Abstract
1. Cells in area 17 of the cat visual cortex were studied with a view towards correlating receptive field properties with layering. A number of receptive field parameters were measured for all units, and nearly every unit was marked with a microlesion to determine accurately the layer in which it was found.
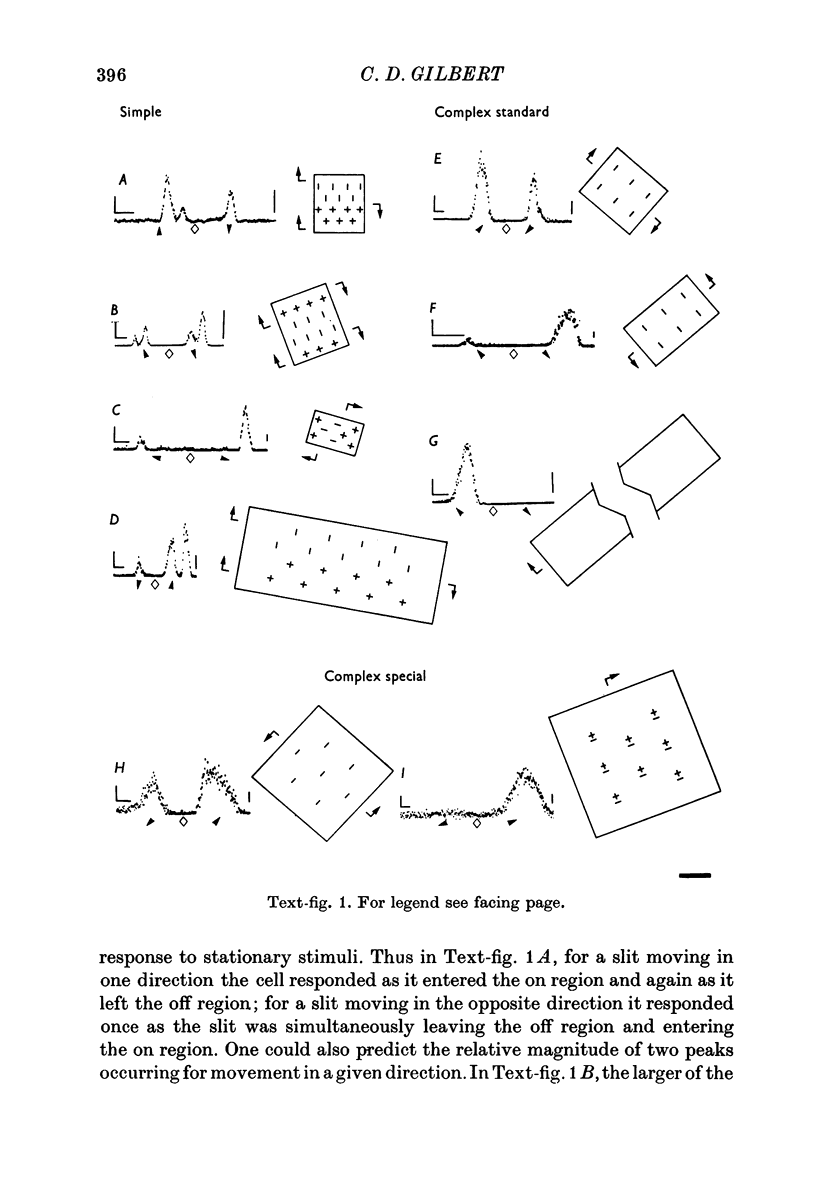
2. Cells were defined as simple or complex by mapping with stationary stimuli, using the criteria of Hubel & Wiesel (1962). Complex cells fell into two groups: those that showed summation for increased slit length (standard complex) and those that did not (special complex).
