Abstract
1. The spectral sensitivity, orientation specificity and inhibitory surround of seventy-three cells were studied in the vervet monkey. The eye was in the dark or illuminated with steady white or spectral light. The cells were in the striate cortex corresponding to the foveal representation. Nearly all the cells gave on- or on/off-responses.
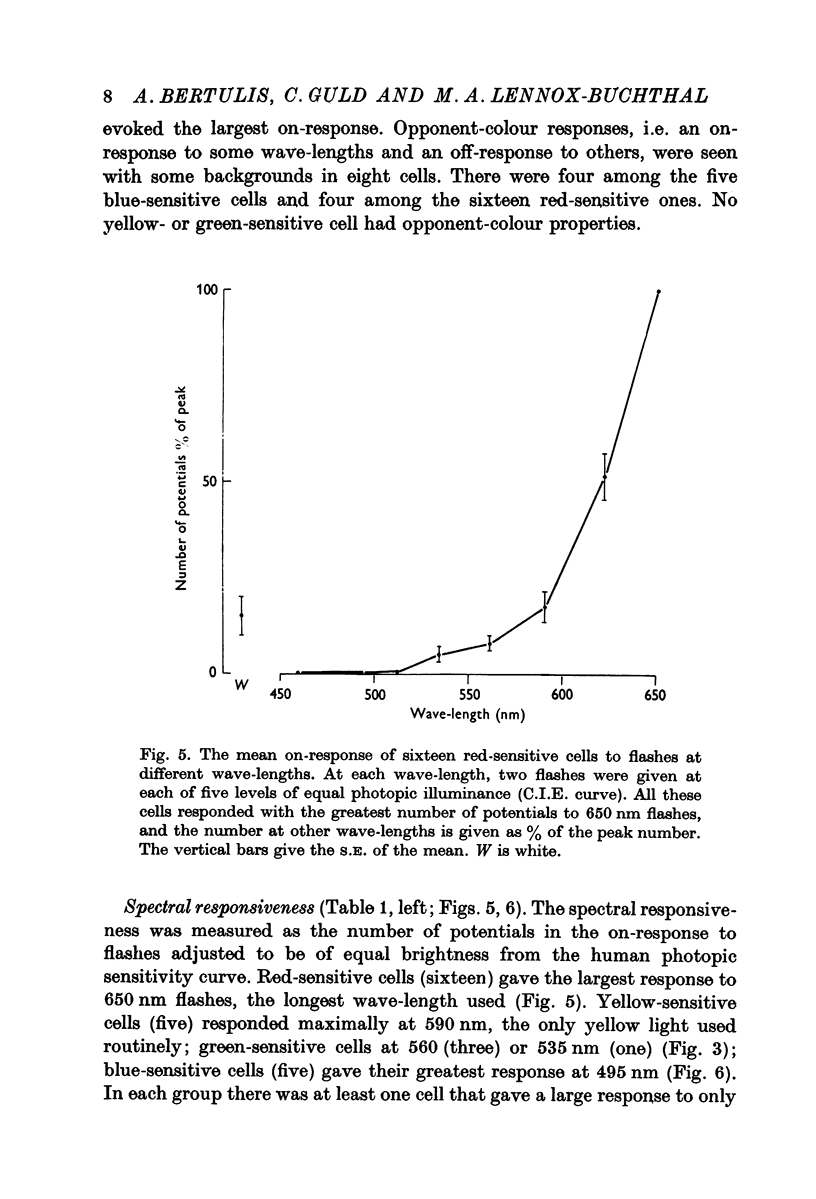
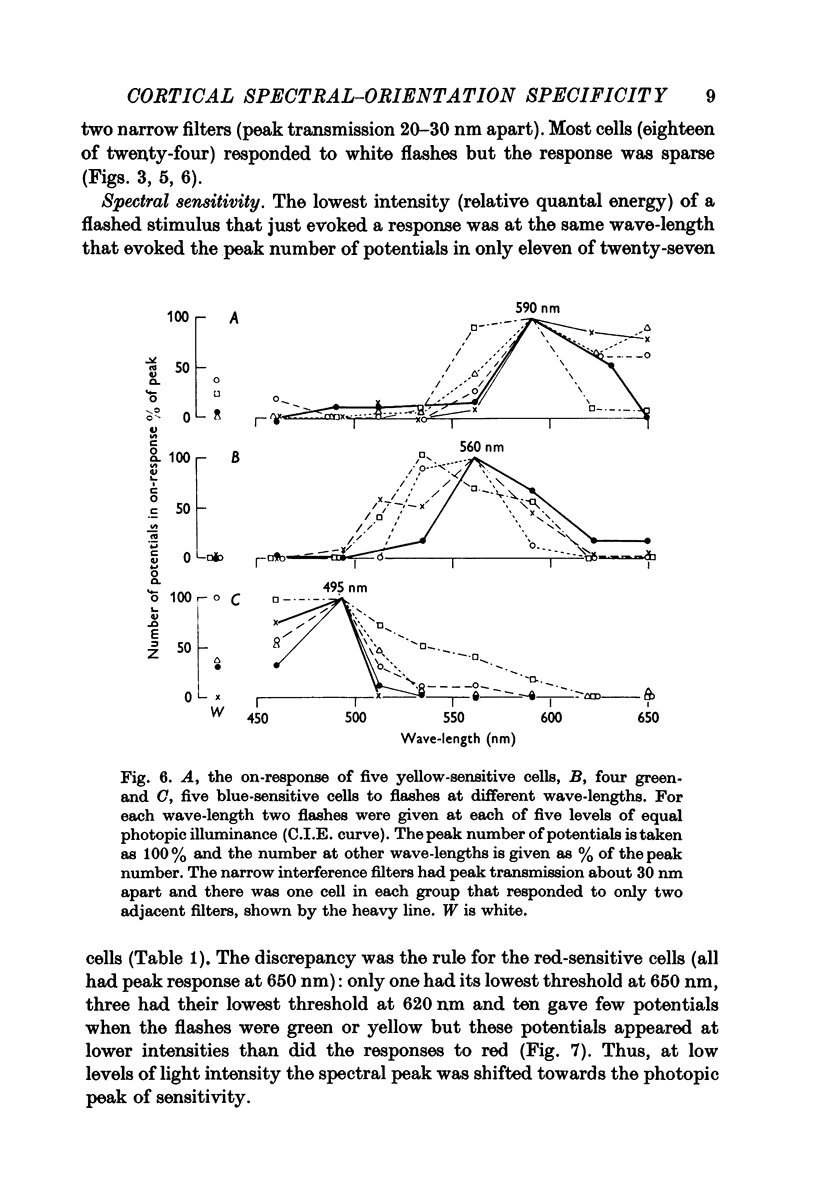
2. More than a third of the cells (41%) responded over a narrow spectral band, in the blue, green, yellow or red section. Three quarters of them were orientation specific with flanking inhibitory surround and half of these were of the `stopped-end' variety as well. The effect of the wave-length of the background indicated that only a half were activated by such excitatory—inhibitory colour pairs as have been described in the geniculate nucleus.
3. A third of the cells (36%) responded to most colours but with the greatest response to green, yellow or red. Less than half were orientation specific. Unlike the narrowband cells, the response decreased with the intensity of the light. Two thirds were activated by the excitatory-inhibitory colour pairs that have been described in the geniculate nucleus.
