Abstract
1. Isolated rat superior cervical ganglia were continuously superfused with 42K (or 86Rb) solution and the amount of radioactivity taken up was monitored using scintillation counting.
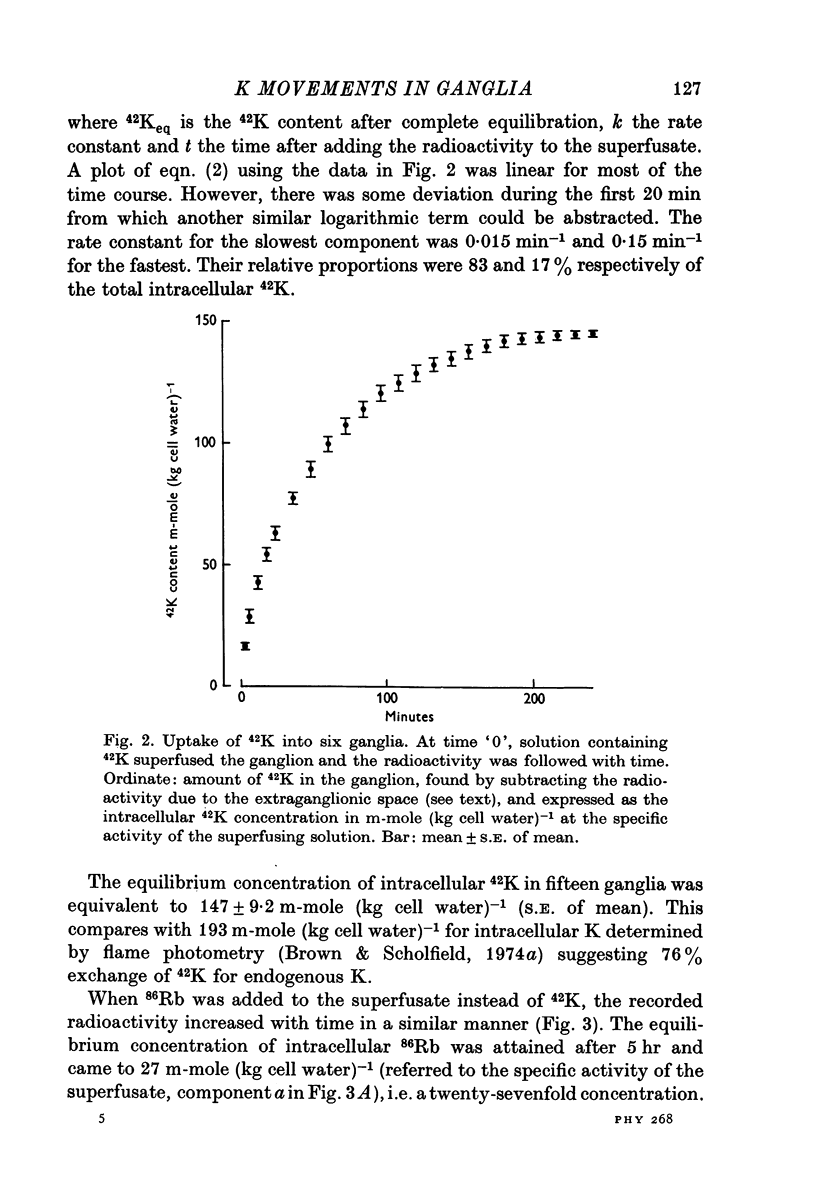
2. Entry of 42K into the ganglia could be resolved into two components, one amounting to 83% of the total 42K uptake, with a rate constant of 0·015 min-1, and the other of 17% of the total, with a rate constant of 0·15 min-1.
