Abstract
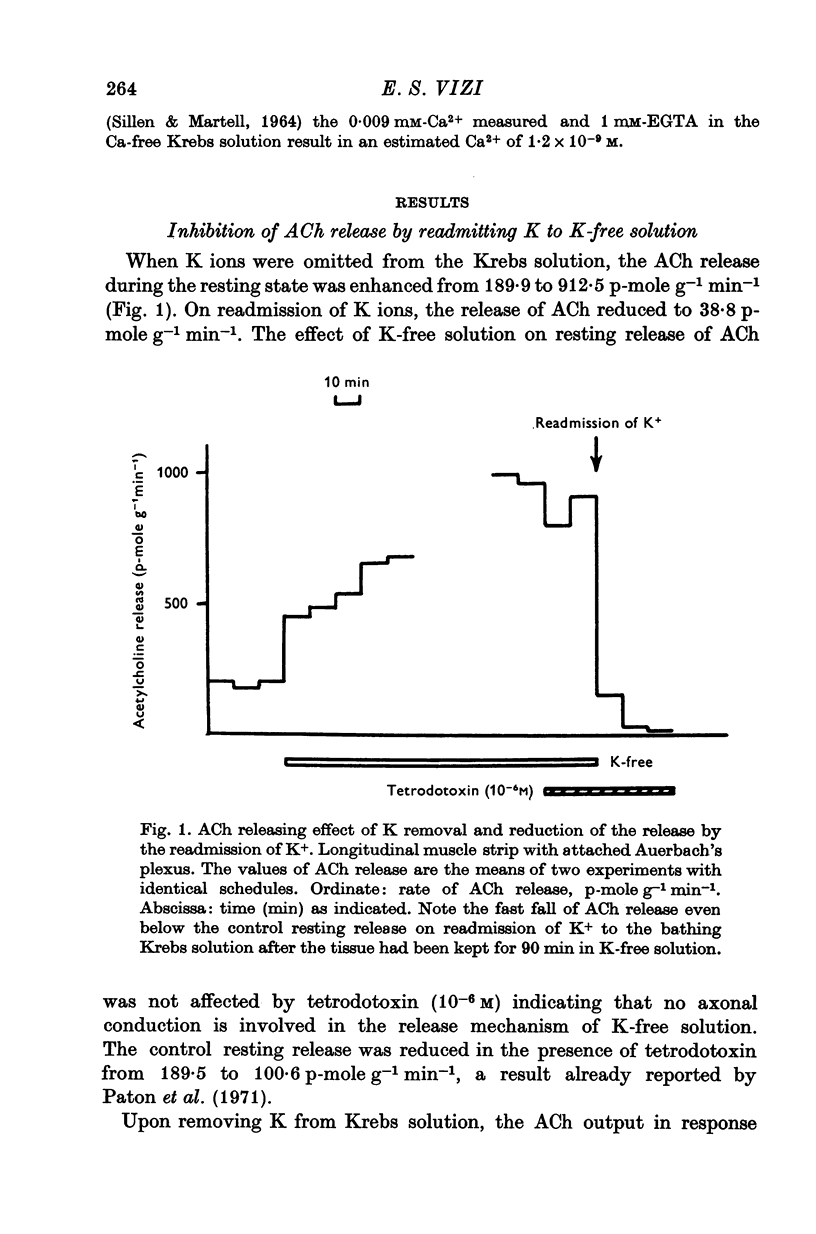
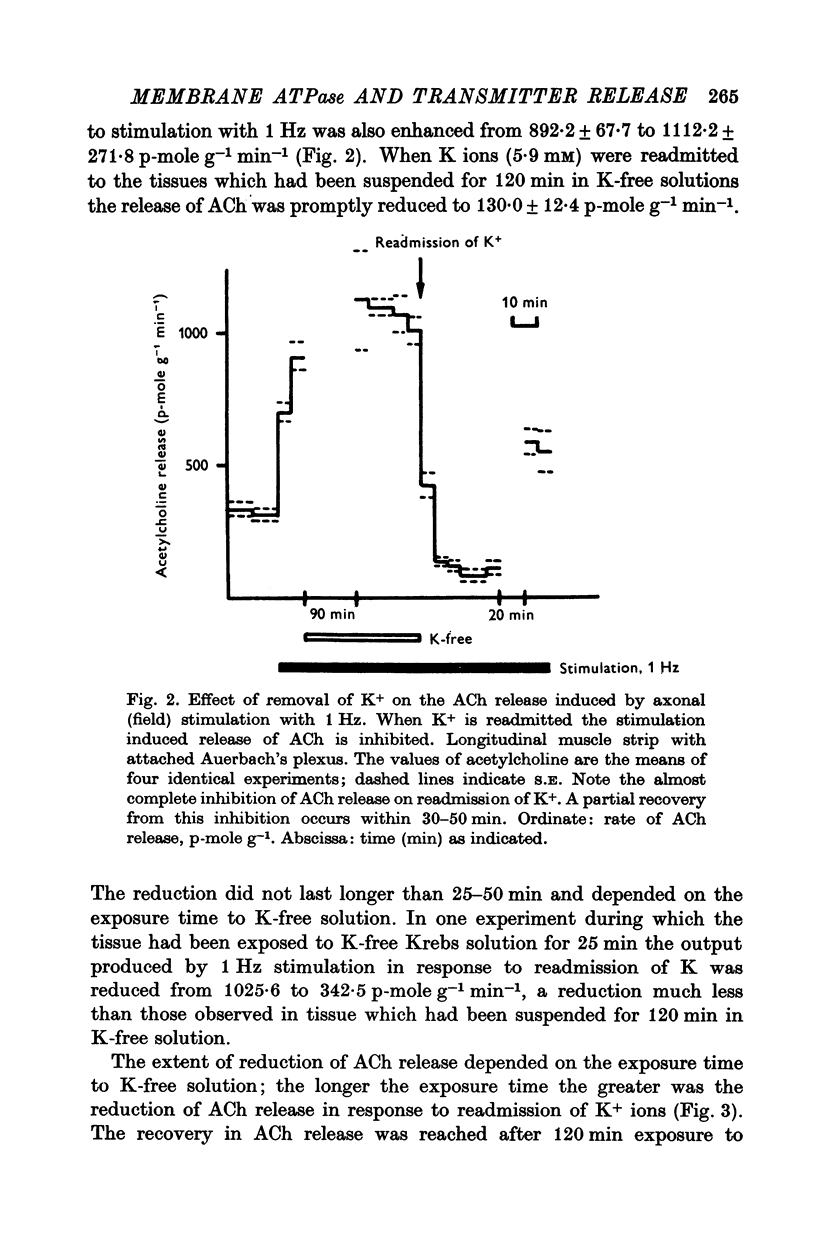
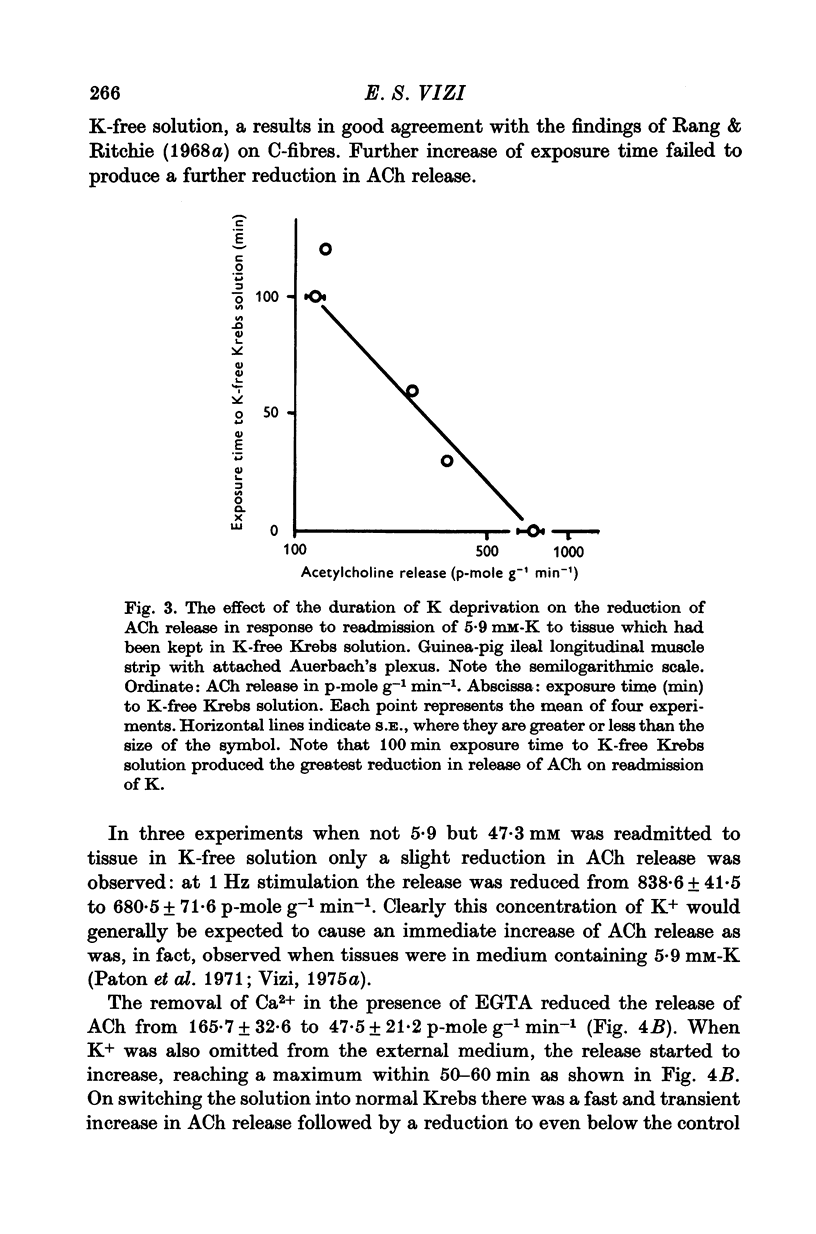
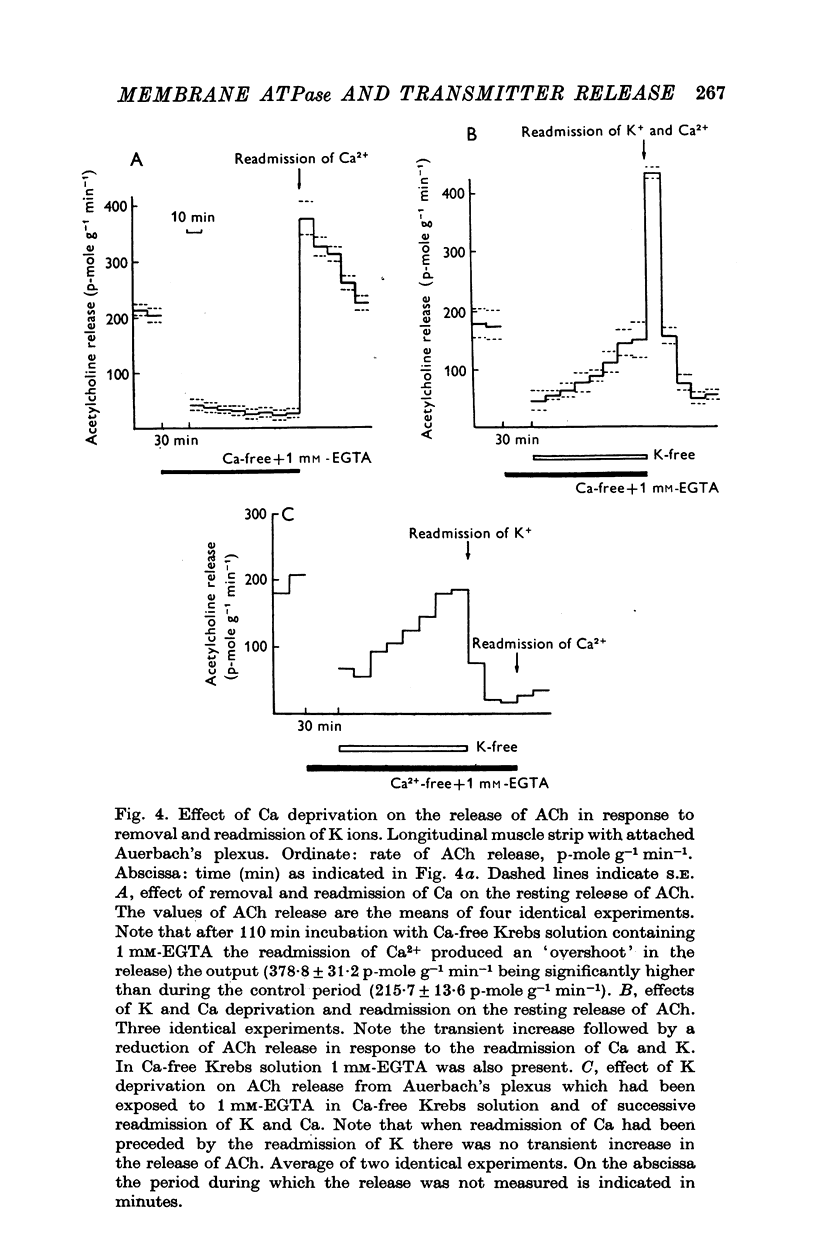
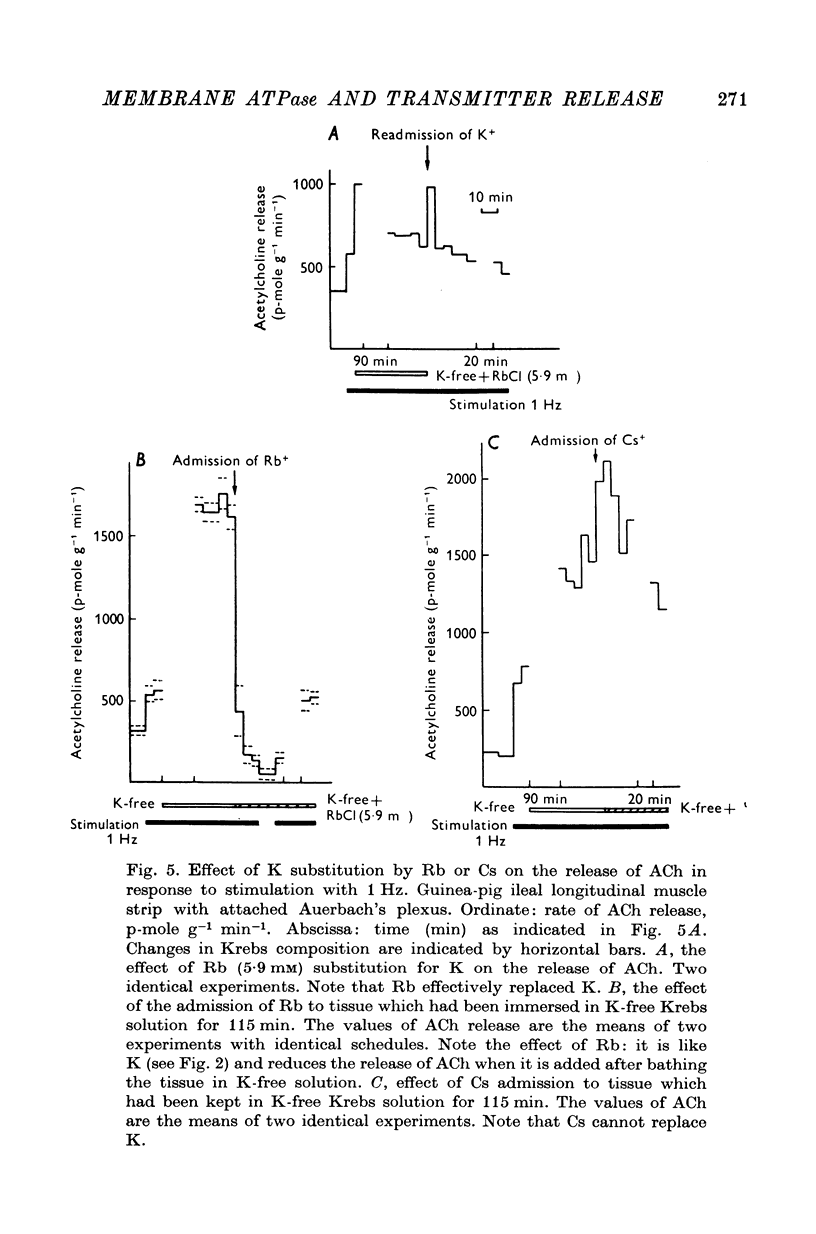
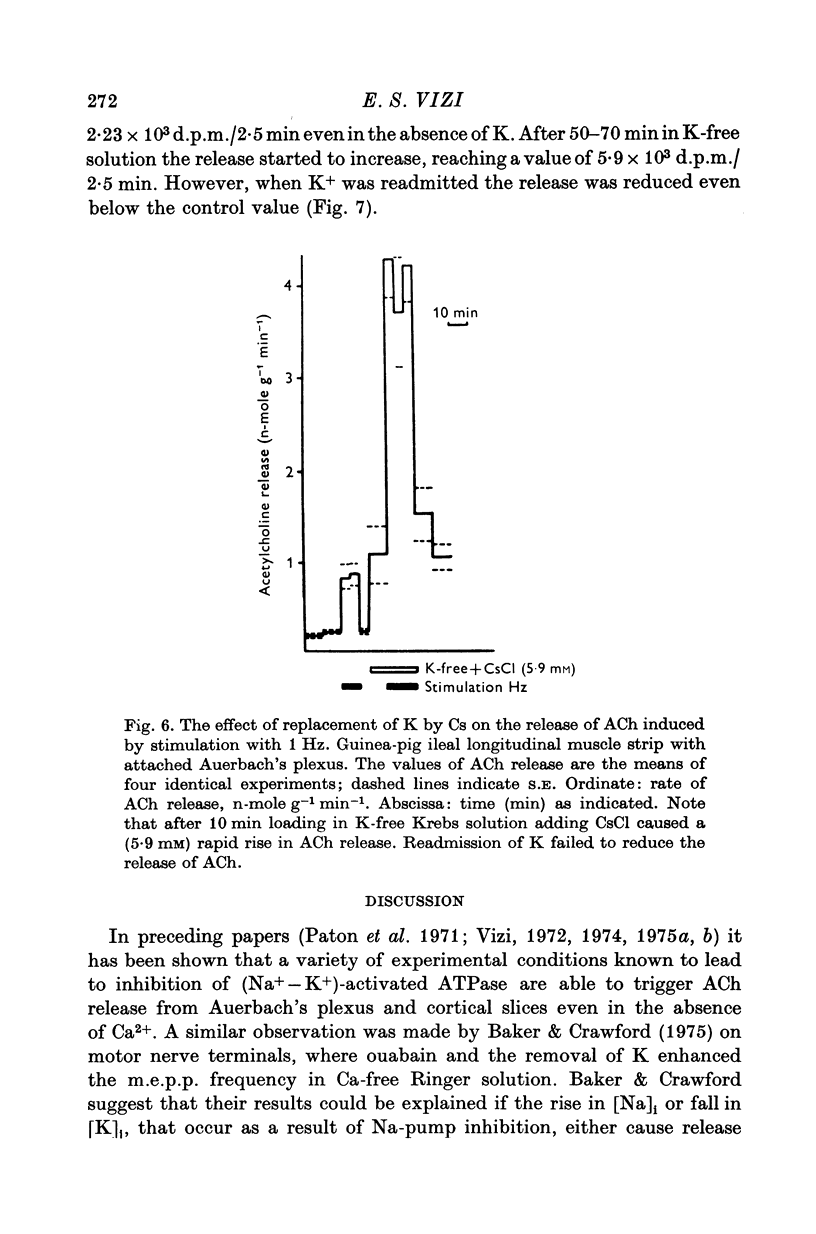
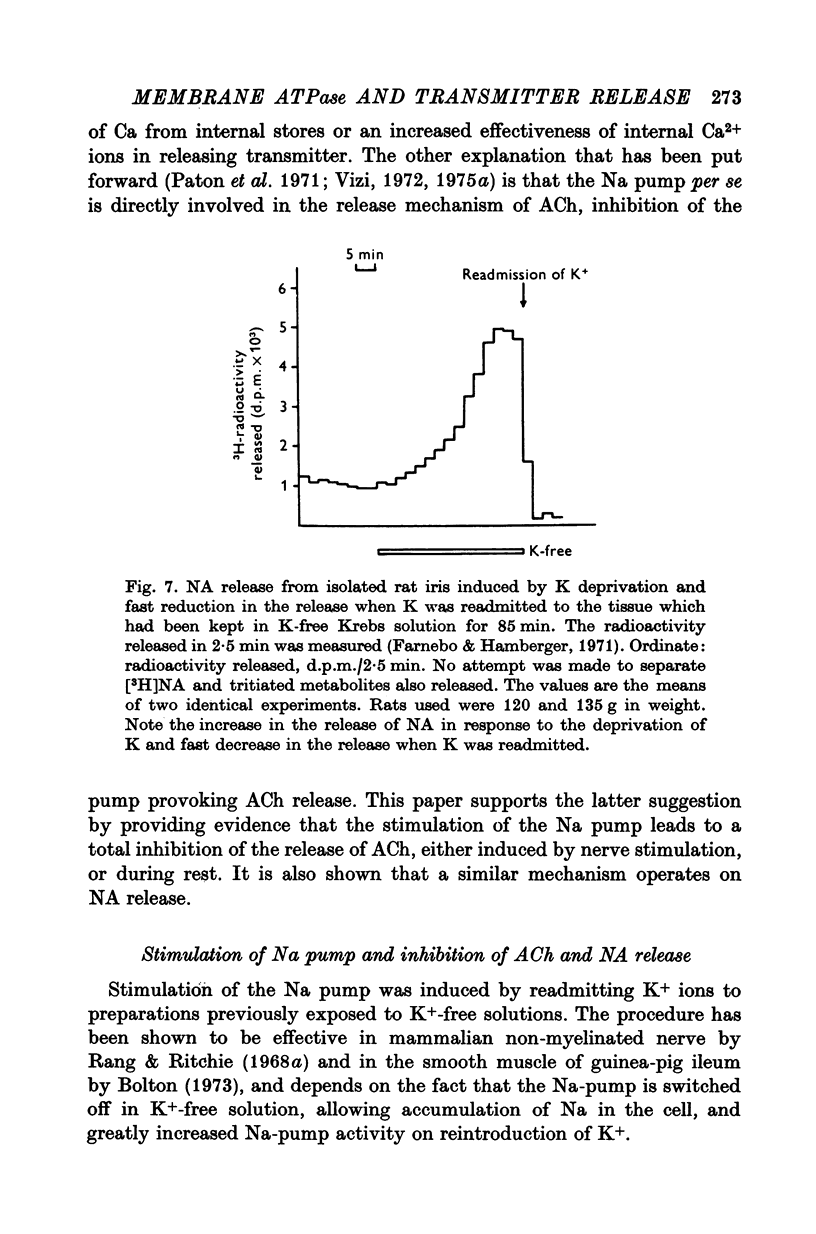
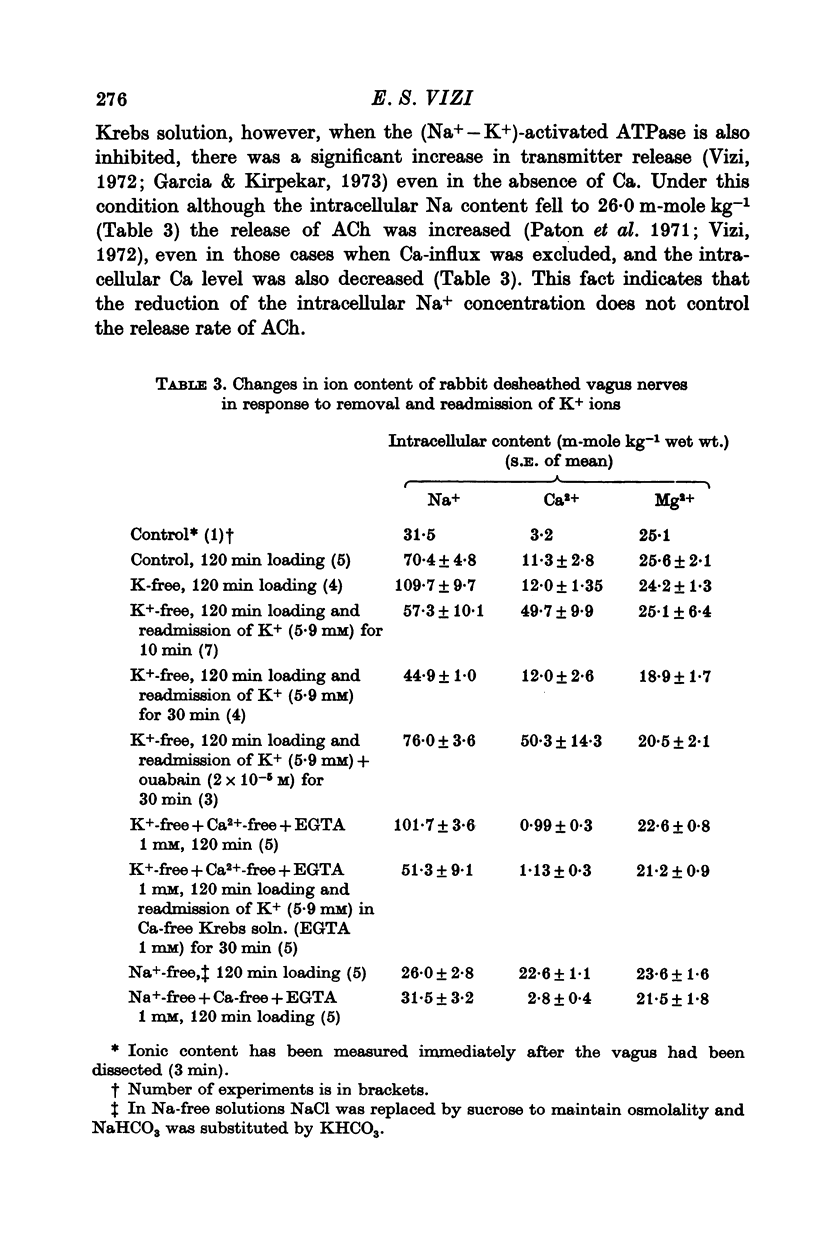
1. The release of acetylcholine (ACh) from Auerbach's plexus of guinea-pig ileum has been measured in eserinized Krebs solution using longitudinal muscle strip preparations. 2. Removal of the external K ions enhanced both the resting and stimulated release of ACh from the plexus. This effect was not affected by tetrodotoxin. 3. On readmission of K+ to tissues which had been suspended in K-free Krebs solution the release of ACh was promptly reduced in both stimulated and unstimulated tissues. The extent of the reduction of ACh release depended on the exposure time to K-free solution, the recovery being delayed by longer exposure. 