Abstract
1. The effect of 4-aminopyridine (4-AP) on the K outward and inward currents in perfused giant axons of Loligo forbesi has been studied with the voltage-clamp technique.
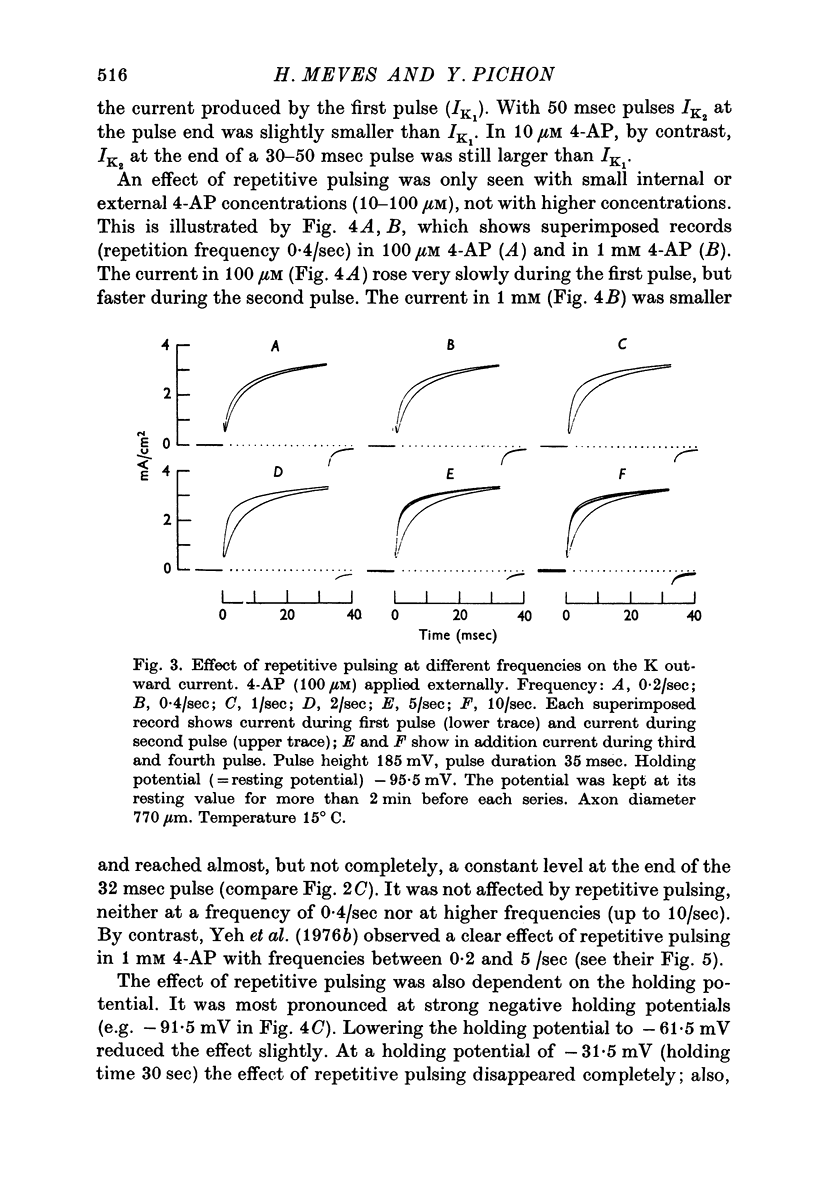
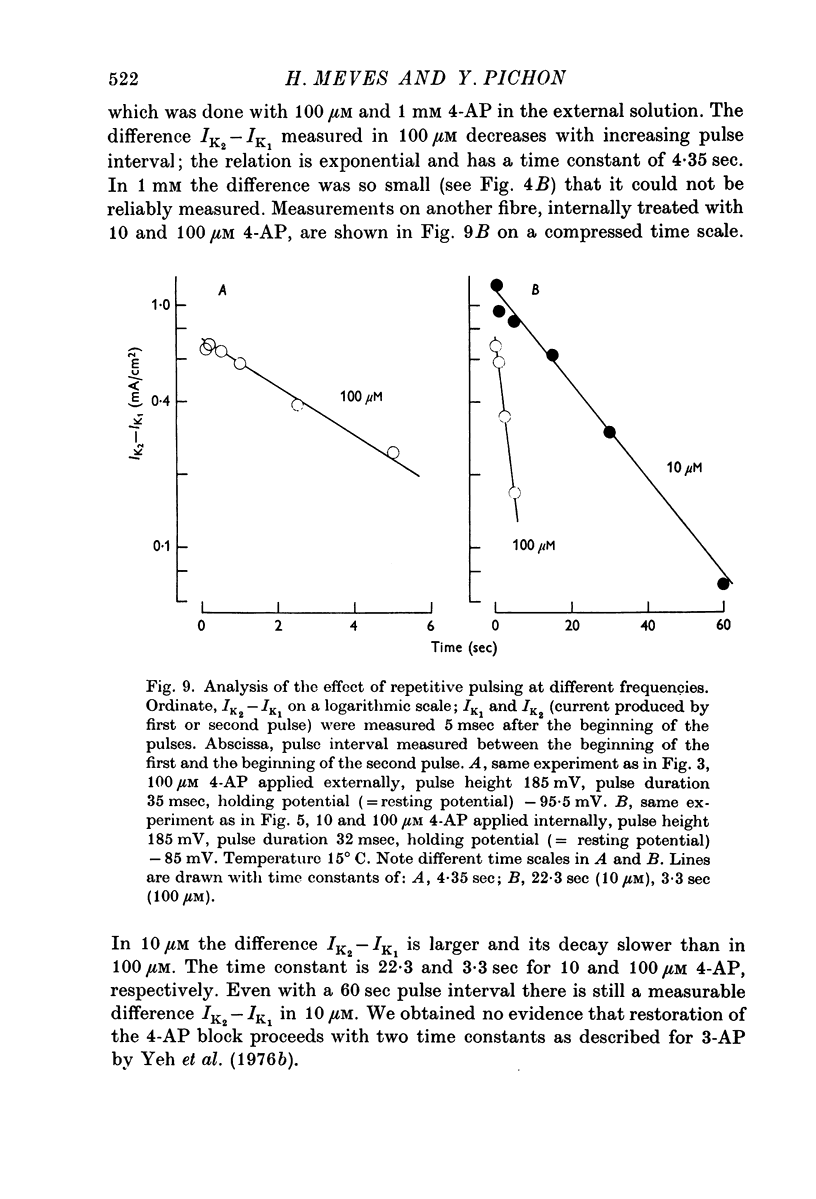
