Abstract
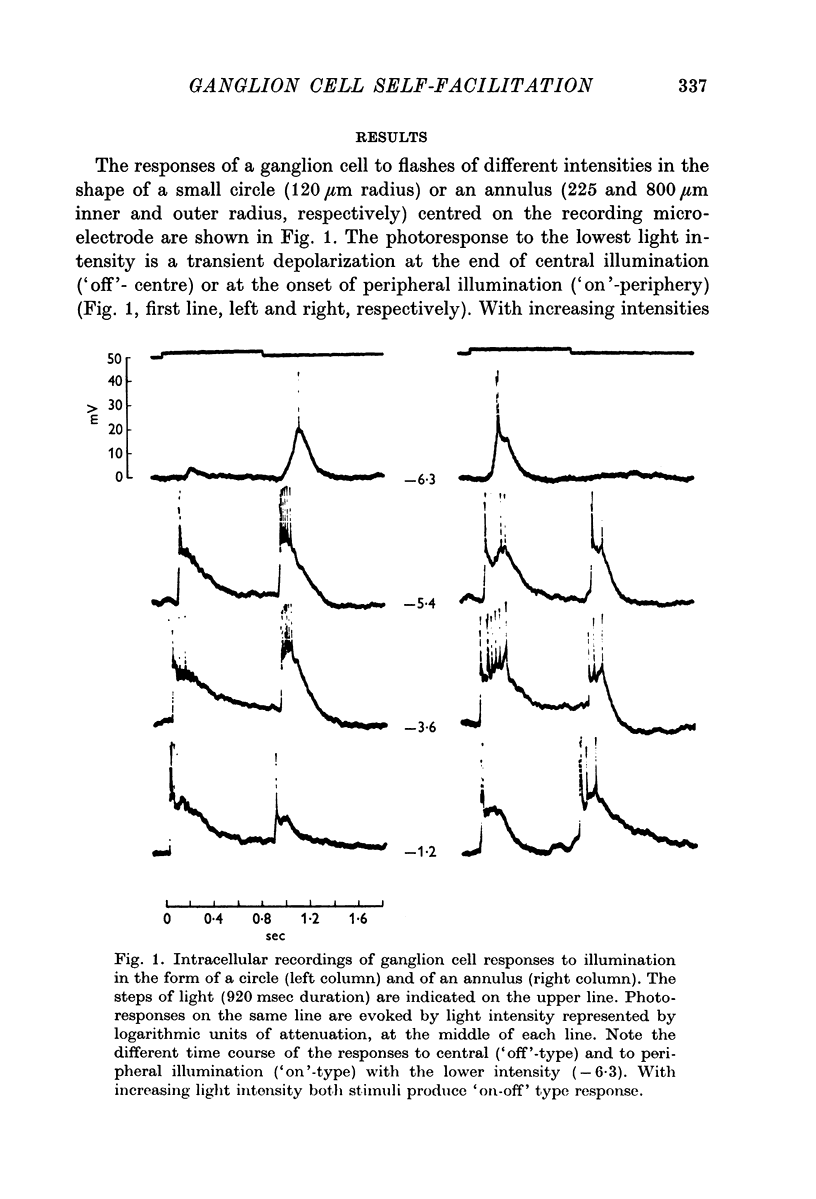
1. Ganglion cells responses to illumination and to optic nerve stimulation were recorded intracellularly from the retina of the turtle. All ganglion cells were identified by their antidromic responses to optic nerve stimulation.