4. The ACh releasing effect of (1,1-dimethyl-4-phenyl-piperazinium iodide (DMPP) was completely inhibited by the readmission of K ions to tissue which had been kept in K-free Krebs solution. 5. Rb+ substitution for K+ produced no change in ACh release and addition of 5-9 mM-Rb after K removal reduced the release of ACh as K did readmission. When the K ions were substituted by Cs+, both the resting and stimulated release were enhanced. The amount of ACh released by a stimulus was enhanced both at low and high frequency of sustained stimulation. 6. Removal of the external K ions increased the release of tritiated noradrenaline (NA), from isolated rat iris; however, when K+ (5-9 mM) was readmitted the release was reduced even below the control value. 7. It is concluded that the stimulation of (Na+-K+)-activated ATP-ase in the membrane inhibits the release of transmitter, and under physiological condition Ca-fluxes and the subsequent inhibition of membrane ATP-ase may be involved in triggering the release of transmitter.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
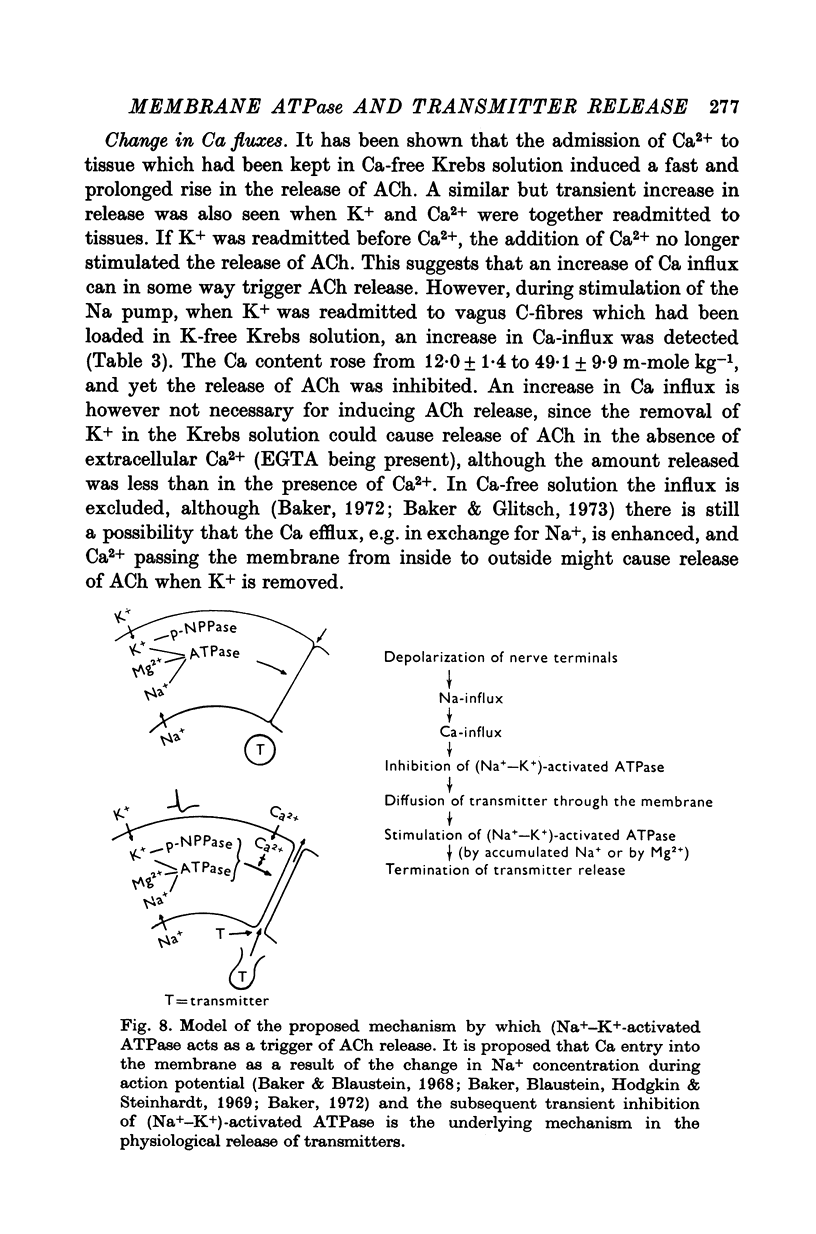
- Baker P. F., Blaustein M. P. Sodium-dependent uptake of calcium by crab nerve. Biochim Biophys Acta. 1968 Jan 3;150(1):167–170. doi: 10.1016/0005-2736(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Crawford A. C. A note of the mechanism by which inhibitors of the sodium pump accelerate spontaneous release of transmitter from motor nerve terminals. J Physiol. 1975 May;247(1):209–226. doi: 10.1113/jphysiol.1975.sp010928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Crawford A. C. Mobility and transport of magnesium in squid giant axons. J Physiol. 1972 Dec;227(3):855–874. doi: 10.1113/jphysiol.1972.sp010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Glitsch H. G. Does metabolic energy participate directly in the Na+-dependent extrusion of Ca2+ -Ca2+ ions from squid giant axons? J Physiol. 