2. Small internal or external 4-AP concentrations (10-100 μM) considerably delay the rise of the K outward current. Repetitive pulsing with a pulse interval of 0·1-5 sec leads to a faster rise of the K current; in 10 μM 4-AP a small effect is visible even with a pulse interval of 60 sec.
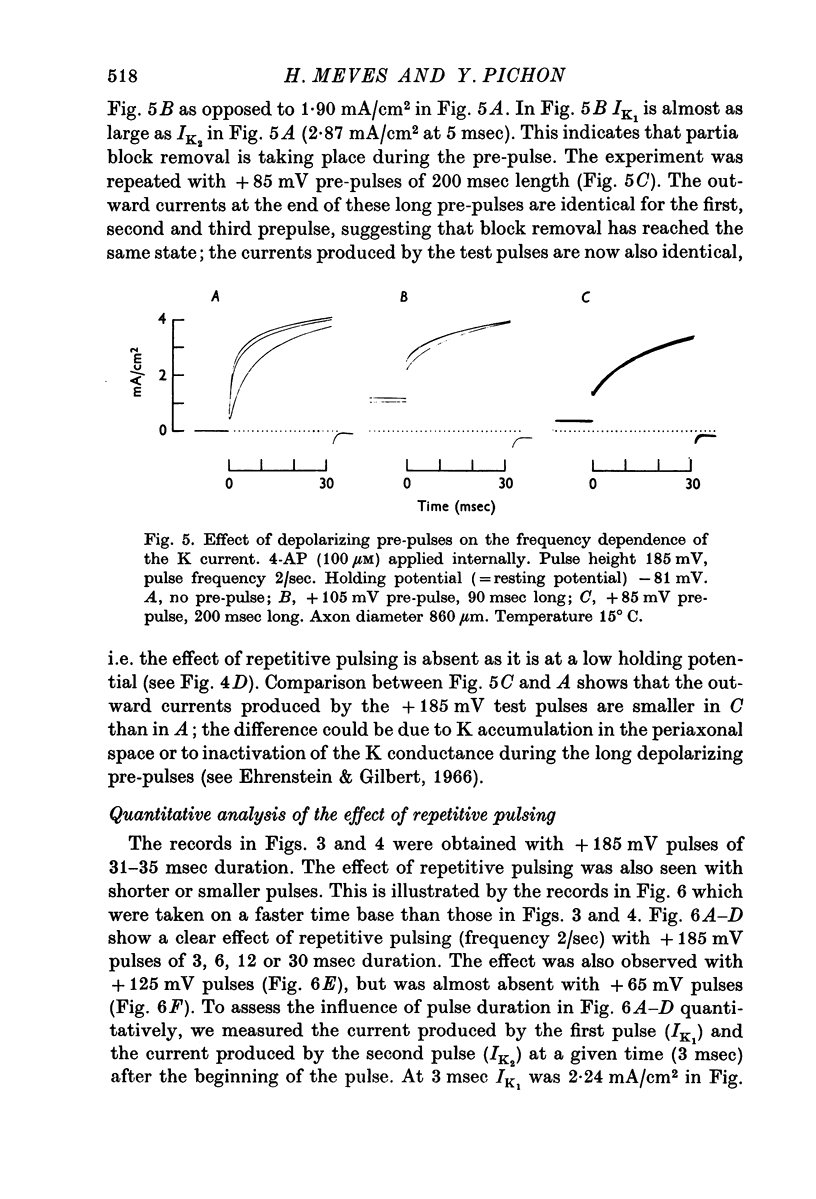
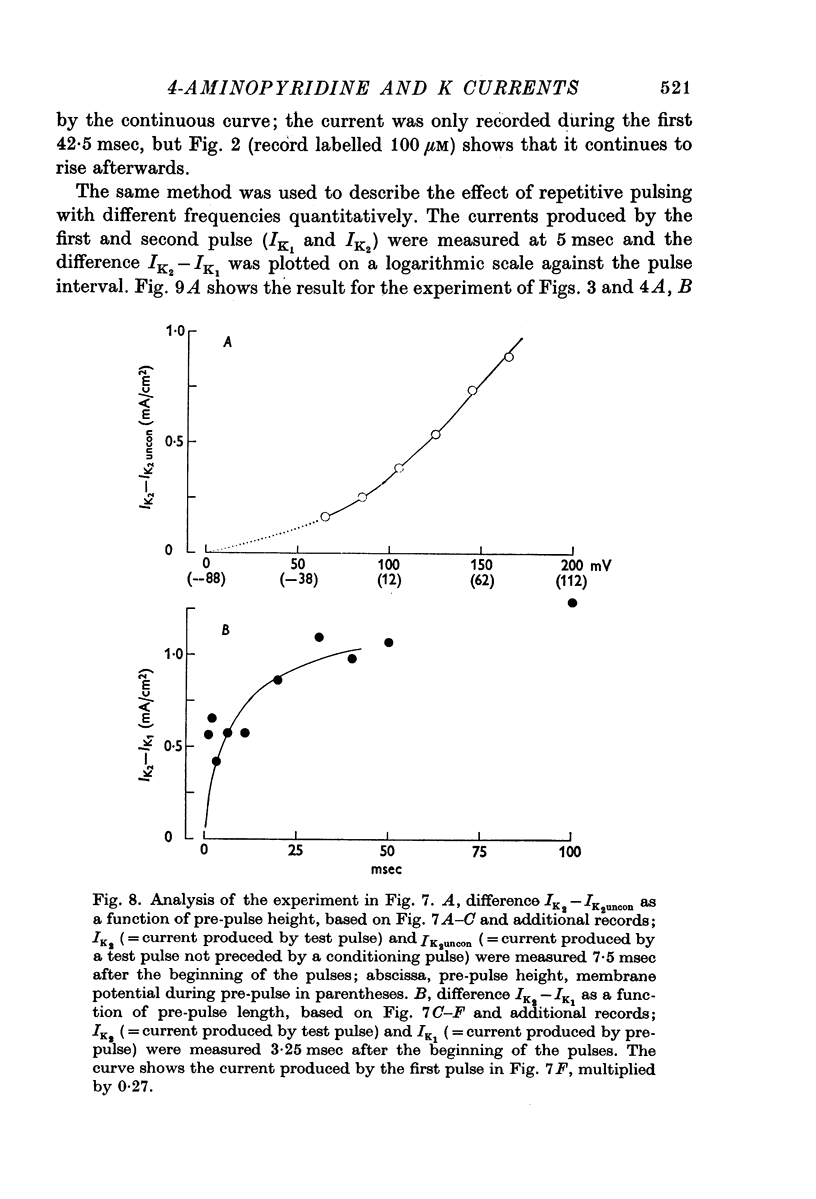
3. The phenomenon has been studied quantitatively by using a prepulse of varying height and duration, followed after 5 sec by a constant test pulse. The effect of changing the holding potential has been investigated.
