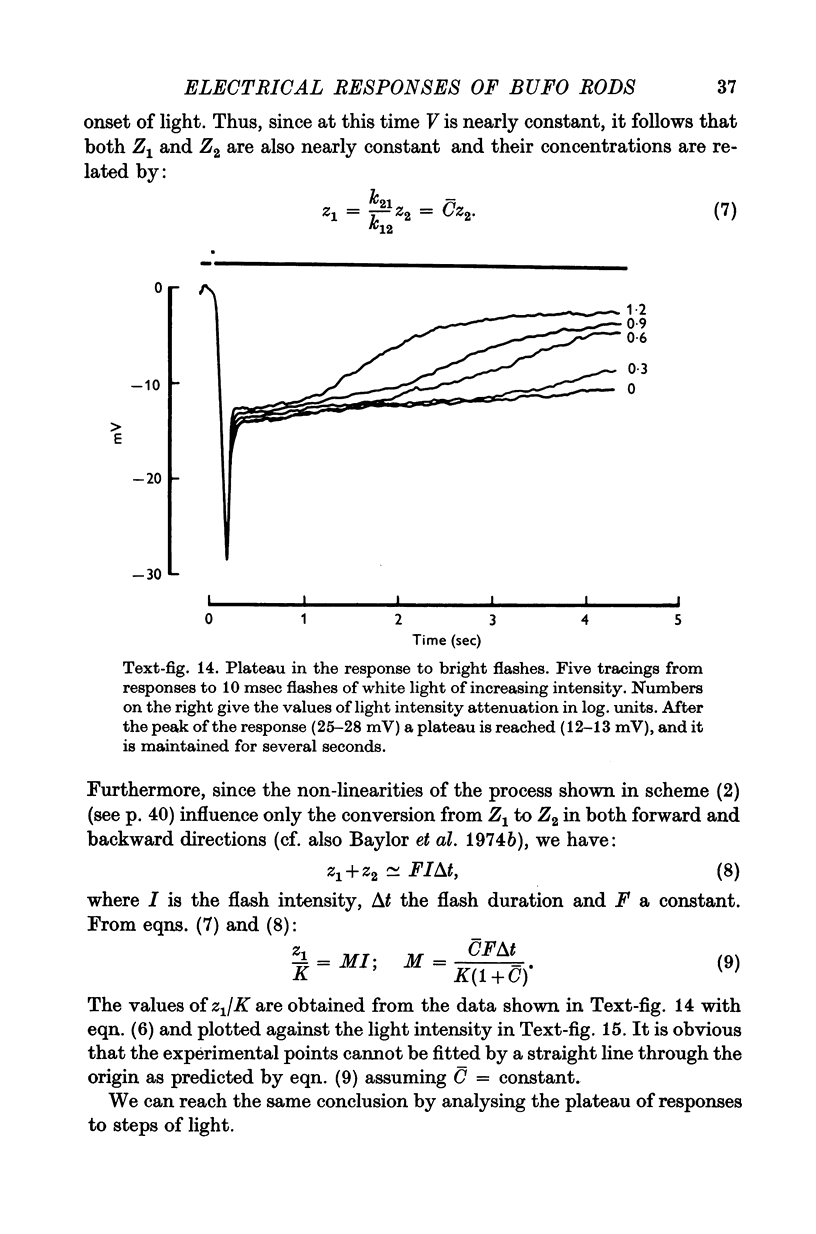
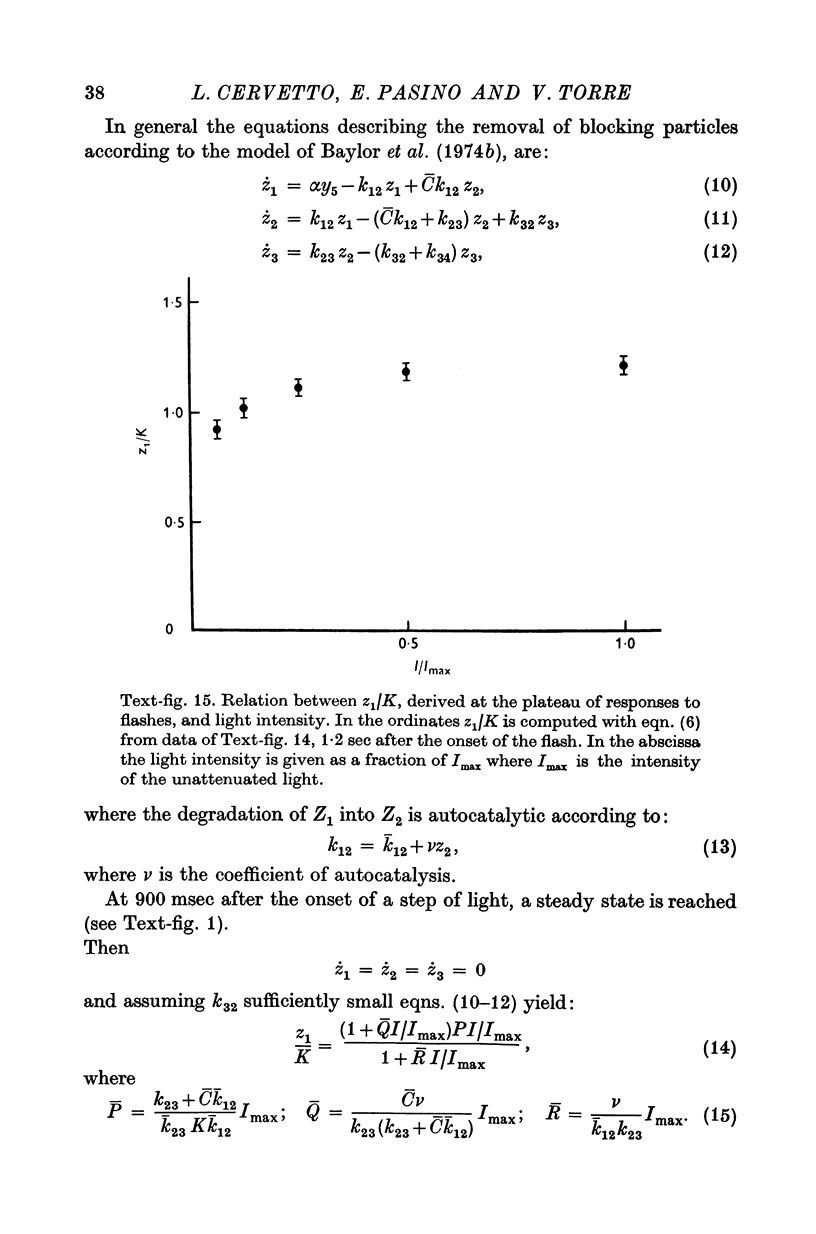
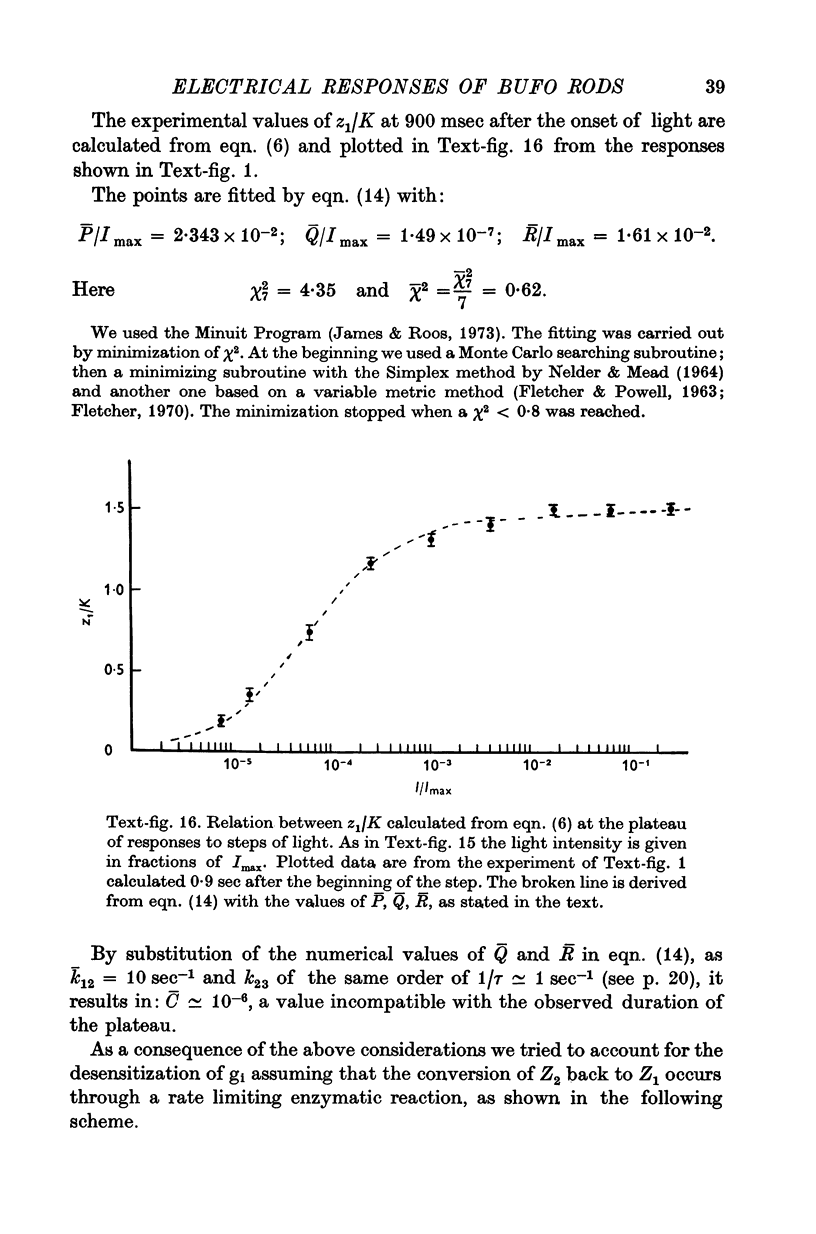
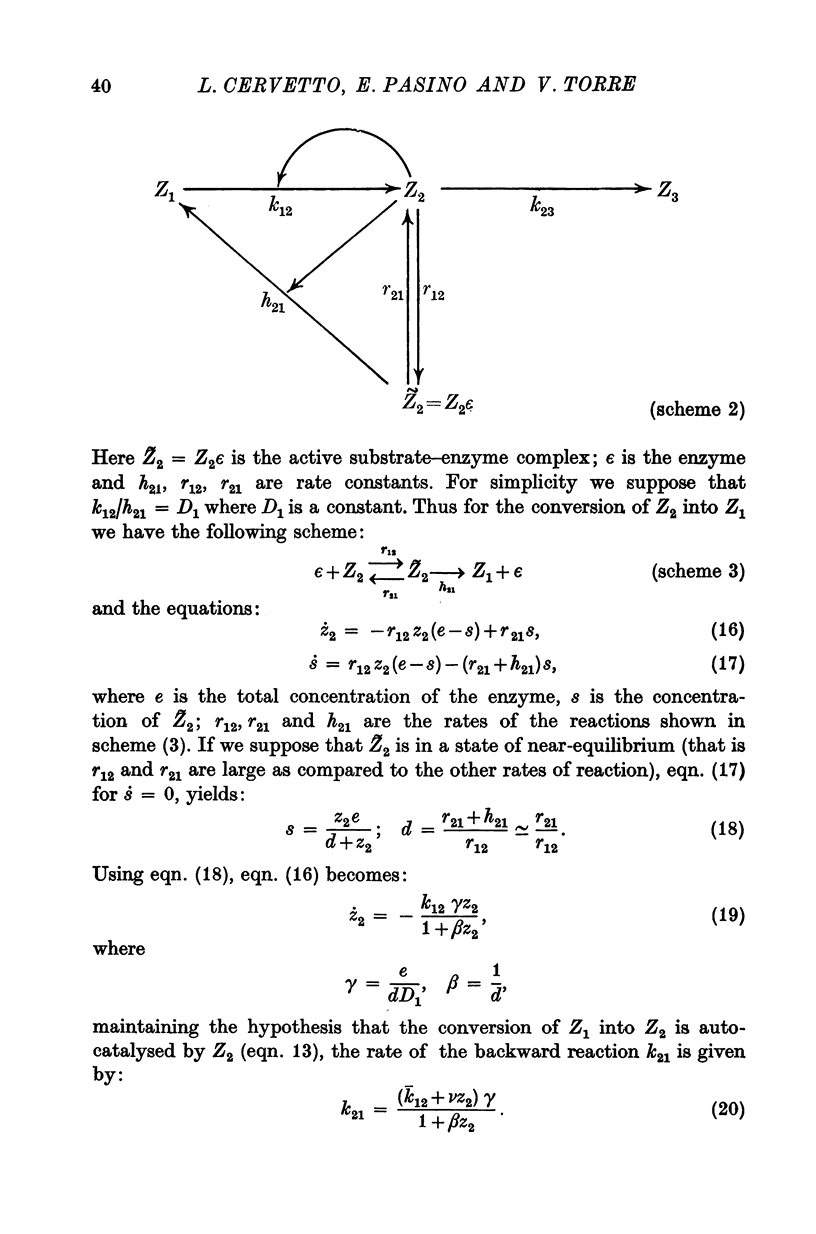






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Fuortes M. G. Electrical responses of single cones in the retina of the turtle. J Physiol. 1970 Mar;207(1):77–92. doi: 10.1113/jphysiol.1970.sp009049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Changes in time scale and sensitivity in turtle photoreceptors. J Physiol. 1974 Nov;242(3):729–758. doi: 10.1113/jphysiol.1974.sp010732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Detection and resolution of visual stimuli by turtle photoreceptors. J Physiol. 1973 Oct;234(1):163–198. doi: 10.1113/jphysiol.1973.sp010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L., Lamb T. D. Reconstruction of the electrical responses of turtle cones to flashes and steps of light. J Physiol. 1974 Nov;242(3):759–791. doi: 10.1113/jphysiol.1974.sp010733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L., Lamb T. D. The electrical response of turtle cones to flashes and steps of light. J Physiol. 1974 Nov;242(3):685–727. doi: 10.1113/jphysiol.1974.sp010731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortoff A., Norton A. L. An electrical model of the vertebrate photoreceptor cell. Vision Res. 1967 Mar;7(3):253–263. doi: 10.1016/0042-6989(67)90089-2. [DOI] [PubMed] [Google Scholar]