1973 Aug;233(1):44P–46P. [PubMed] [Google Scholar]
- Baker P. F. Transport and metabolism of calcium ions in nerve. Prog Biophys Mol Biol. 1972;24:177–223. doi: 10.1016/0079-6107(72)90007-7. [DOI] [PubMed] [Google Scholar]
- Birks R. I., Cohen M. W. The action of sodium pump inhibitors on neuromuscular transmission. Proc R Soc Lond B Biol Sci. 1968 Jul 9;170(1021):381–399. doi: 10.1098/rspb.1968.0046. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. Effects of electrogenic sodium pumping on the membrane potential of longitudinal smooth muscle from terminal ileum of guinea-pig. J Physiol. 1973 Feb;228(3):693–712. doi: 10.1113/jphysiol.1973.sp010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnebo L. O., Hamberger B. Drug-induced changes in the release of ( 3 H)-noradrenaline from field stimulated rat iris. Br J Pharmacol. 1971 Sep;43(1):97–106. doi: 10.1111/j.1476-5381.1971.tb07160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia A. G., Kirpekar S. M. Letter: Inhibition of Na, K-activated ATPase and release of neurotransmitters. Nature. 1975 Oct 23;257(5528):722–722. doi: 10.1038/257722b0. [DOI] [PubMed] [Google Scholar]
- Garcia A. G., Kirpekar S. M. Release of noradrenaline from the cat spleen by sodium deprivation. Br J Pharmacol. 1973 Apr;47(4):729–747. doi: 10.1111/j.1476-5381.1973.tb08200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Willis W. D. The effects of depolarization of motor nerve terminals upon the release of transmitter by nerve impulses. J Physiol. 1968 Feb;194(2):381–405. doi: 10.1113/jphysiol.1968.sp008414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D. The ionic fluxes in frog muscle. Proc R Soc Lond B Biol Sci. 1954 May 27;142(908):359–382. doi: 10.1098/rspb.1954.0030. [DOI] [PubMed] [Google Scholar]