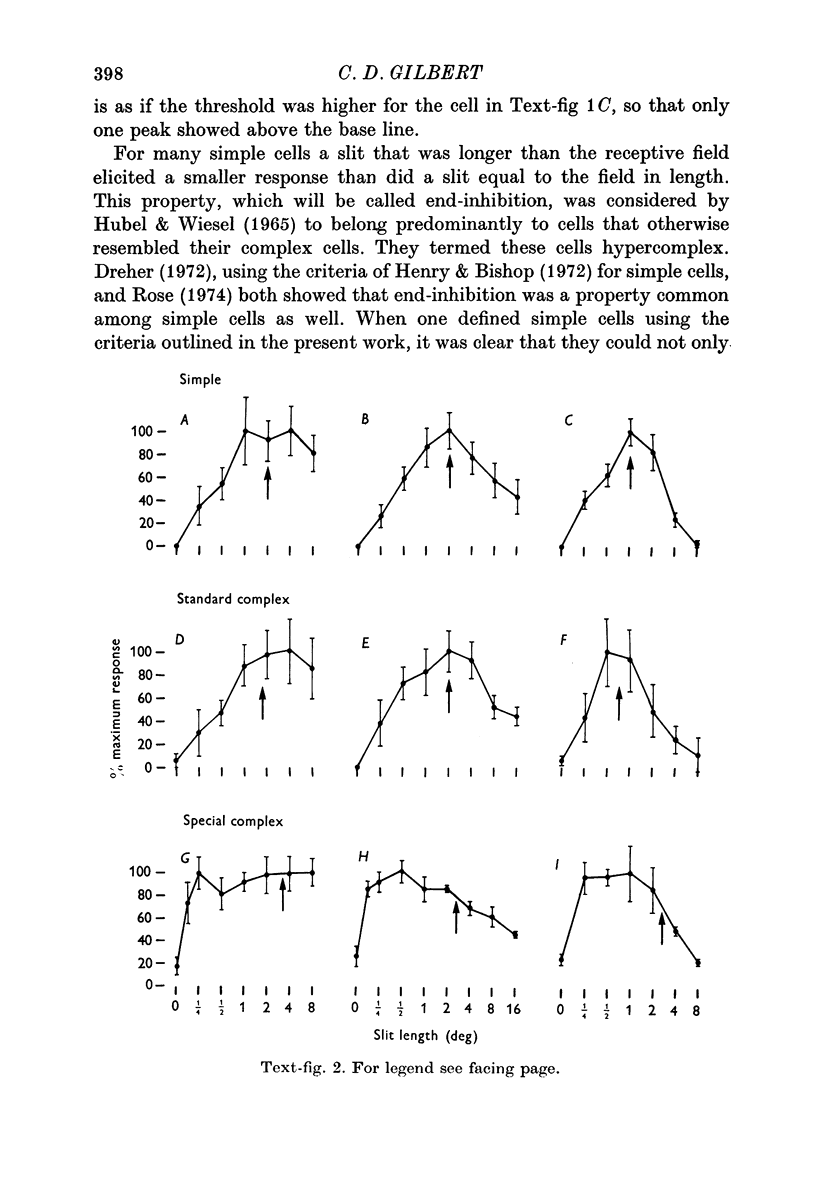
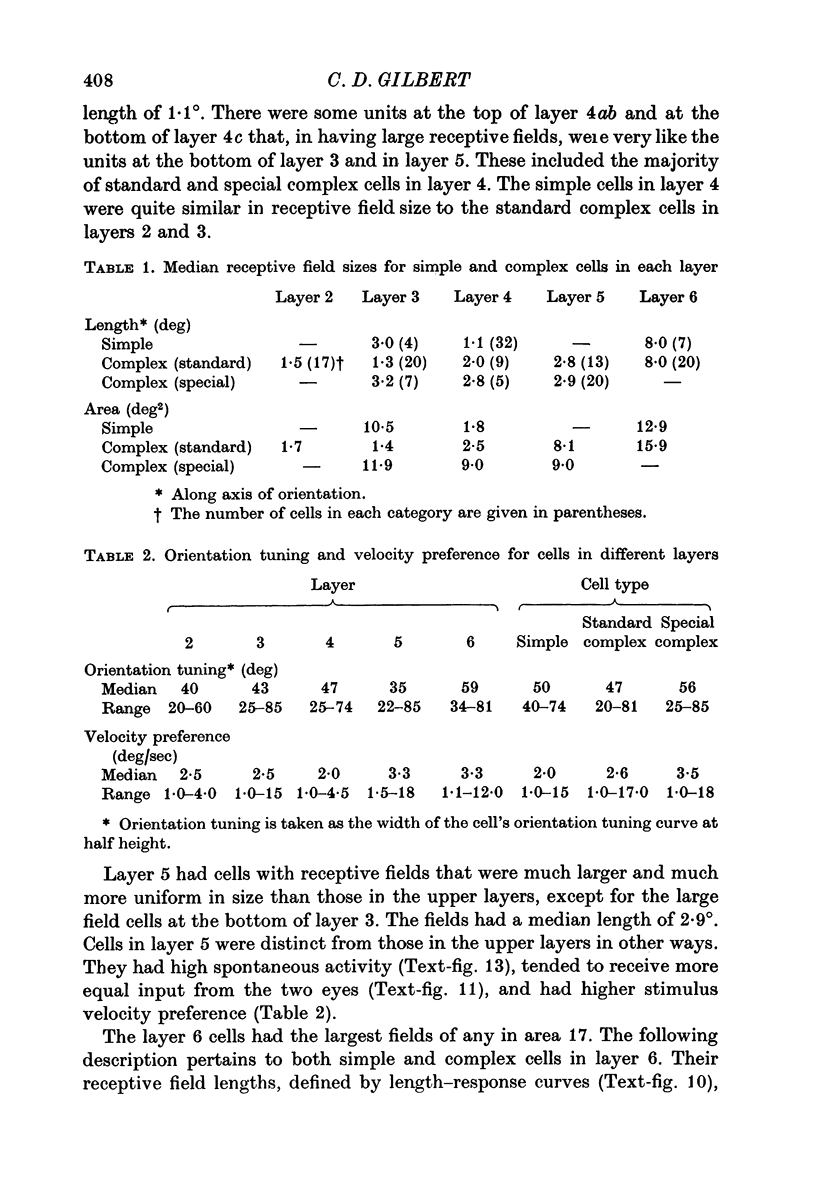
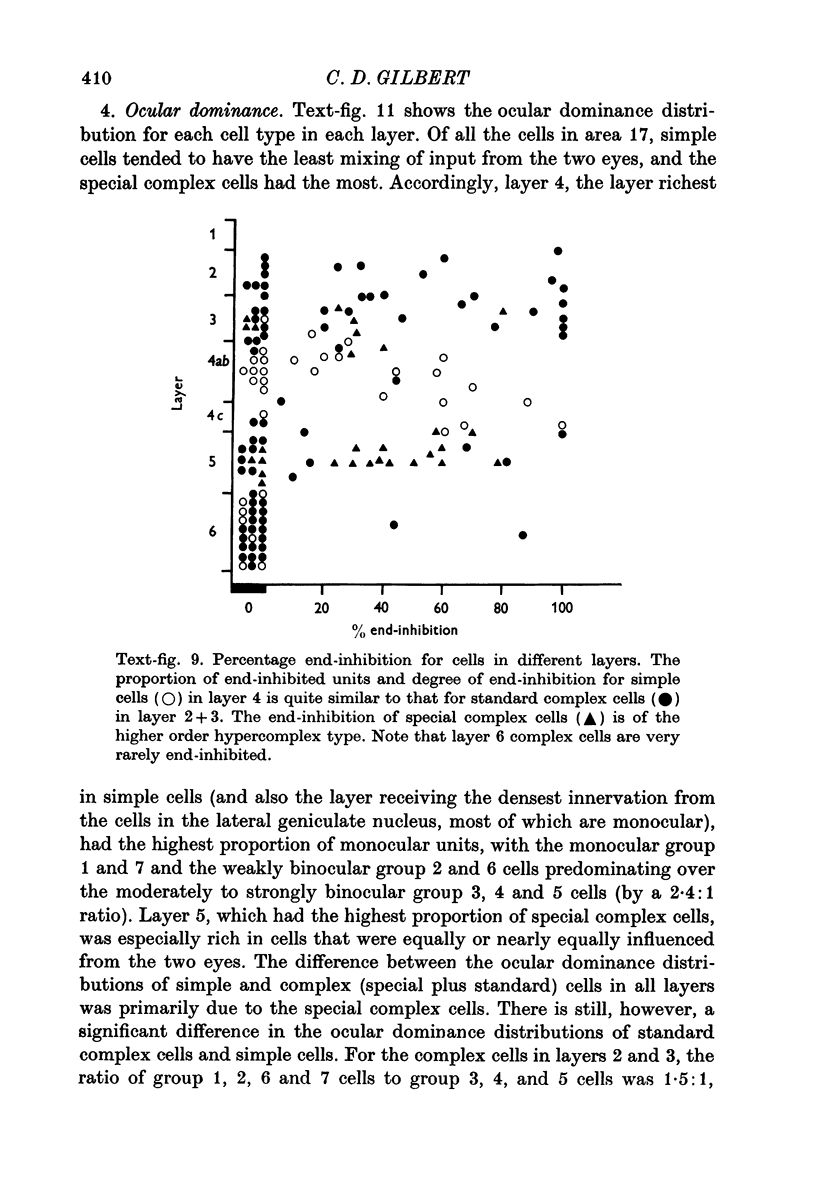
3. The simple cells were located in the deep part of layer 3, in layer 4, and in layer 6. This corresponds to the distribution of afferents from the dorsal layers of the lateral geniculate nucleus. In these cortical layers the simple cells differed primarily with respect to their receptive field size, cells in layer 4 having the smallest, layer 3 intermediate, and layer 6 the largest fields. Layer 4 was the only layer in which simple cells showed end-inhibition (a reduction in response to slits extending beyond the excitatory portion of the receptive field).