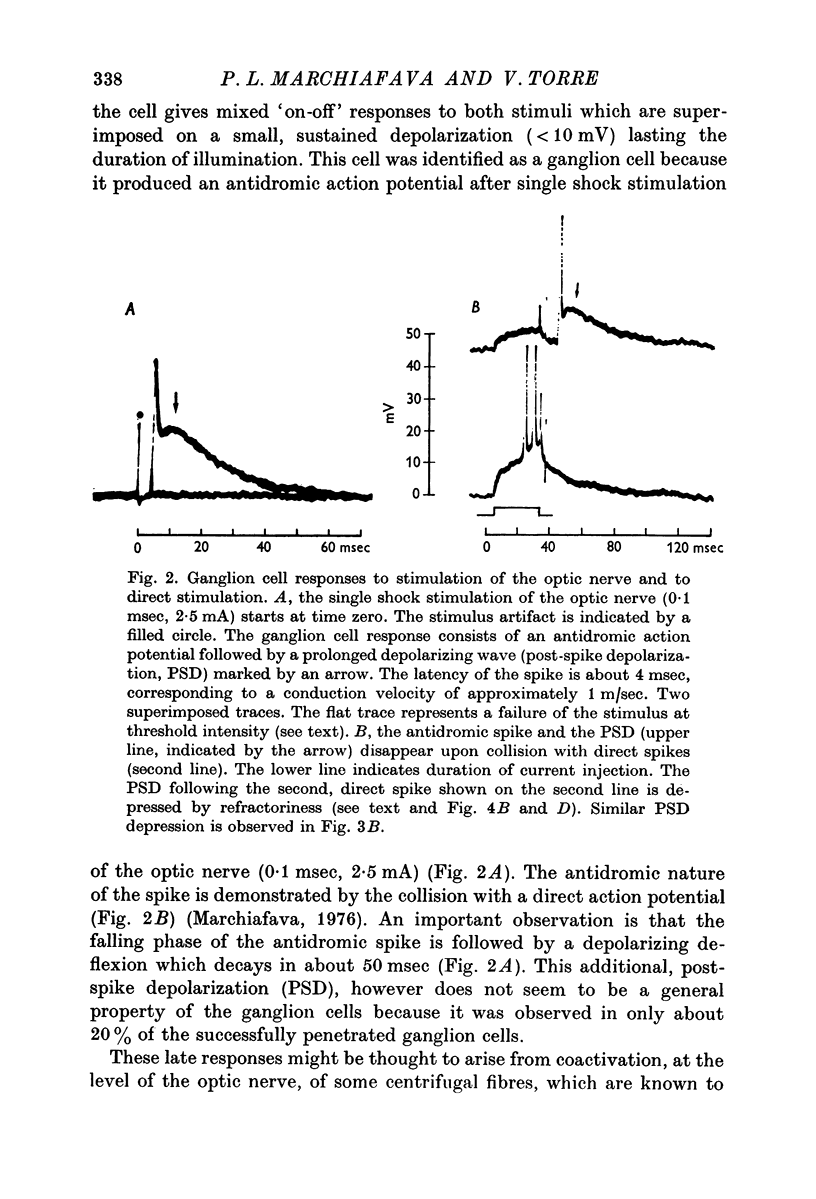
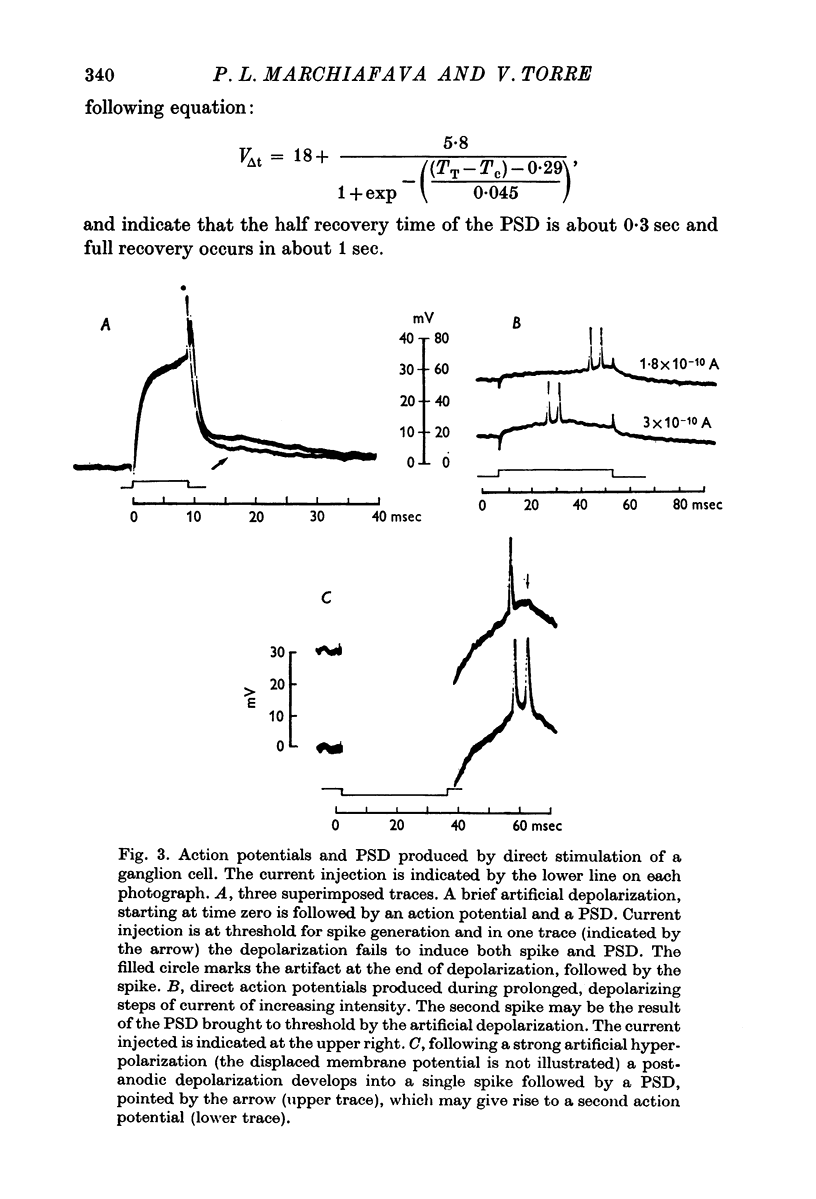
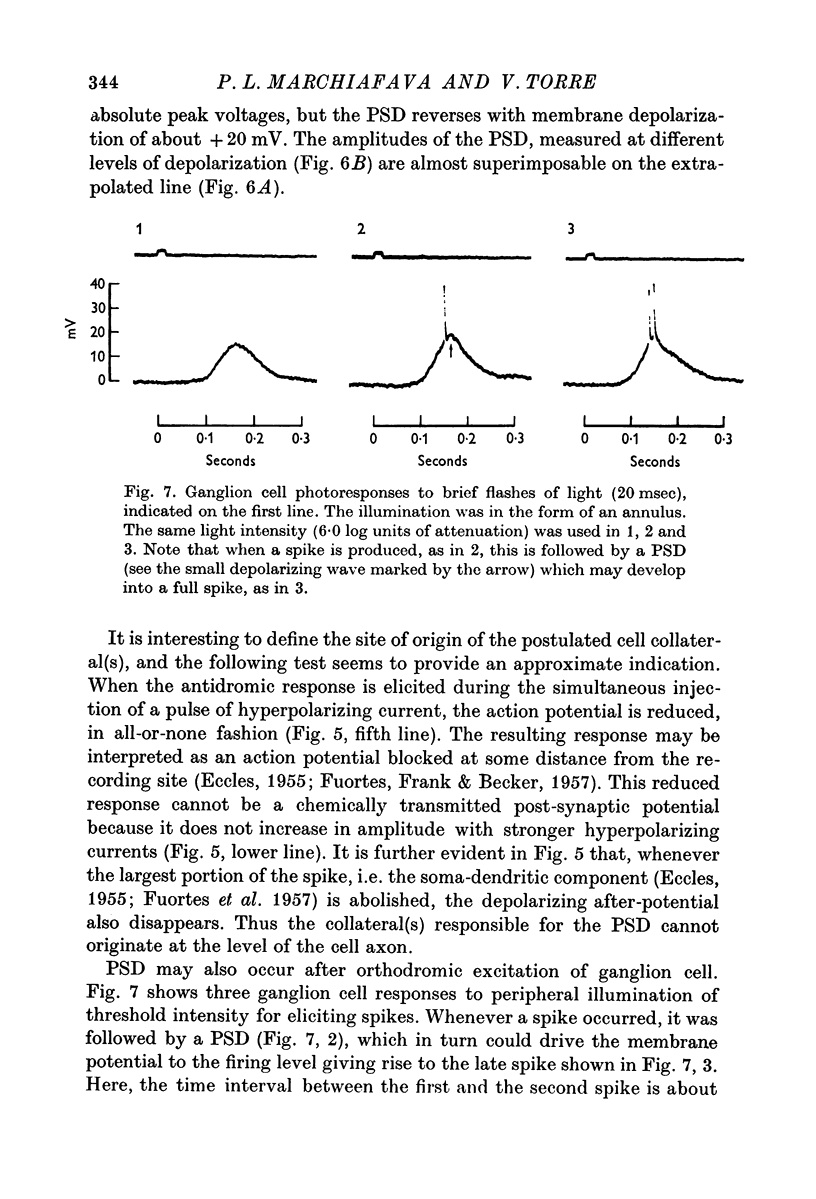
2. When solitary spikes are produced following antidromic, orthodromic or