- Keynes R. D., Ritchie J. M. The movements of labelled ions in mammalian non-myelinated nerve fibres. J Physiol. 1965 Jul;179(2):333–367. doi: 10.1113/jphysiol.1965.sp007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. The effects of presynaptic polarization on the spontaneous activity at the mammalian neuromuscular junction. J Physiol. 1956 Nov 28;134(2):427–443. doi: 10.1113/jphysiol.1956.sp005655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. The quantal components of the mammalian end-plate potential. J Physiol. 1956 Sep 27;133(3):571–587. doi: 10.1113/jphysiol.1956.sp005610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer S. Z. The metabolism of (3H)noradrenaline released by electrical stimulation from the isolated nictitating membrane of the cat and from the vas deferens of the rat. J Physiol. 1970 Jul;208(3):515–546. doi: 10.1113/jphysiol.1970.sp009135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton W. D., Vizi E. S. The inhibitory action of noradrenaline and adrenaline on acetylcholine output by guinea-pig ileum longitudinal muscle strip. Br J Pharmacol. 1969 Jan;35(1):10–28. doi: 10.1111/j.1476-5381.1969.tb07964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton W. D., Vizi E. S., Zar M. A. The mechanism of acetylcholine release from parasympathetic nerves. J Physiol. 1971 Jul;215(3):819–848. doi: 10.1113/jphysiol.1971.sp009500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. On the electrogenic sodium pump in mammalian non-myelinated nerve fibres and its activation by various external cations. J Physiol. 1968 May;196(1):183–221. doi: 10.1113/jphysiol.1968.sp008502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. The ionic content of mammalian non-myelinated nerve fibres and its alteration as a result of electrical activity. J Physiol. 1968 May;196(1):223–236. doi: 10.1113/jphysiol.1968.sp008503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Electrical changes in pre- and postsynaptic axons of the giant synapse of Loligo. J Gen Physiol. 1962 Jul;45:1181–1193. doi: 10.1085/jgp.45.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T., Yamamoto T. Effects of removing the external potassium on the smooth muscle of guinea-pig taenia coli. J Physiol. 1971 Feb;212(3):851–868. doi: 10.1113/jphysiol.1971.sp009360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizi E. S. Stimulation, by inhibition of (Na + -K + -Mg 2+ )-activated ATP-ase, of acetylcholine release in cortical slices from rat brain. J Physiol. 1972 Oct;226(1):95–117. doi: 10.1113/jphysiol.1972.sp009975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wespi H. H. Active transport and passive fluxes of K, Na, and Li in mammalian non-myelinated nerve fibres. Pflugers Arch. 1969;306(3):262–280. doi: 10.1007/BF00592437. [DOI] [PubMed] [Google Scholar]