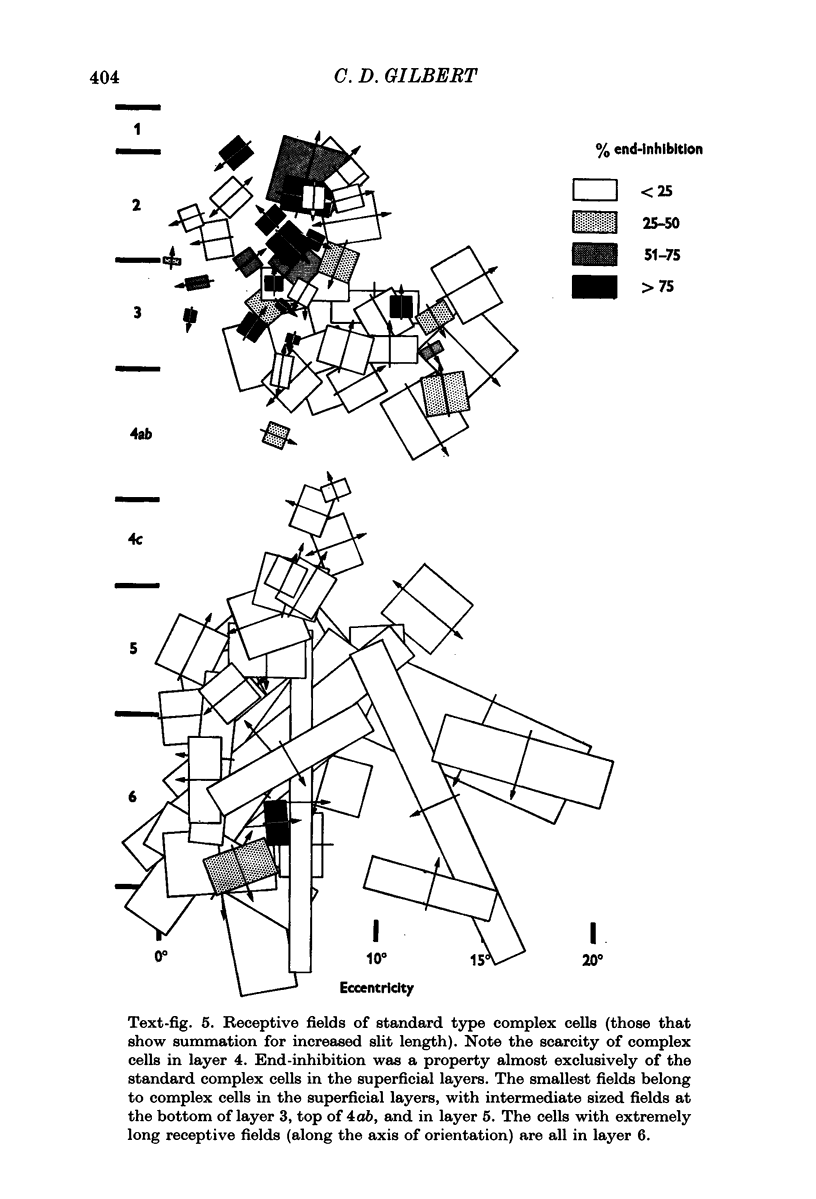
4. The standard complex cells were found in all layers, but were quite scarce in layer 4. As with the simple cells, field size varied with layer: in layer 2+3 they had small to intermediate field sizes, in layer 5 intermediate, and in layer 6 very large. Layer 6 cells showed summation for slits of increased length up to very large values, and responded best when the slits were centred in the receptive field.