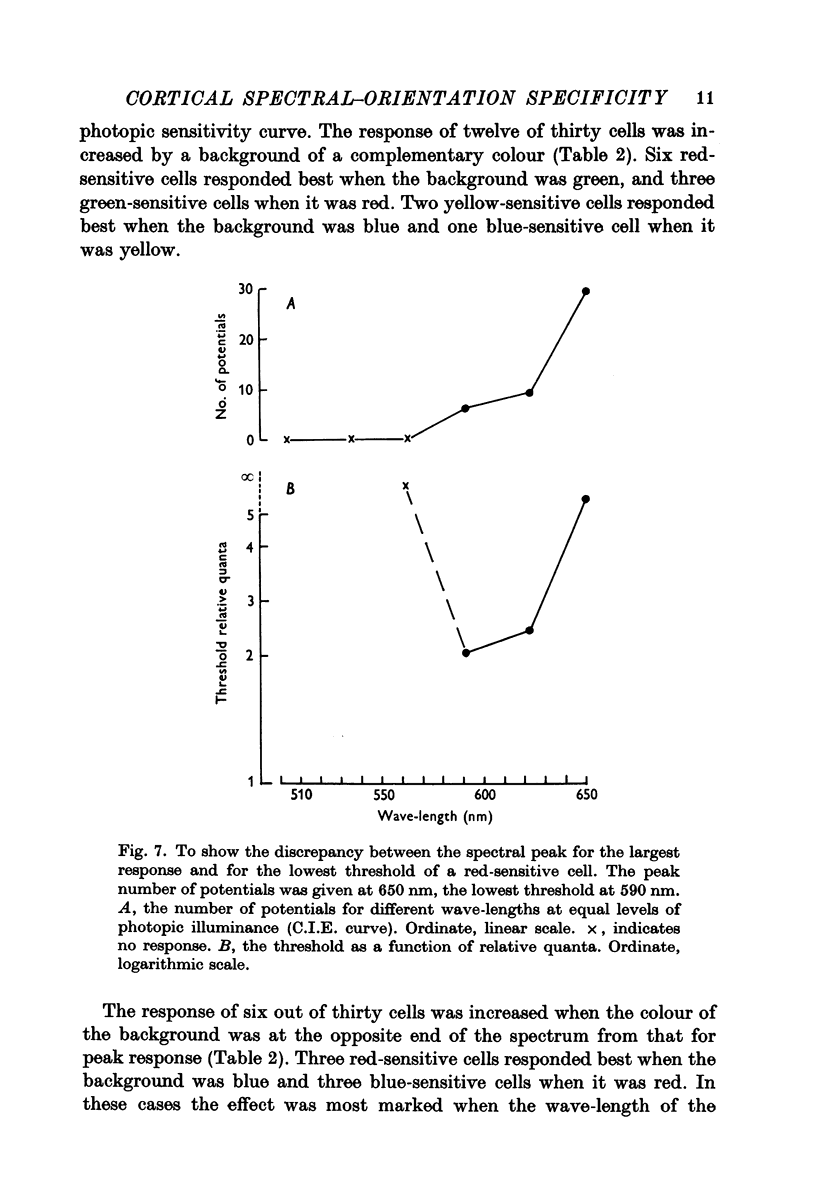
4. In both groups of cells the wave-length of the spectral peak could be different when the stimuli were weak compared with when they were strong.
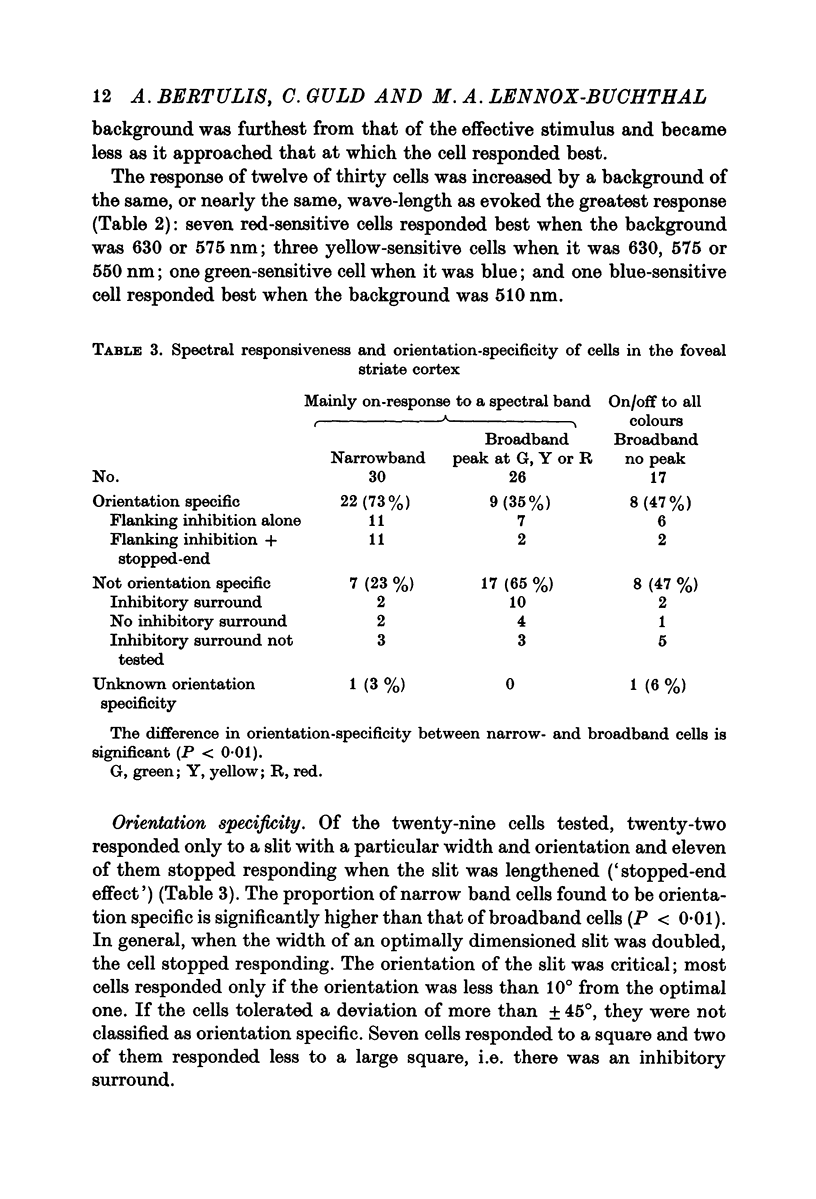
