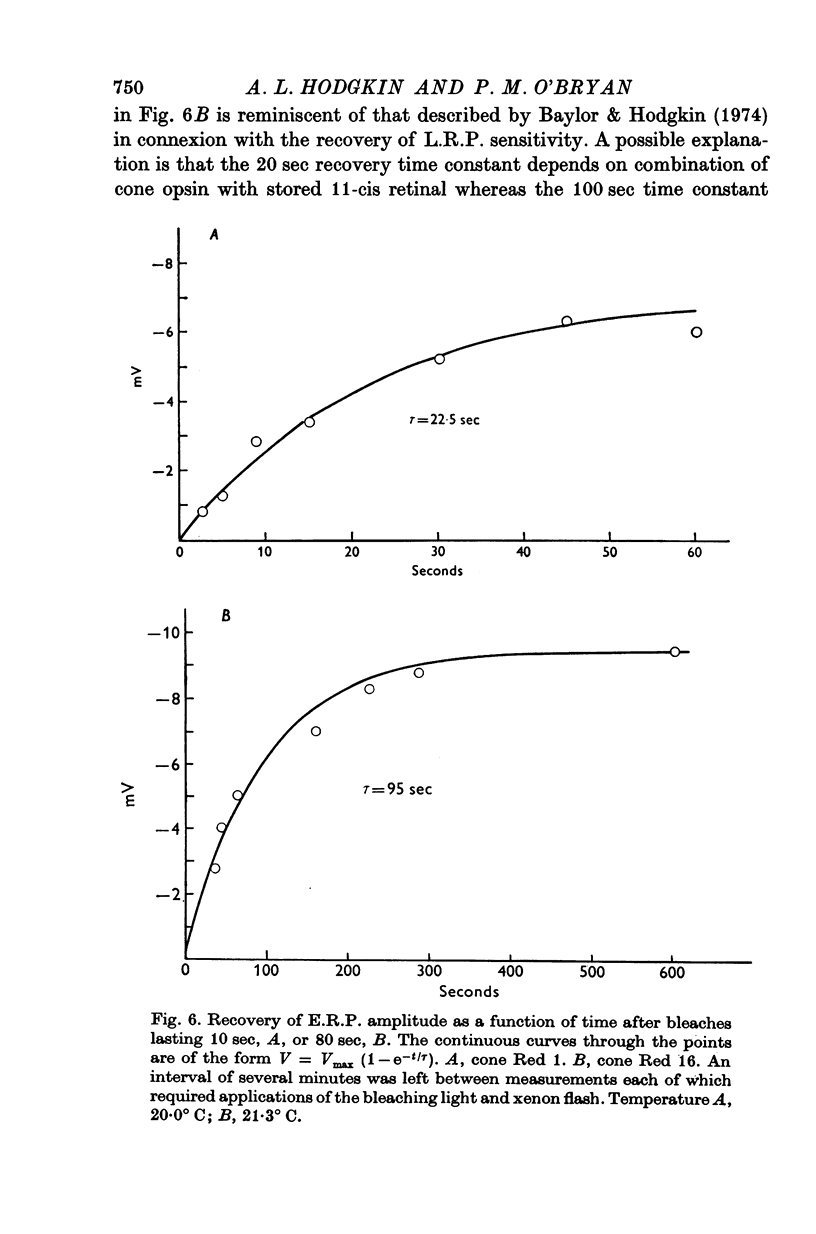
Abstract
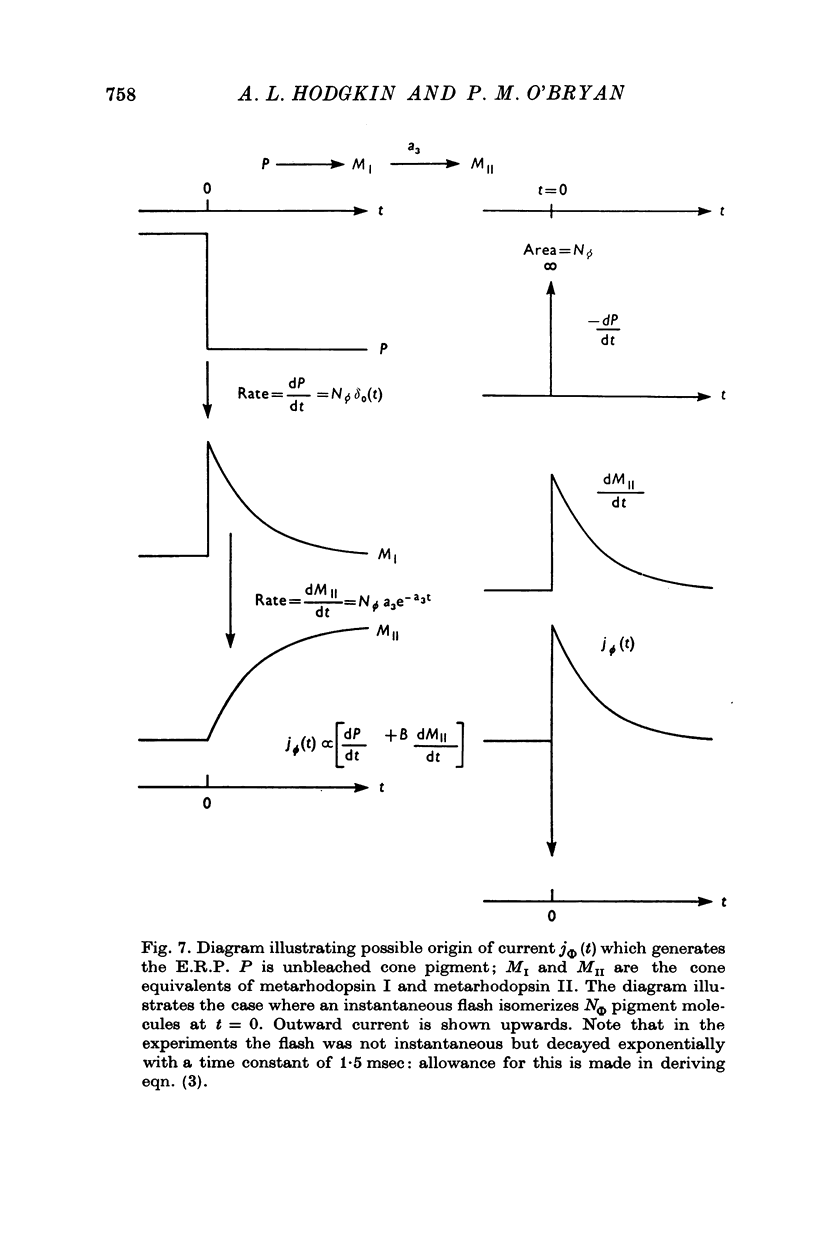
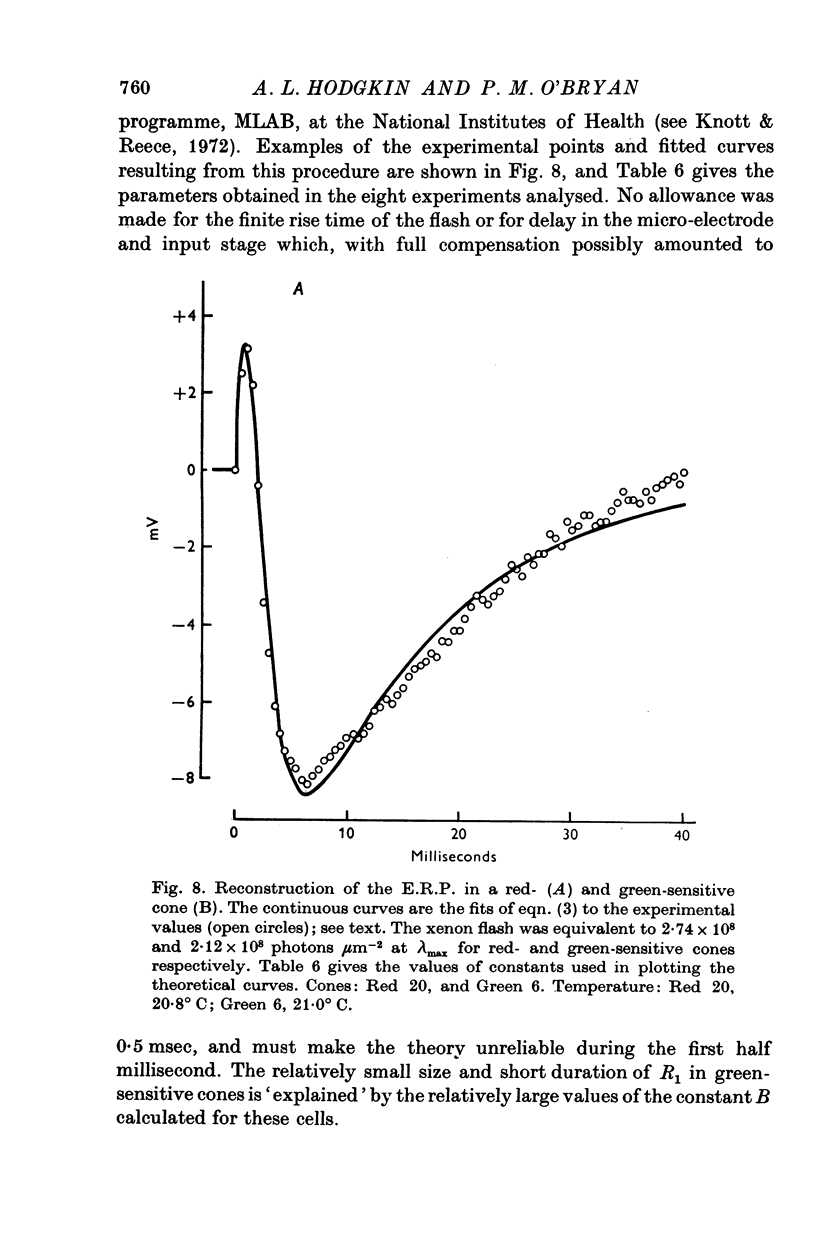
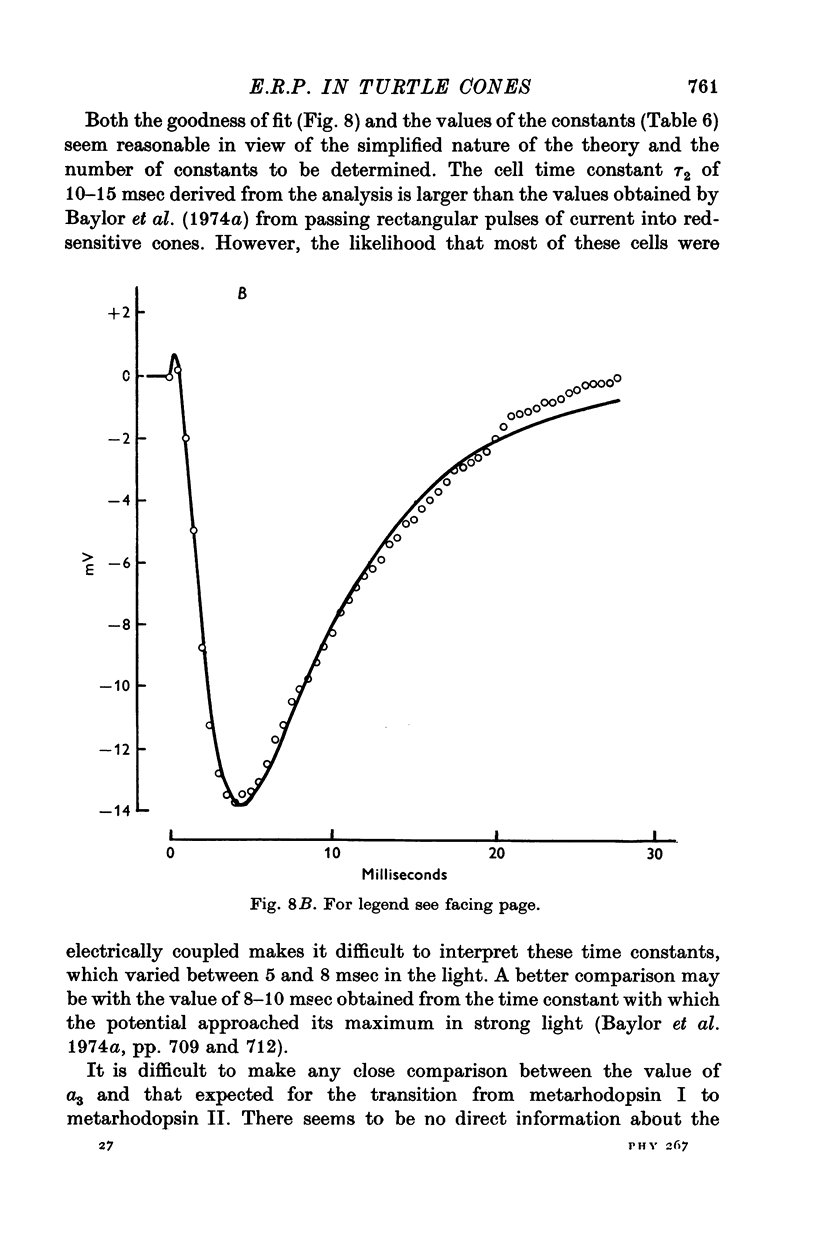
1. Early receptor potentials (E.R.P.s) were recorded with internal electrodes in turtle cones by applying brief flashes from a xenon tube with a maximum photon density equivalent to 2-3 x 10(8) photons micronm-2 at the optimum wave-length. 2. The E.R.P. was separated from the late receptor potential (L.R.P.) by superposing in flash on a step of light which was strong enough to saturate the L.R.P. 3. In red-sensitive cones the E.R.P. consisted of a brief depolarizing phase (R1) followed by a hyperpolarizing phase (R2) of maximum amplitude 10 mV and duration 30-40 msec. R1 was small or absent in green-sensitive cones. 4. With flashes of increasing intensity the E.R.P. approached its maximum exponentially with an exponential constant Q of about 10(8) photons micronm-2 which is of the same order as the reciprocal of the photosensitivity of porphyropsin; the implication of this result, which is considered in the theoretical section, is the the E.R.P. is proportional to the number of photoisomerizations. 5. When tested with a constant xenon flash at varying times after the beginning of a bleaching light the E.R.P. declined exponentially with a similar value of Q. 6. After prolonged bleaches the E.R.P. recovered with a time constant of about 100 sec but much quicker recoveries were observed after relatively brief bleaches. 7. The form and size of the E.R.P. are consistent with the accepted view that it arises from a redistribution of charge in the cone pigment molecule. 8. The effect of a single photoisomerization in an isolated cone was estimated as about 10(-10) V or one electronic charge through about 10% of the membrane.