intracellular stimulation, about 20% of the recorded ganglion cells show an additional depolarization along the falling phase of the action potential (post-spike depolarization, PSD).
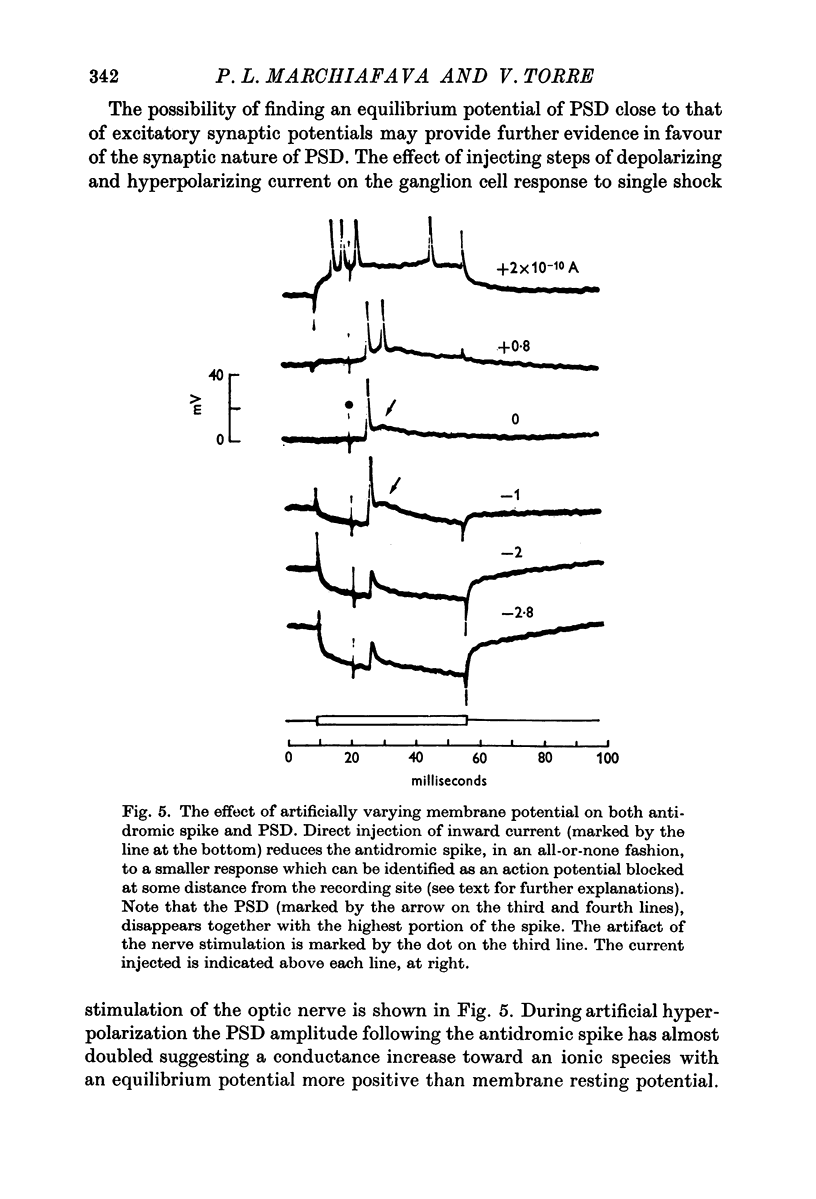
3. The PSD following the antidromic action potential disappears upon collision with a direct spike or when the antidromic spike is prevented from invading the cell soma.