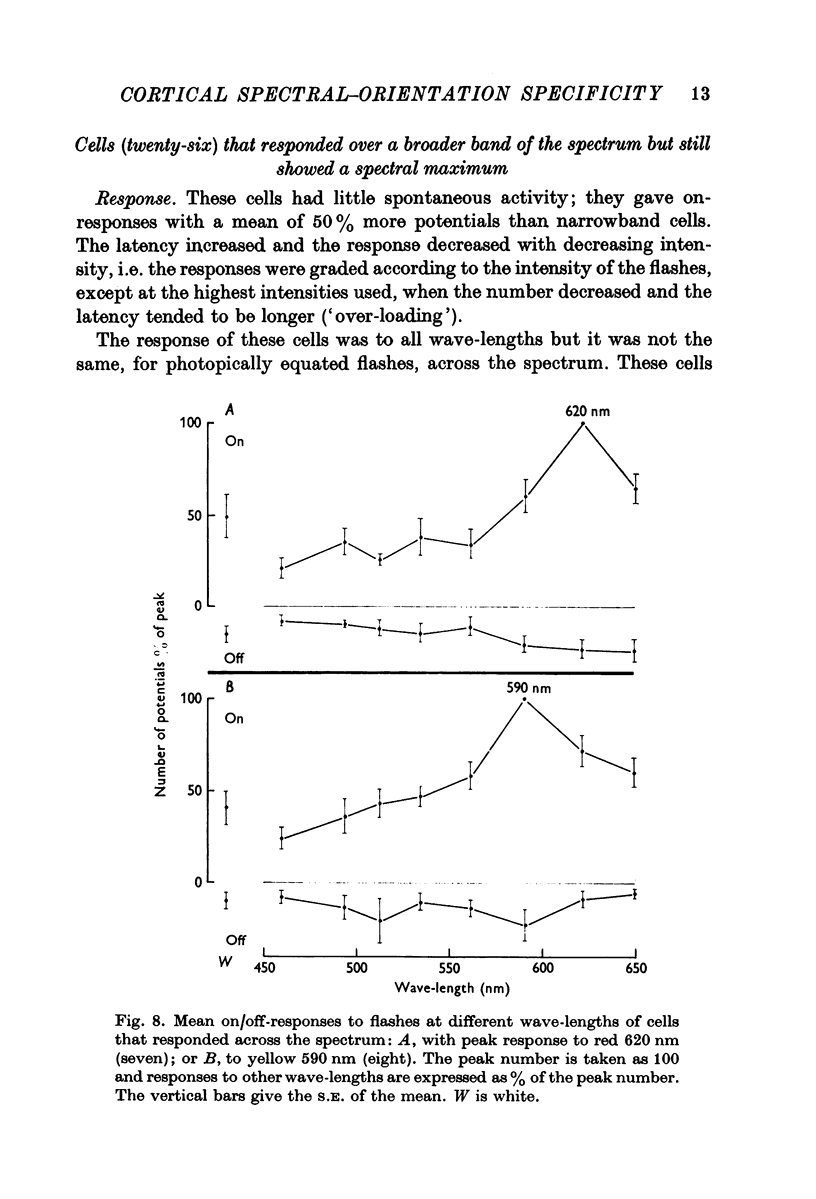
5. One quarter of the cells (23%) gave on/off-responses to all spectral flashes; half were not orientation specific. The difference in orientation specificity between narrow- and broadband cells is significant (P < 0·01).