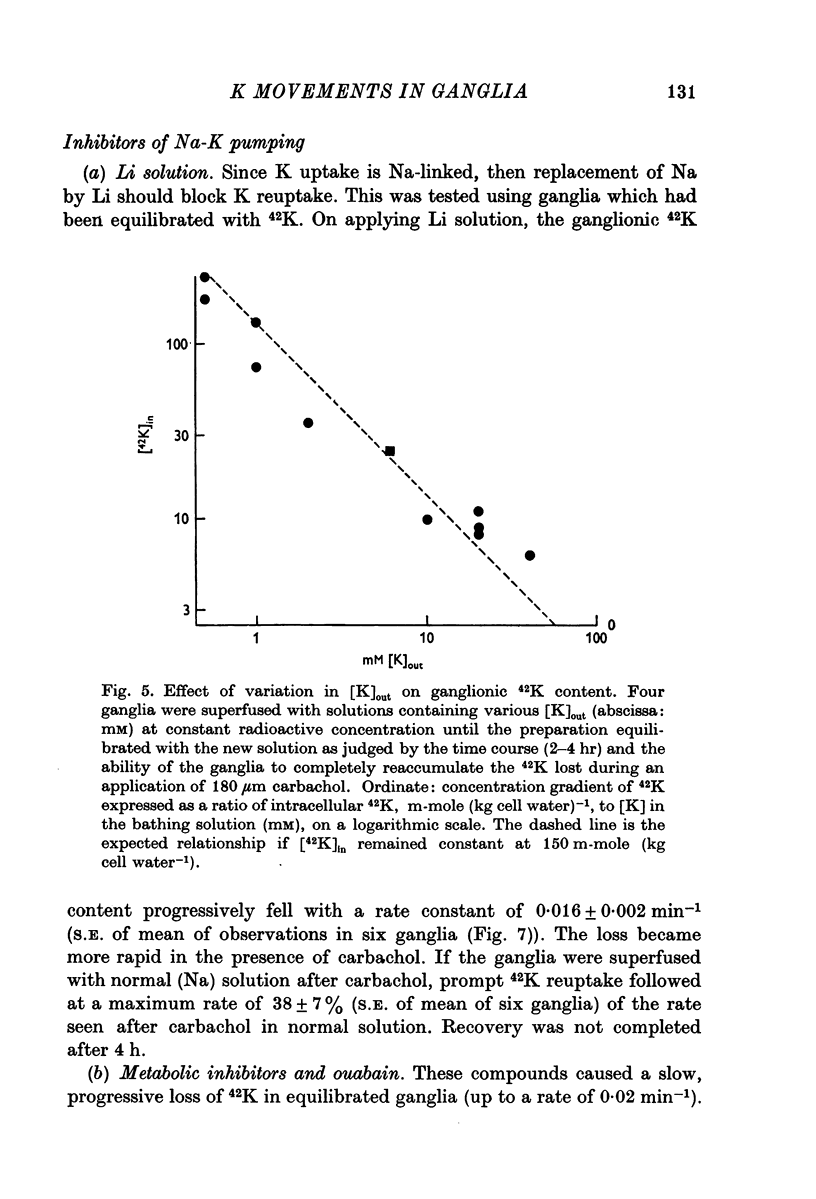
3. With 6 mM-K in the bathing solution, the equilibrium uptake of 42K after 4 hr corresponded to an intracellular concentration of 147 mM-K. Changes in the K concentration of the bathing solution (0·5-20 mM) had little effect on this value.