The only standard complex cells that showed end-inhibition were those in layer 2+3, and these were similar to the layer 4 simple cells in terms of proportion of end-inhibited units and degree of end-inhibition.
5. The special complex cells, originally described by Palmer & Rosenquist (1974), were found in two tiers: the upper one at the layer 3/layer 4 border and the lower one in layer 5. They were different from the standard complex cells in having a high spontaneous activity, high velocity preference, and large fields which were similar in size (at a given eccentricity) from one cell to the next. Many showed reduced response to slits of increasing length, even for slits that did not extend beyond the borders of the responsive region.
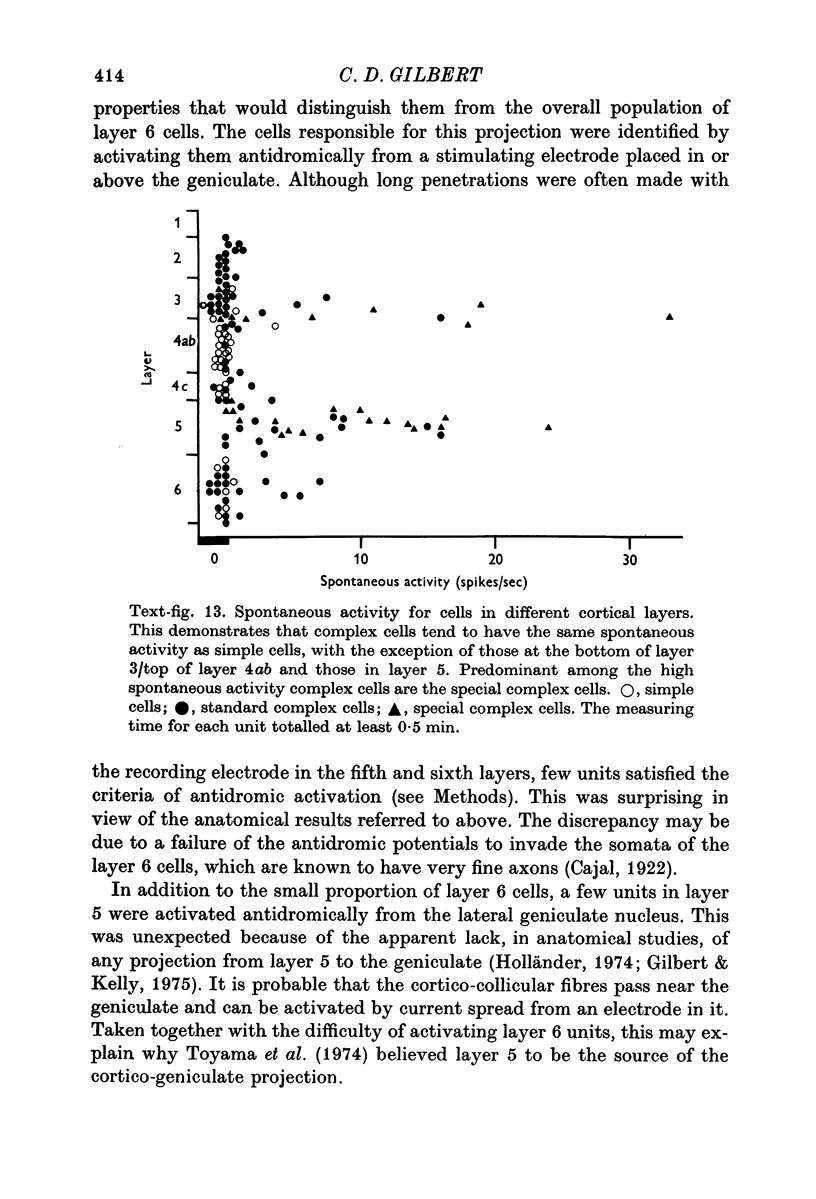
6. Cells in layer 6 (the origin of the corticogeniculate projection) were antidromically activated from the lateral geniculate nucleus. The antidromically activated units included both simple and complex cells, and they had the long receptive fields characteristic of the overall population of cells in layer 6.