Full text
PDF

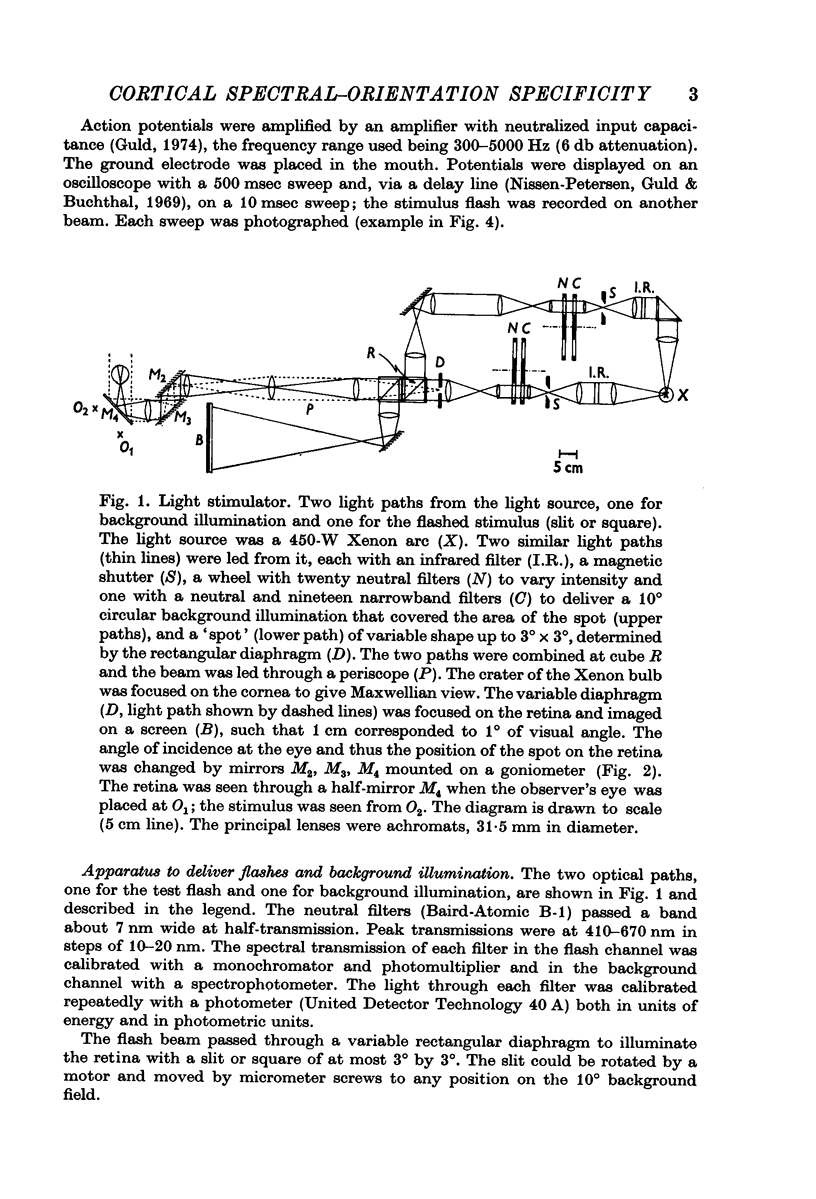
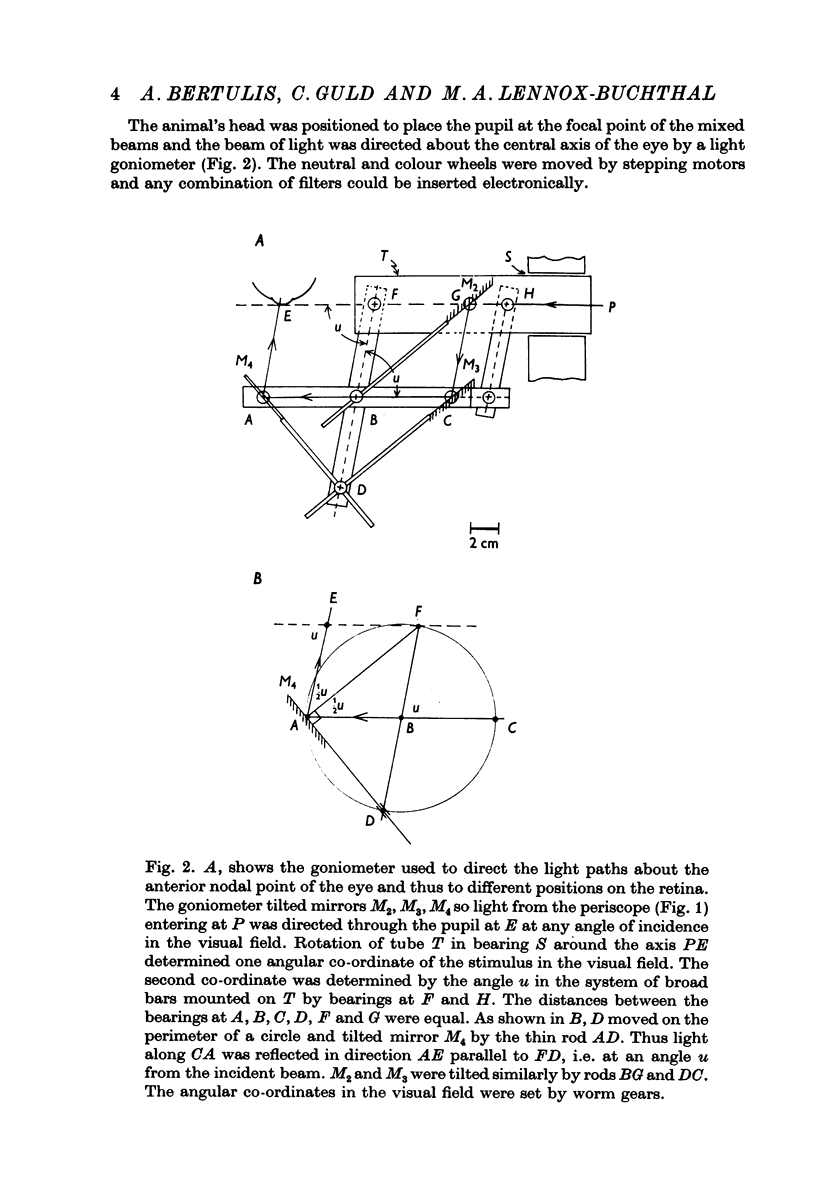

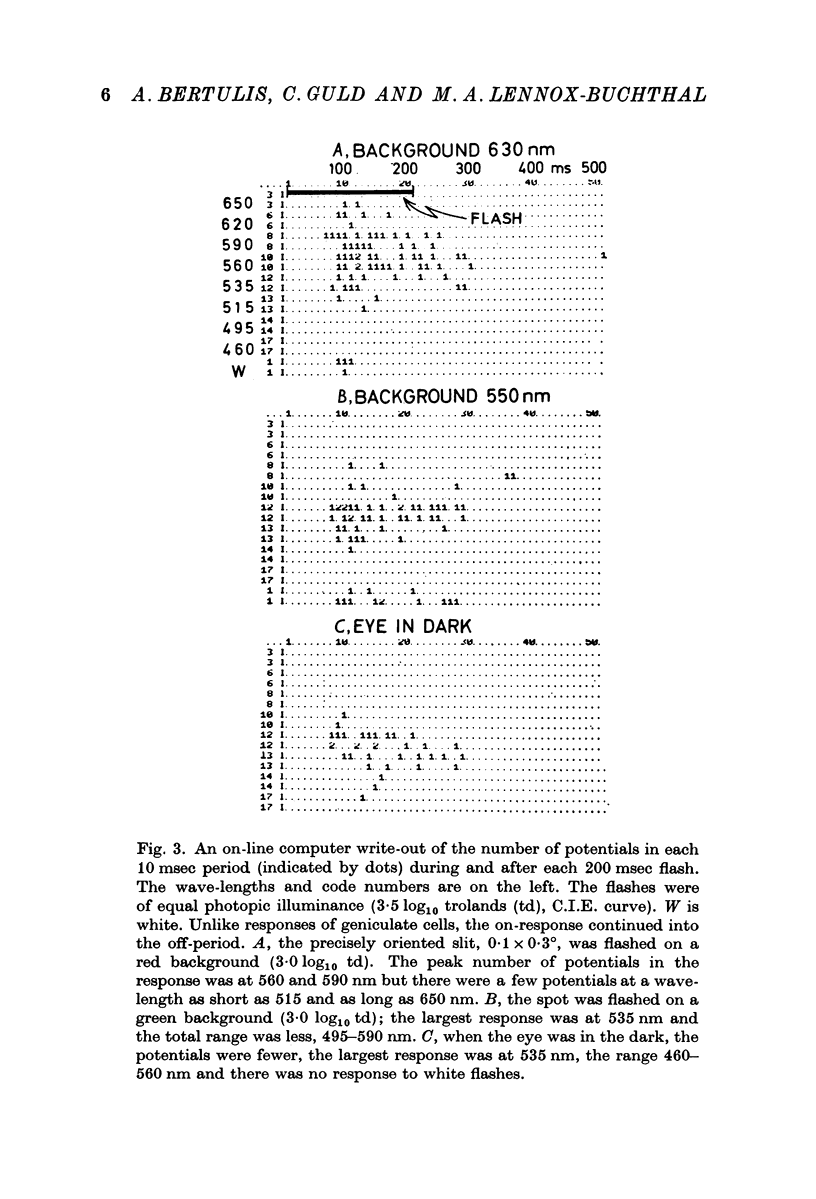
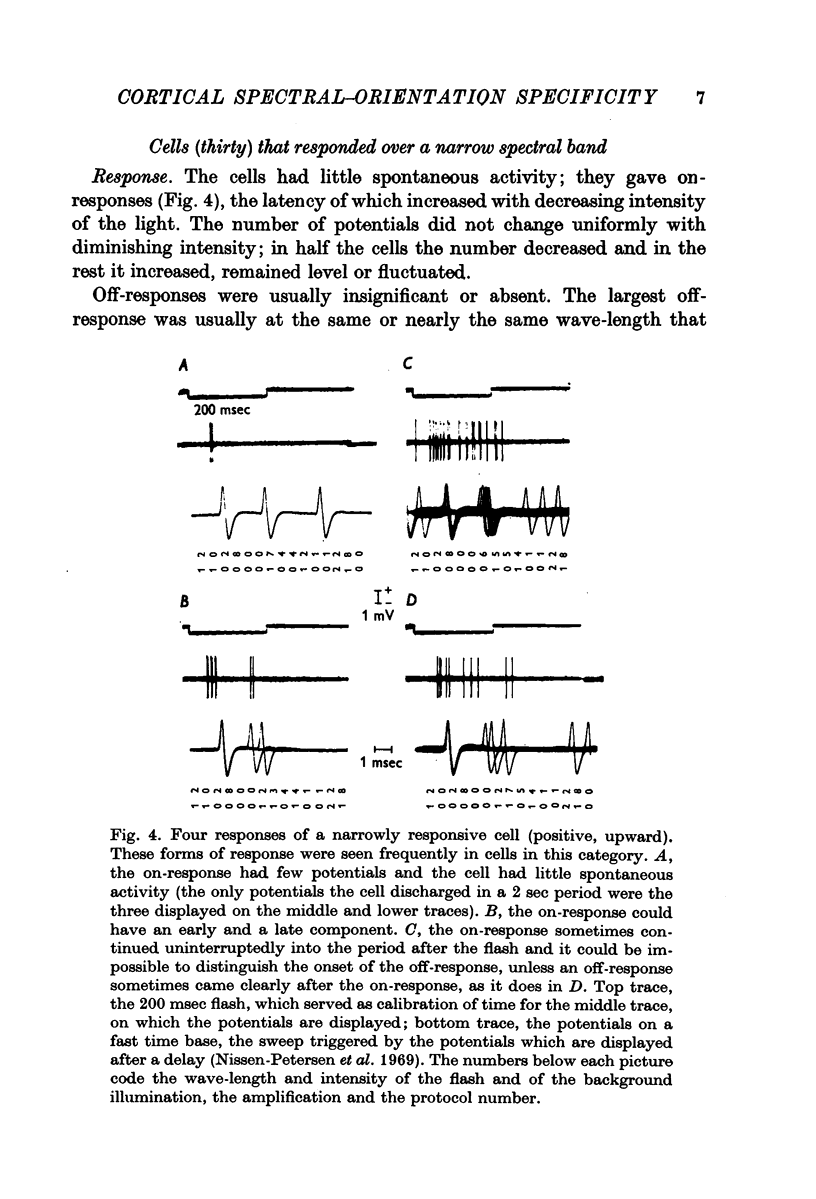








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN V. O., LAURSEN A. M. Automatic transport device for microelectrodes. Electroencephalogr Clin Neurophysiol. 1959 Feb;11(1):172–173. doi: 10.1016/0013-4694(59)90025-2. [DOI] [PubMed] [Google Scholar]
- De Valois R. L. Processing of intensity and wavelength information by the visual system. Invest Ophthalmol. 1972 Jun;11(6):417–427. [PubMed] [Google Scholar]
- Dow B. M., Gouras P. Color and spatial specificity of single units in Rhesus monkey foveal striate cortex. J Neurophysiol. 1973 Jan;36(1):79–100. doi: 10.1152/jn.1973.36.1.79. [DOI] [PubMed] [Google Scholar]