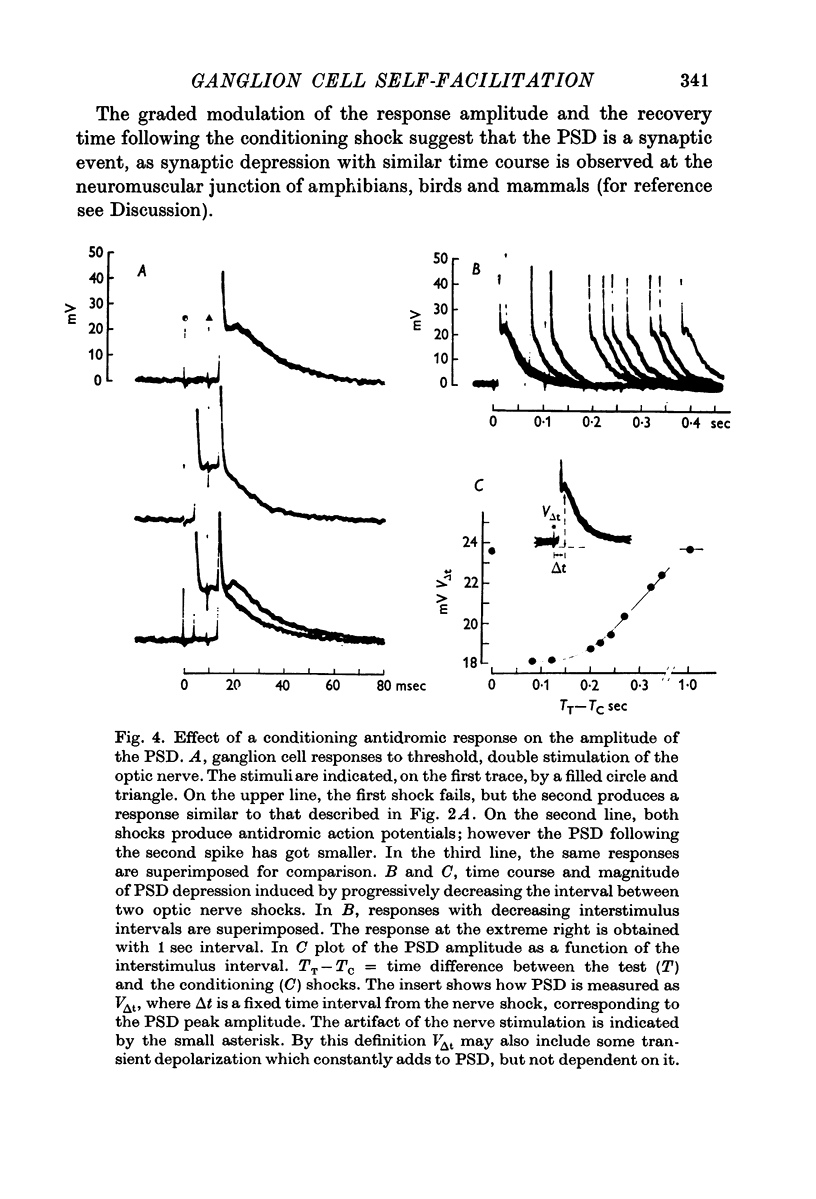
4. By pairing two optic nerve stimuli the PSD is depressed with brief interstimulus intervals, but gradually recovers to the control amplitude 600-800 msec after the conditioning shock.