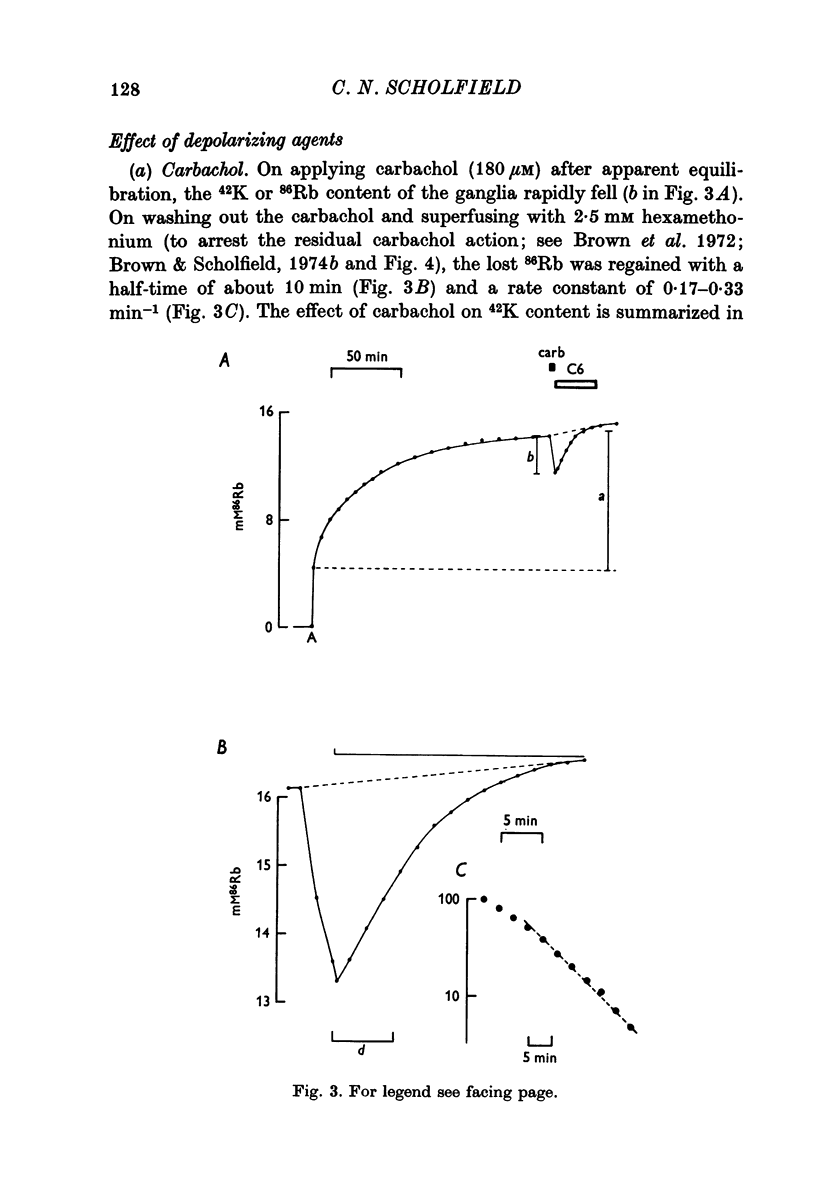
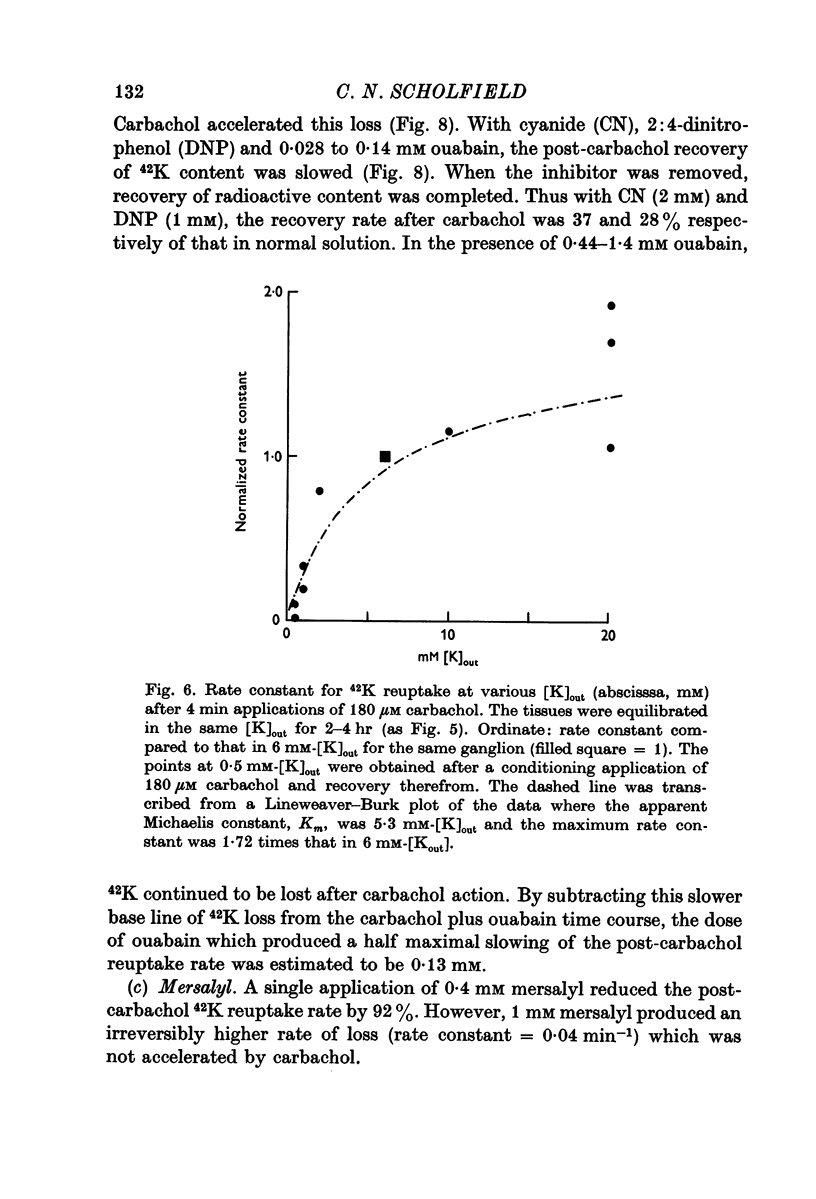
4. Carbachol or nicotine caused a rapid net loss of 42K. 42K was recaptured on washing out the depolarizing agents, with a rate constant of about 0·3 min-1. This recapture rate was slowed by ouabain, dinitrophenol, cyanide, mersalyl and by reducing the K concentration in the bathing solution.