- Gouras P. Opponent-colour cells in different layers of foveal striate cortex. J Physiol. 1974 May;238(3):583–602. doi: 10.1113/jphysiol.1974.sp010545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guld C., Bertulis A. Contact lenses for animals used in vision research. Vision Res. 1975 Mar;15(3):441–442. doi: 10.1016/0042-6989(75)90096-6. [DOI] [PubMed] [Google Scholar]
- Guld C., Bertulis A. Representation of fovea in the striate cortex of vervet monkey, Cercopithecus aethiops pygerythrus. Vision Res. 1976;16(6):629–631. doi: 10.1016/0042-6989(76)90010-9. [DOI] [PubMed] [Google Scholar]
- Guld C., Lennox-Buchthal M. Effect of direct current on the responses to colored flashes of single cells in monkey cortex. Acta Physiol Scand. 1968 Sep-Oct;74(1):142–152. doi: 10.1111/j.1748-1716.1968.tb04223.x. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968 Mar;195(1):215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollough C. Color Adaptation of Edge-Detectors in the Human Visual System. Science. 1965 Sep 3;149(3688):1115–1116. doi: 10.1126/science.149.3688.1115. [DOI] [PubMed] [Google Scholar]
- Nissen-Petersen H., Guld C., Buchthal F. A delay line to record random action potentials. Electroencephalogr Clin Neurophysiol. 1969 Jan;26(1):100–106. doi: 10.1016/0013-4694(69)90040-6. [DOI] [PubMed] [Google Scholar]
- Petersen J. C., Butterfield B. O. Depth gauge for microelectrodes. IEEE Trans Biomed Eng. 1968 Apr;15(2):129–130. [PubMed] [Google Scholar]
- Poggio G. F., Baker F. H., Mansfield R. J., Sillito A., Grigg P. Spatial and chromatic properties of neurons subserving foveal and parafoveal vision in rhesus monkey. Brain Res. 1975 Dec 12;100(1):25–59. doi: 10.1016/0006-8993(75)90240-1. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. J Neurophysiol. 1966 Nov;29(6):1115–1156. doi: 10.1152/jn.1966.29.6.1115. [DOI] [PubMed] [Google Scholar]
- Yates J. T. Chromatic information processing in the foveal projection (area striata) of unanesthetized primate. Vision Res. 1974 Feb;14(2):163–173. doi: 10.1016/0042-6989(74)90097-2. [DOI] [PubMed] [Google Scholar]
- Zeki S. M. Colour coding in rhesus monkey prestriate cortex. Brain Res. 1973 Apr 27;53(2):422–427. doi: 10.1016/0006-8993(73)90227-8. [DOI] [PubMed] [Google Scholar]