Full text
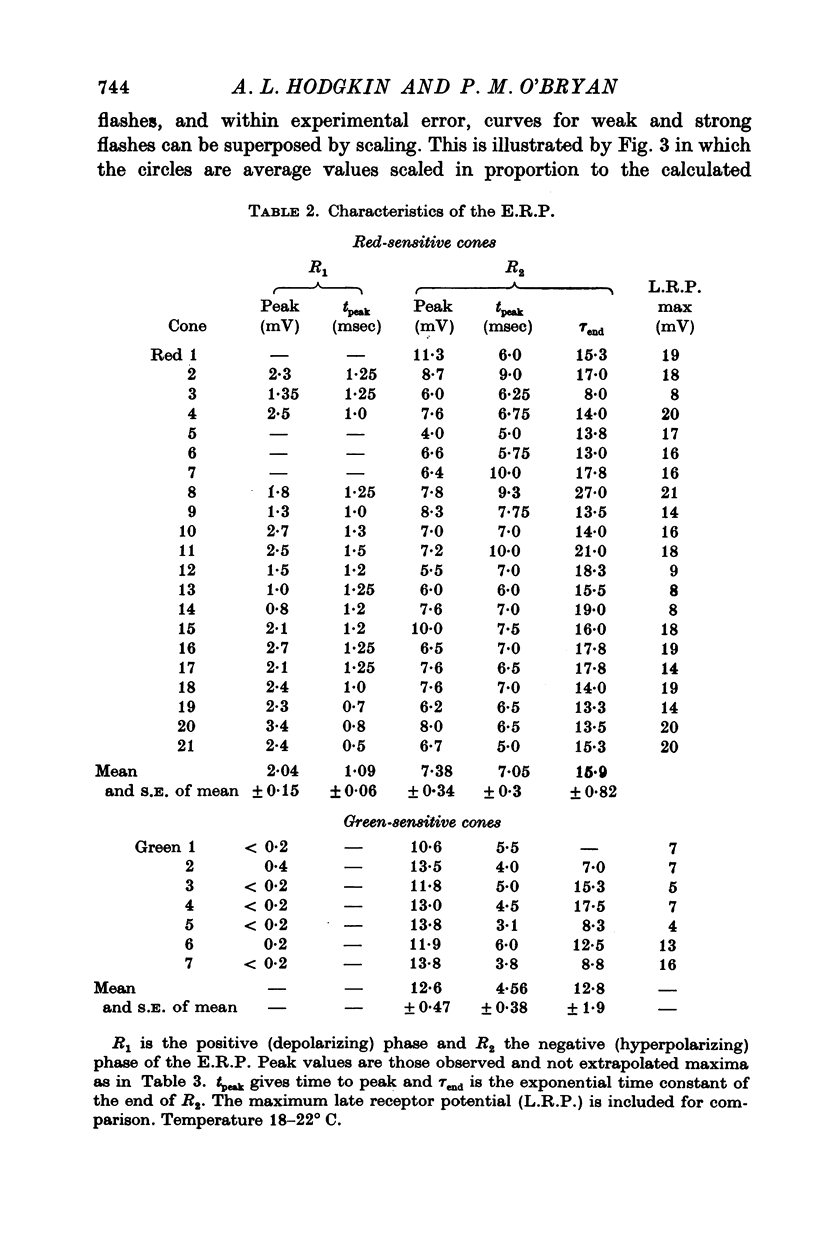
PDF



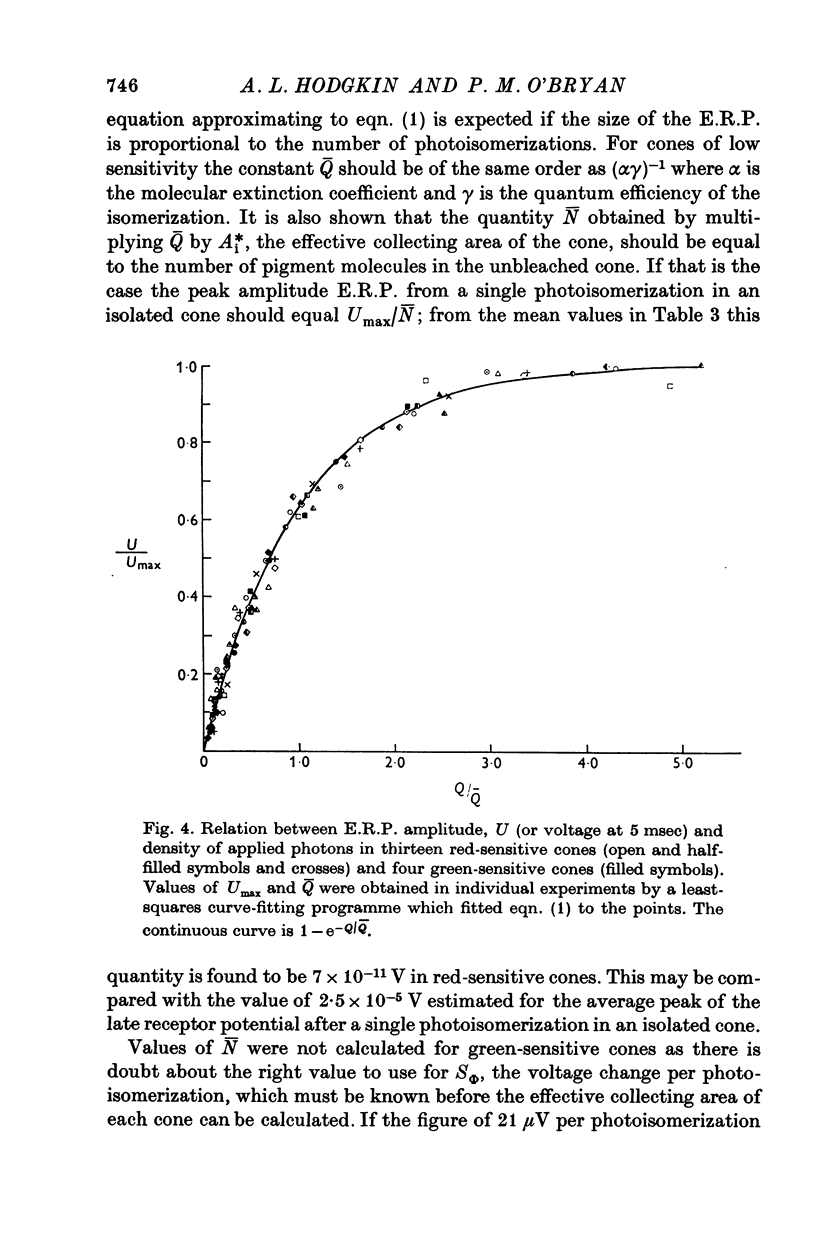
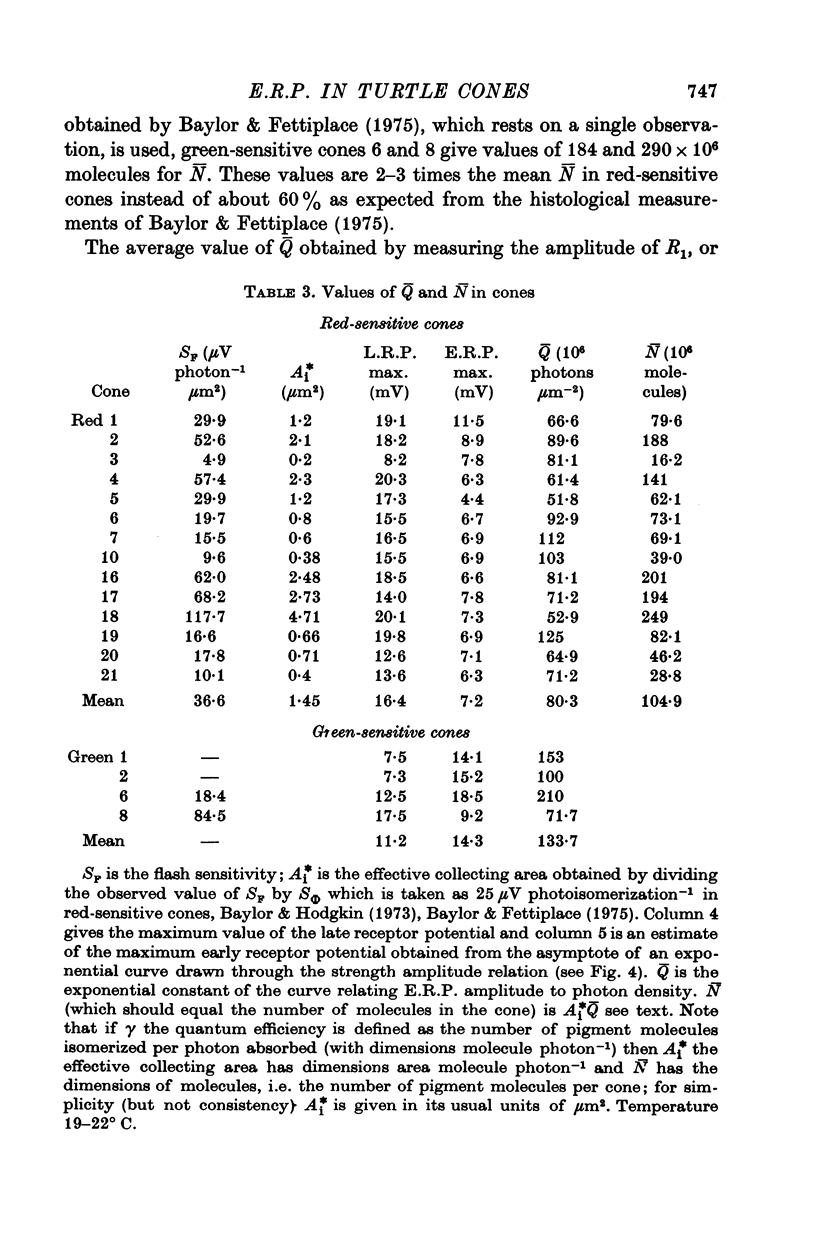
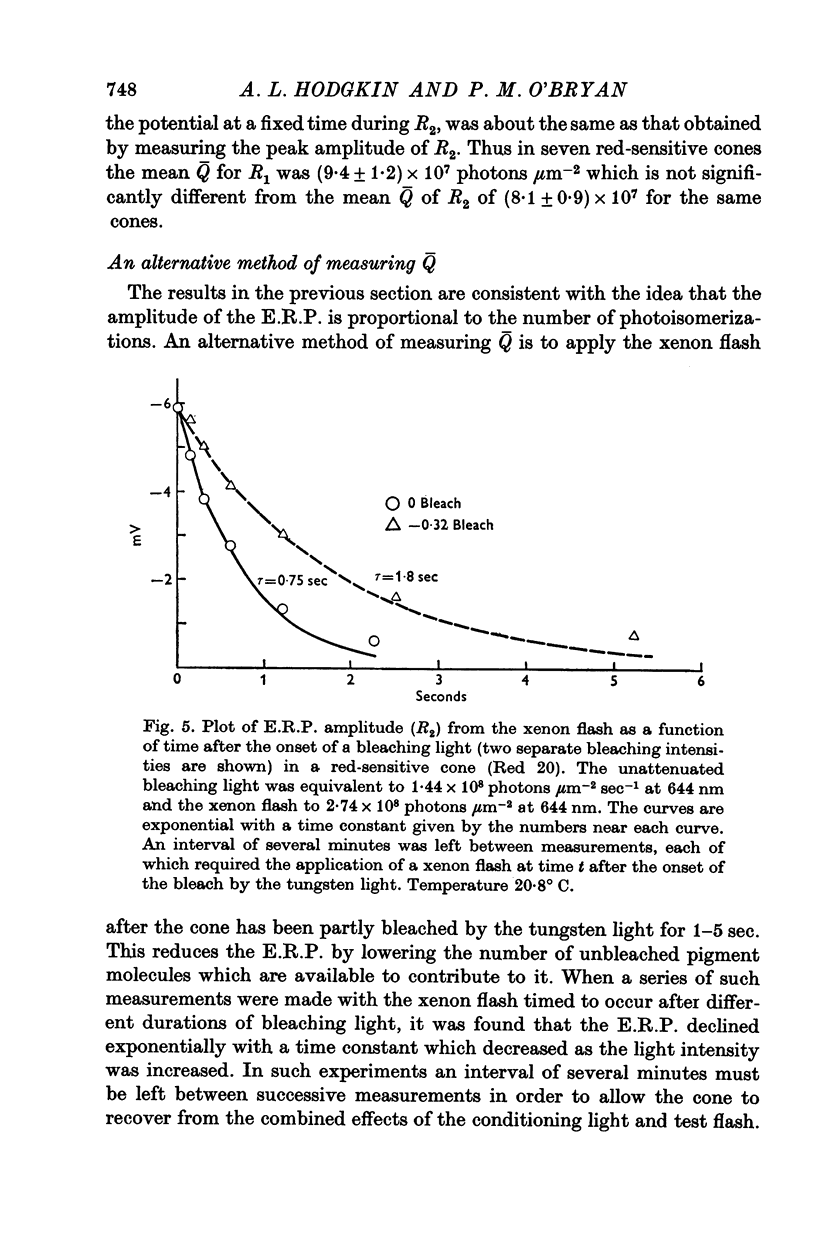













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN K. T., MURAKAMI M. A NEW RECEPTOR POTENTIAL OF THE MONKEY RETINA WITH NO DETECTABLE LATENCY. Nature. 1964 Feb 8;201:626–628. doi: 10.1038/201626a0. [DOI] [PubMed] [Google Scholar]
- Baumann C. The formation of metarhodospin380 in the retinal rods of the frog. J Physiol. 1976 Jul;259(2):357–366. doi: 10.1113/jphysiol.1976.sp011470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fettiplace R. Light path and photon capture in turtle photoreceptors. J Physiol. 1975 Jun;248(2):433–464. doi: 10.1113/jphysiol.1975.sp010983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Changes in time scale and sensitivity in turtle photoreceptors. J Physiol. 