- Brown H. M., Hagiwara S., Koike H., Meech R. M. Membrane properties of a barnacle photoreceptor examined by the voltage clamp technique. J Physiol. 1970 Jun;208(2):385–413. doi: 10.1113/jphysiol.1970.sp009127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Pinto L. H. Ionic mechanism for the photoreceptor potential of the retina of Bufo marinus. J Physiol. 1974 Feb;236(3):575–591. doi: 10.1113/jphysiol.1974.sp010453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretta A., Cavaggioni A. On the metabolism of the rod outer segments. J Physiol. 1976 Jun;257(3):687–697. doi: 10.1113/jphysiol.1976.sp011392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaggioni A., Sorbi R. T., Turini S. Efflux of potassium from isolated rod outer segments: a photic effect. J Physiol. 1973 Aug;232(3):609–620. doi: 10.1113/jphysiol.1973.sp010288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervetto L. Influence of sodium, potassium and chloride ions on the intracellular responses of turtle photoreceptors. Nature. 1973 Feb 9;241(5389):401–403. doi: 10.1038/241401a0. [DOI] [PubMed] [Google Scholar]
- Copenhagen D. R., Owen W. G. Coupling between rod photoreceptors in a vertebrate retina. Nature. 1976 Mar 4;260(5546):57–59. doi: 10.1038/260057a0. [DOI] [PubMed] [Google Scholar]
- Dartnall H. J. The photosensitivities of visual pigments in the presence of hydroxylamine. Vision Res. 1968 Apr;8(4):339–358. doi: 10.1016/0042-6989(68)90104-1. [DOI] [PubMed] [Google Scholar]
- FRANK K., FUORTES M. G. Stimulation of spinal motoneurones with intracellular electrodes. J Physiol. 1956 Nov 28;134(2):451–470. doi: 10.1113/jphysiol.1956.sp005657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUORTES M. G., HODGKIN A. L. CHANGES IN TIME SCALE AND SENSITIVITY IN THE OMMATIDIA OF LIMULUS. J Physiol. 1964 Aug;172:239–263. doi: 10.1113/jphysiol.1964.sp007415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L. Quantum sensitivity of rods in the toad retina. Science. 1975 Mar 7;187(4179):838–841. doi: 10.1126/science.1114328. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W. A., Penn R. D., Yoshikami S. Dark current and photocurrent in retinal rods. Biophys J. 1970 May;10(5):380–412. doi: 10.1016/S0006-3495(70)86308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hárosi F. I. Absorption spectra and linear dichroism of some amphibian photoreceptors. J Gen Physiol. 