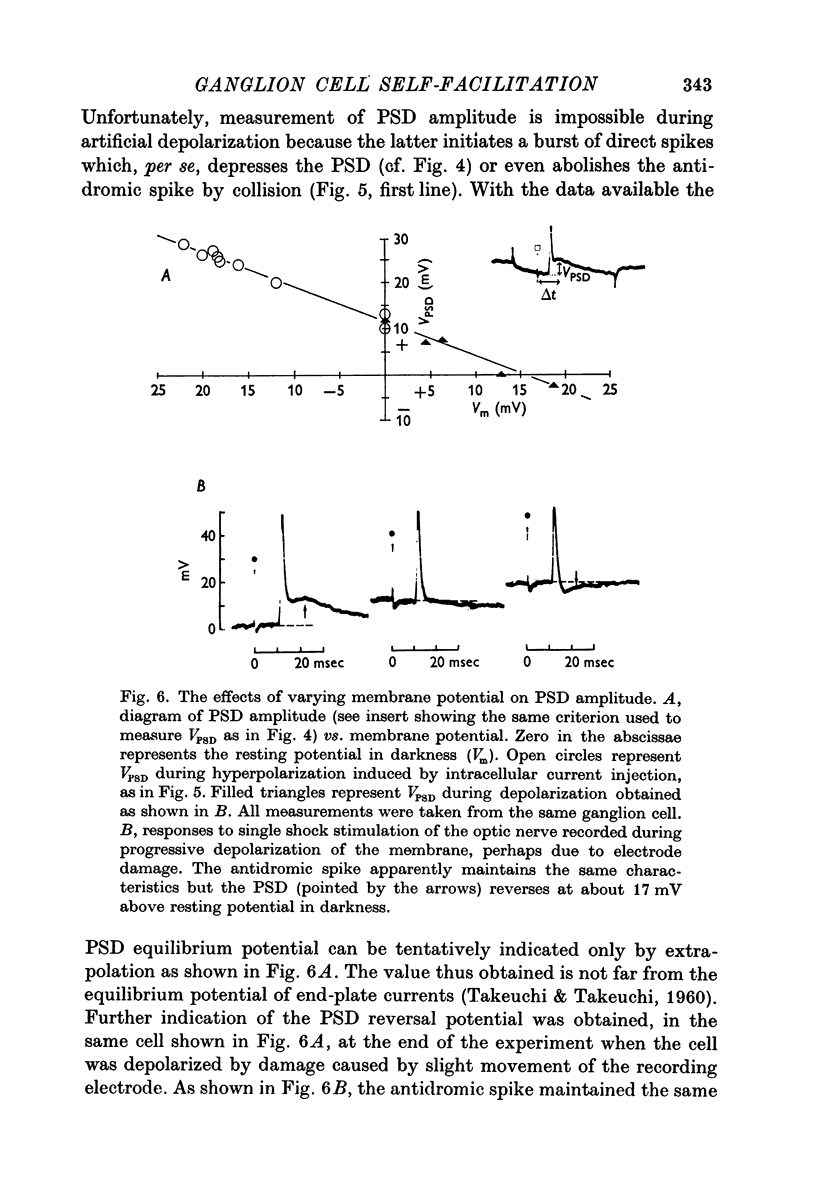
5. The PSD is tentatively interpreted as an e.p.s.p. transmitted by ganglion cell collaterals originating at the level of the soma dendritic complex of the recorded cell.