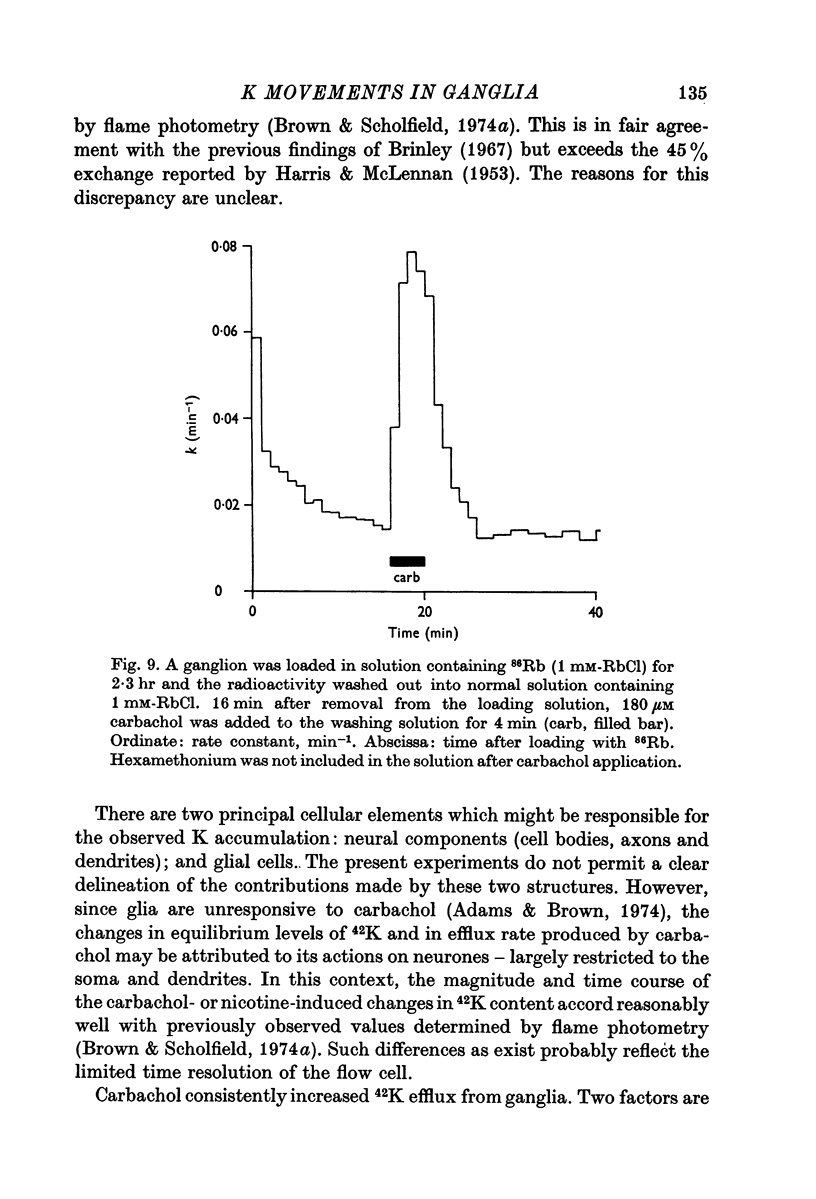
5. Efflux of 42K from preloaded ganglia occurred with a rate constant of 0·017 min-1. This rate was increased about sixfold by 180 μM carbachol in 6 mM-K but not in 150 mM-K suggesting that the increase in efflux was mainly a consequence of the depolarization caused by carbachol.