1975 Sep;66(3):357–382. doi: 10.1085/jgp.66.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenbrot J. I., Cone R. A. Dark ionic flux and the effects of light in isolated rod outer segments. J Gen Physiol. 1972 Jul;60(1):20–45. doi: 10.1085/jgp.60.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasansky A., Marchiafava P. L. Light-induced resistance changes in retinal rods and cones of the tiger salamander. J Physiol. 1974 Jan;236(1):171–191. doi: 10.1113/jphysiol.1974.sp010429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister R. E., Noble D., Tsien R. W. Reconstruction of the electrical activity of cardiac Purkinje fibres. J Physiol. 1975 Sep;251(1):1–59. doi: 10.1113/jphysiol.1975.sp011080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millecchia R., Mauro A. The ventral photoreceptor cells of Limulus. 3. A voltage-clamp study. J Gen Physiol. 1969 Sep;54(3):331–351. doi: 10.1085/jgp.54.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millecchia R., Mauro A. The ventral photoreceptor cells of Limulus. II. The basic photoresponse. J Gen Physiol. 1969 Sep;54(3):310–330. doi: 10.1085/jgp.54.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. S-potentials from colour units in the retina of fish (Cyprinidae). J Physiol. 1966 Aug;185(3):536–555. doi: 10.1113/jphysiol.1966.sp008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Cones excite rods in the retina of the turtle. J Physiol. 1975 Apr;246(3):639–651. doi: 10.1113/jphysiol.1975.sp010908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Responses of single rods in the retina of the turtle. J Physiol. 1973 Aug;232(3):503–514. doi: 10.1113/jphysiol.1973.sp010283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda J., Hashimoto H., Anno H., Tomita T. The rod response in the frog and studies by intracellular recording. Vision Res. 1970 Nov;10(11):1093–1100. doi: 10.1016/0042-6989(70)90026-x. [DOI] [PubMed] [Google Scholar]
- Toyoda J., Nosaki H., Tomita T. Light-induced resistance changes in single photoreceptors of Necturus and Gekko. Vision Res. 1969 Apr;9(4):453–463. doi: 10.1016/0042-6989(69)90134-5. [DOI] [PubMed] [Google Scholar]
- Werblin F. S. Regenerative hyperpolarization in rods. J Physiol. 1975 Jan;244(1):53–81. doi: 10.1113/jphysiol.1975.sp010784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman R. Mechanisms of photoreceptor current generation in light and darkness. Nat New Biol. 1971 Nov 3;234(44):29–31. doi: 10.1038/newbio234029a0. [DOI] [PubMed] [Google Scholar]