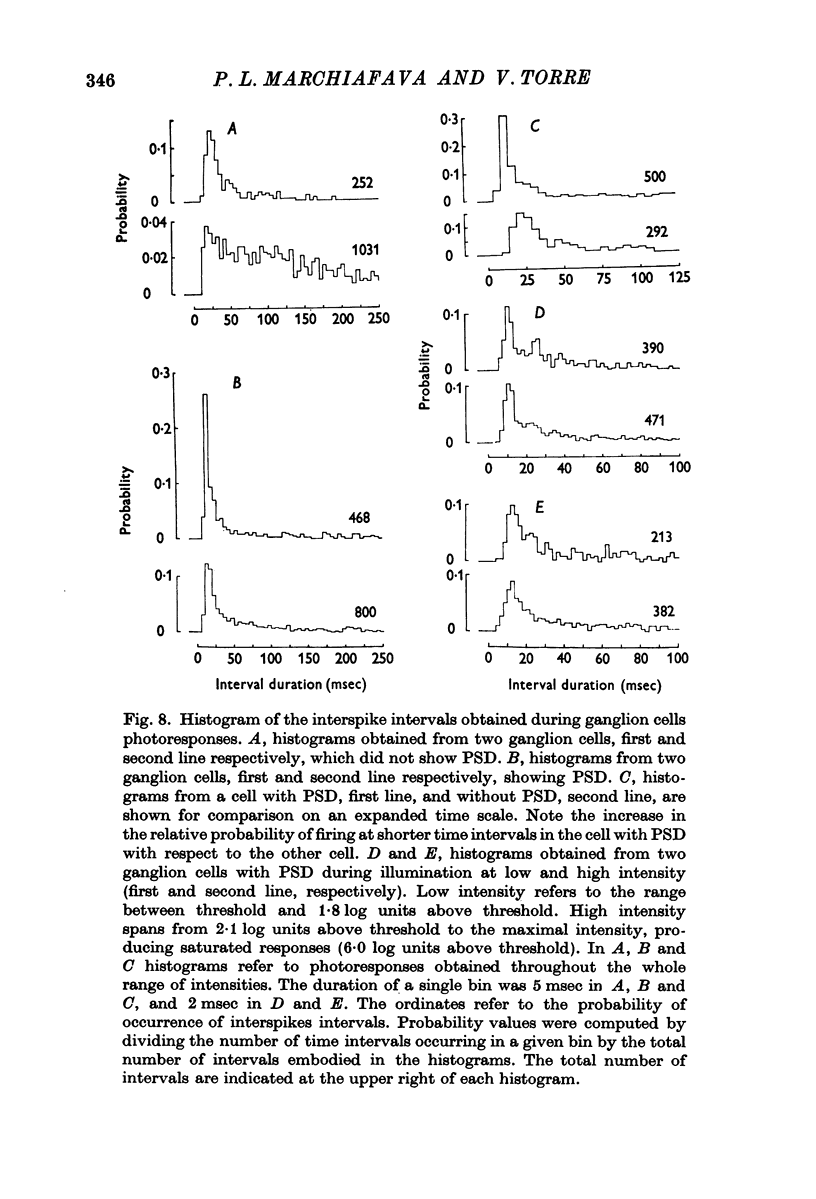
6. The interspike interval histogram of ganglion cells showing PSD is characterized by a peak at about 10 msec, as opposed to a peak between 12 and 100 msec observed in cells without PSD. It is suggested that the occurrence of PSD facilitate the onset of additional action potentials at brief interspikes intervals, thus potentiating ganglion cell discharges.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldissera F. Relationships between the spike components and the delayed depolarization in cat spinal neurones. J Physiol. 1976 Jul;259(2):325–338. doi: 10.1113/jphysiol.1976.sp011468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Changes in the maintained discharge with adaptation level in the cat retina. J Physiol. 1969 Jun;202(3):699–718. doi: 10.1113/jphysiol.1969.sp008836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W. J. Depression of transmitter release at the neuromuscular junction of the frog. J Physiol. 1970 Mar;206(3):629–644. doi: 10.1113/jphysiol.1970.sp009034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C. The central action of antidromic impulses in motor nerve fibres. Pflugers Arch. 1955;260(5):385–415. doi: 10.1007/BF00363548. [DOI] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956 Feb 28;131(2):341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUORTES M. G., FRANK K., BECKER M. C. Steps in the production of motoneuron spikes. J Gen Physiol. 1957 May 20;40(5):735–752. doi: 10.1085/jgp.40.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERSTEIN G. L., MANDELBROT B. RANDOM WALK MODELS FOR THE SPIKE ACTIVITY OF A SINGLE NEURON. Biophys J. 1964 Jan;4:41–68. doi: 10.1016/s0006-3495(64)86768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., KERNELL D., SMITH R. S. DELAYED DEPOLARIZATION AND THE REPETITIVE RESPONSE TO INTRACELLULAR STIMULATION OF MAMMALIAN MOTONEURONES. J Physiol. 1963 Oct;168:890–910. doi: 10.1113/jphysiol.1963.sp007229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENGARD P., STRAUB R. W. After-potentials in mammalian non-myelinated nerve fibres. J Physiol. 1958 Dec 30;144(3):442–462. doi: 10.1113/jphysiol.1958.sp006112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego A., Cruz J. Mammalian Retina: Associational Nerve Cells in Ganglion Cell Layer. Science. 1965 Dec 3;150(3701):1313–1314. doi: 10.1126/science.150.3701.1313. [DOI] [PubMed] [Google Scholar]