7. The results showed that there are different types of simple and complex cells, and that cells in different layers have different properties. Taken together with their differences in site of projection, this demonstrates that the anatomical lamination pattern is reflected in functional differences between cells in different layers.
Full text
PDF
























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cleland B. G., Morstyn R., Wagner H. G., Levick W. R. Long-latency retinal input to lateral geniculate neurones of the cat. Brain Res. 1975 Jun 27;91(2):306–310. doi: 10.1016/0006-8993(75)90553-3. [DOI] [PubMed] [Google Scholar]
- Daw N. W., Pearlman A. L. Cat colour vision: evidence for more than one cone process. J Physiol. 1970 Nov;211(1):125–137. doi: 10.1113/jphysiol.1970.sp009270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher B. Hypercomplex cells in the cat's striate cortex. Invest Ophthalmol. 1972 May;11(5):355–356. [PubMed] [Google Scholar]
- Fukuda Y., Stone J. Retinal distribution and central projections of Y-, X-, and W-cells of the cat's retina. J Neurophysiol. 1974 Jul;37(4):749–772. doi: 10.1152/jn.1974.37.4.749. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D., Kelly J. P. The projections of cells in different layers of the cat's visual cortex. J Comp Neurol. 1975 Sep;163(1):81–105. doi: 10.1002/cne.901630106. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H. Single unit activity in lateral geniculate body and optic tract of unrestrained cats. J Physiol. 1960 Jan;150:91–104. doi: 10.1113/jphysiol.1960.sp006375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Integrative action in the cat's lateral geniculate body. J Physiol. 1961 Feb;155:385–398. doi: 10.1113/jphysiol.1961.sp006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS AND FUNCTIONAL ARCHITECTURE IN TWO NONSTRIATE VISUAL AREAS (18 AND 19) OF THE CAT. J Neurophysiol. 1965 Mar;28:229–289. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields of single neurones in the cat's striate cortex. J Physiol. 1959 Oct;148:574–591. doi: 10.1113/jphysiol.1959.sp006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry G. H., Bishop P. O. Striate neurons: receptive field organization. Invest Ophthalmol. 1972 May;11(5):357–368. [PubMed] [Google Scholar]
- Hoffman K. P., Stone J. Conduction velocity of afferents to cat visual cortex: a correlation with cortical receptive field properties. Brain Res. 1971 Sep 24;32(2):460–466. doi: 10.1016/0006-8993(71)90340-4. [DOI] [PubMed] [Google Scholar]
- Hollander H. Autoradiographic evidence for a projection from the striate cortex to the dorsal part of the lateral geniculate nucleus in the cat. Brain Res. 1972 Jun 22;41(2):464–466. doi: 10.1016/0006-8993(72)90516-1. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Laminar and columnar distribution of geniculo-cortical fibers in the macaque monkey. J Comp Neurol. 1972 Dec;146(4):421–450. doi: 10.1002/cne.901460402. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968 Mar;195(1):215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Uniformity of monkey striate cortex: a parallel relationship between field size, scatter, and magnification factor. J Comp Neurol. 1974 Dec 1;158(3):295–305. doi: 10.1002/cne.901580305. [DOI] [PubMed] [Google Scholar]
- Kelly J. P., Van Essen D. C. Cell structure and function in the visual cortex of the cat. J Physiol. 1974 May;238(3):515–547. doi: 10.1113/jphysiol.1974.sp010541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVay S., Gilbert C. D. Laminar patterns of geniculocortical projection in the cat. Brain Res. 1976 Aug 20;113(1):1–19. doi: 10.1016/0006-8993(76)90002-0. [DOI] [PubMed] [Google Scholar]