6. 86Rb fluxes and the effects of carbachol thereon were similar.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A. Proceedings: Some observations on electrically-inexcitable cells (neuroglia?) in rat sympathetic ganglia. Br J Pharmacol. 1974 May;51(1):131P–132P. [PMC free article] [PubMed] [Google Scholar]
- Adams P. R. Kinetics of agonist conductance changes during hyperolarization at frog endplates. Br J Pharmacol. 1975 Feb;53(2):308–310. doi: 10.1111/j.1476-5381.1975.tb07364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinley F. J., Jr Potassium accumulation and transport in the rat sympathetic ganglion. J Neurophysiol. 1967 Nov;30(6):1531–1560. doi: 10.1152/jn.1967.30.6.1531. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Brownstein M. J., Scholfield C. N. Origin of the after-hyperpolarization that follows removal of depolarizing agents from the isolated superior cervical ganglion of the rat. Br J Pharmacol. 1972 Apr;44(4):651–671. doi: 10.1111/j.1476-5381.1972.tb07305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Scholfield C. N. Changes of intracellular sodium and potassium ion concentrations in isolated rat superior cervical ganglia induced by depolarizing agents. J Physiol. 1974 Oct;242(2):307–319. doi: 10.1113/jphysiol.1974.sp010709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Scholfield C. N. Movements of labelled sodium ions in isolated rat superior cervical ganglia. J Physiol. 1974 Oct;242(2):321–351. doi: 10.1113/jphysiol.1974.sp010710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Scholfield C. N. Nicotine washout rates from isolated rat ganglia in relation to recovery from nicotine depolarization. Br J Pharmacol. 1972 May;45(1):29–36. doi: 10.1111/j.1476-5381.1972.tb09573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DURBIN R. P., JENKINSON D. H. The effect of carbachol on the permeability of depolarized smooth muscle to inorganic ions. J Physiol. 1961 Jun;157:74–89. doi: 10.1113/jphysiol.1961.sp006706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS E. J., McLENNAN H. Cation exchanges in sympathetic ganglia. J Physiol. 1953 Sep;121(3):629–637. doi: 10.1113/jphysiol.1953.sp004970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINSON D. H., NICHOLLS J. G. Contractures and permeability changes produced by acetylcholine in depolarized denervated muscle. J Physiol. 1961 Nov;159:111–127. doi: 10.1113/jphysiol.1961.sp006796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., LEWIS P. R. The resting exchange of radioactive potassium in crab nerve. J Physiol. 1951 Mar;113(1):73–98. doi: 10.1113/jphysiol.1951.sp004557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester H. A., Changeux J. P., Sheridan R. E. Conductance increases produced by bath application of cholinergic agonists to Electrophorus electroplaques. J Gen Physiol. 1975 Jun;65(6):797–816. doi: 10.1085/jgp.65.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons T. J. Potassium: potassium exchange catalysed by the sodium pump in human red cells. J Physiol. 1974 Feb;237(1):123–155. doi: 10.1113/jphysiol.1974.sp010474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C., Simon W., Oehme M. Lithium accumulation by snail neurones measured by a new Li+-sensitive microelectrode. Nature. 1975 Dec 25;258(5537):754–756. doi: 10.1038/258754a0. [DOI] [PubMed] [Google Scholar]