- Kuno M., Llinás R. Enhancement of synaptic transmission by dendritic potentials in chromatolysed motoneurones of the cat. J Physiol. 1970 Nov;210(4):807–821. doi: 10.1113/jphysiol.1970.sp009243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W., NORTH K. A. An electrical investigation of effects of repetitive stimulation on mammalian neuromuscular junction. J Neurophysiol. 1953 Sep;16(5):509–527. doi: 10.1152/jn.1953.16.5.509. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A., QUILISCH H. Presynaptic potentiation and depression of neuromuscular transmission in frog and rat. Acta Physiol Scand Suppl. 1953;111:111–120. [PubMed] [Google Scholar]
- Lasansky A., Marchiafava P. L. Light-induced resistance changes in retinal rods and cones of the tiger salamander. J Physiol. 1974 Jan;236(1):171–191. doi: 10.1113/jphysiol.1974.sp010429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchiafava P. L. Centrifugal actions on amacrine and ganglion cells in the retina of the turtle. J Physiol. 1976 Feb;255(1):137–155. doi: 10.1113/jphysiol.1976.sp011273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maturana H. R., Frenk S. Synaptic connections of the centrifugal fibers in the pigeon retina. Science. 1965 Oct 15;150(3694):359–361. doi: 10.1126/science.150.3694.359. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. On the permeability of end-plate membrane during the action of transmitter. J Physiol. 1960 Nov;154:52–67. doi: 10.1113/jphysiol.1960.sp006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI A. The long-lasting depression in neuromuscular transmission of frog. Jpn J Physiol. 1958 Jun 15;8(2):102–113. doi: 10.2170/jjphysiol.8.102. [DOI] [PubMed] [Google Scholar]
- THIES R. E. NEUROMUSCULAR DEPRESSION AND THE APPARENT DEPLETION OF TRANSMITTER IN MAMMALIAN MUSCLE. J Neurophysiol. 1965 May;28:428–442. doi: 10.1152/jn.1965.28.3.427. [DOI] [PubMed] [Google Scholar]
- Zucker R. S. Crayfish escape behavior and central synapses. 3. Electrical junctions and dendrite spikes in fast flexor motoneurons. J Neurophysiol. 1972 Sep;35(5):638–651. doi: 10.1152/jn.1972.35.5.638. [DOI] [PubMed] [Google Scholar]