- Movshon J. A. The velocity tuning of single units in cat striate cortex. J Physiol. 1975 Aug;249(3):445–468. doi: 10.1113/jphysiol.1975.sp011025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimi K., Miki M., Kawamura S. Ascending projections of the superior colliculus in the cat. Okajimas Folia Anat Jpn. 1970 Dec;47(5):269–287. doi: 10.2535/ofaj1936.47.5_269. [DOI] [PubMed] [Google Scholar]
- Noda H. Depression in the excitability of relay cells of lateral geniculate nucleus following saccadic eye movements in the cat. J Physiol. 1975 Jul;249(1):87–102. doi: 10.1113/jphysiol.1975.sp011004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda H. Discharges of relay cells in lateral geniculate nucleus of the cat during spontaneous eye movements in light and darkness. J Physiol. 1975 Sep;250(3):579–595. doi: 10.1113/jphysiol.1975.sp011071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTSUKA R., HASSLER R. [On the structure and segmentation of the cortical center of vision in the cat]. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1962;203:212–234. doi: 10.1007/BF00352744. [DOI] [PubMed] [Google Scholar]
- Palmer L. A., Rosenquist A. C. Visual receptive fields of single striate corical units projecting to the superior colliculus in the cat. Brain Res. 1974 Feb 15;67(1):27–42. doi: 10.1016/0006-8993(74)90295-9. [DOI] [PubMed] [Google Scholar]
- Pettigrew J. D., Nikara T., Bishop P. O. Responses to moving slits by single units in cat striate cortex. Exp Brain Res. 1968;6(4):373–390. doi: 10.1007/BF00233185. [DOI] [PubMed] [Google Scholar]
- Rose D. Proceedings: The hypercomplex cell classification in the cat's striate cortex. J Physiol. 1974 Oct;242(2):123P–125P. [PubMed] [Google Scholar]
- Schiller P. H., Finlay B. L., Volman S. F. Quantitative studies of single-cell properties in monkey striate cortex. I. Spatiotemporal organization of receptive fields. J Neurophysiol. 1976 Nov;39(6):1288–1319. doi: 10.1152/jn.1976.39.6.1288. [DOI] [PubMed] [Google Scholar]
- Schneider G. E. Two visual systems. Science. 1969 Feb 28;163(3870):895–902. doi: 10.1126/science.163.3870.895. [DOI] [PubMed] [Google Scholar]
- Sterling P., Wickelgren B. G. Visual receptive fields in the superior colliculus of the cat. J Neurophysiol. 1969 Jan;32(1):1–15. doi: 10.1152/jn.1969.32.1.1. [DOI] [PubMed] [Google Scholar]
- Stone J., Dreher B. Projection of X- and Y-cells of the cat's lateral geniculate nucleus to areas 17 and 18 of visual cortex. J Neurophysiol. 1973 May;36(3):551–567. doi: 10.1152/jn.1973.36.3.551. [DOI] [PubMed] [Google Scholar]
- Stone J. Morphology and physiology of the geniculocortical synapse in the cat: the question of parallel input to the striate cortex. Invest Ophthalmol. 1972 May;11(5):338–346. [PubMed] [Google Scholar]
- Toyama K., Matsunami K., Ono T., Tokashiki S. An intracellular study of neuronal organization in the visual cortex. Exp Brain Res. 1974;21(1):45–66. doi: 10.1007/BF00234257. [DOI] [PubMed] [Google Scholar]
- Updyke B. V. The patterns of projection of cortical areas 17, 18, and 19 onto the laminae of the dorsal lateral geniculate nucleus in the cat. J Comp Neurol. 1975 Oct 15;163(4):377–395. doi: 10.1002/cne.901630402. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N. Receptive fields of ganglion cells in the cat's retina. J Physiol. 1960 Oct;153:583–594. doi: 10.1113/jphysiol.1960.sp006557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickelgren B. G., Sterling P. Influence of visual cortex on receptive fields in the superior colliculus of the cat. J Neurophysiol. 1969 Jan;32(1):16–23. doi: 10.1152/jn.1969.32.1.16. [DOI] [PubMed] [Google Scholar]
- Wilson P. D., Stone J. Evidence of W-cell input to the cat's visual cortex via the C laminae of the lateral geniculate nucleus. Brain Res. 1975 Jul 18;92(3):472–478. doi: 10.1016/0006-8993(75)90333-9. [DOI] [PubMed] [Google Scholar]