1974 Nov;242(3):729–758. doi: 10.1113/jphysiol.1974.sp010732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Detection and resolution of visual stimuli by turtle photoreceptors. J Physiol. 1973 Oct;234(1):163–198. doi: 10.1113/jphysiol.1973.sp010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L., Lamb T. D. Reconstruction of the electrical responses of turtle cones to flashes and steps of light. J Physiol. 1974 Nov;242(3):759–791. doi: 10.1113/jphysiol.1974.sp010733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L., Lamb T. D. The electrical response of turtle cones to flashes and steps of light. J Physiol. 1974 Nov;242(3):685–727. doi: 10.1113/jphysiol.1974.sp010731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindley G. S., Gardner-Medwin A. R. The origin of the early receptor potential of the retina. J Physiol. 1966 Jan;182(1):185–194. doi: 10.1113/jphysiol.1966.sp007817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone R. A. Early receptor potential: photoreversible charge displacement in rhodopsin. Science. 1967 Mar 3;155(3766):1128–1131. doi: 10.1126/science.155.3766.1128. [DOI] [PubMed] [Google Scholar]
- Goldstein E. B., Price T. M. Temperature dependence of cone pigment regeneration in the isolated frog retina following flash and continuous bleaches. Vision Res. 1975 Apr;15(4):477–481. doi: 10.1016/0042-6989(75)90024-3. [DOI] [PubMed] [Google Scholar]
- Hagins W. A., McGaughy R. E. Molecular and thermal origins of fast photoelectric effects in the squid retina. Science. 1967 Aug 18;157(3790):813–816. doi: 10.1126/science.157.3790.813. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Granda A. M. Microspectrophotometric measurements of visual pigments in two species of turtle, Pseudemys scripta and Chelonia mydas. Vision Res. 1971 Feb;11(2):105–114. doi: 10.1016/0042-6989(71)90227-6. [DOI] [PubMed] [Google Scholar]
- Murakami M., Pak W. L. Intracellularly recorded early receptor potential of the vertebrate photoreceptors. Vision Res. 1970 Oct;10(10):965–975. doi: 10.1016/0042-6989(70)90074-x. [DOI] [PubMed] [Google Scholar]
- Rushton W. A., Henry G. H. Bleaching and regeneration of cone pigments in man. Vision Res. 1968 Jun;8(6):617–631. doi: 10.1016/0042-6989(68)90040-0. [DOI] [PubMed] [Google Scholar]
- Williams T. P., Breil S. J. Kinetic measurements on rhodopsin solutions during intense flashes. Vision Res. 1968 Jul;8(7):777–786. doi: 10.1016/0042-6989(68)90129-6. [DOI] [PubMed] [Google Scholar]
- Williams T. P. Upper limits to the bleaching of rhodopsin by high intensity flashes. Vision Res. 1974 Aug;14(8):603–607. doi: 10.1016/0042-6989(74)90053-4. [DOI] [PubMed] [Google Scholar]