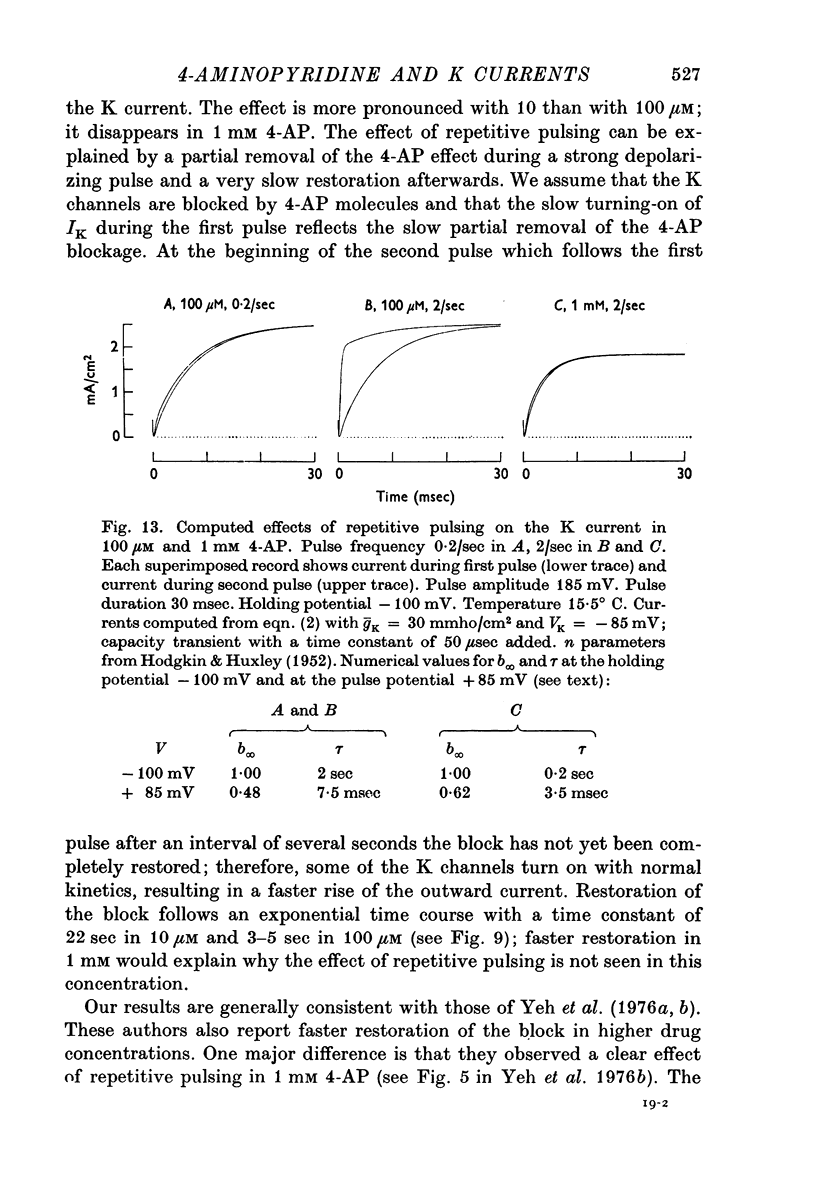
4. The effect of repetitive pulsing disappears in higher 4-AP concentrations; 1-10 mM 4-AP markedly reduce the size of the K outward current; the blocking effect is less pronounced for large depolarizing pulses than for small.
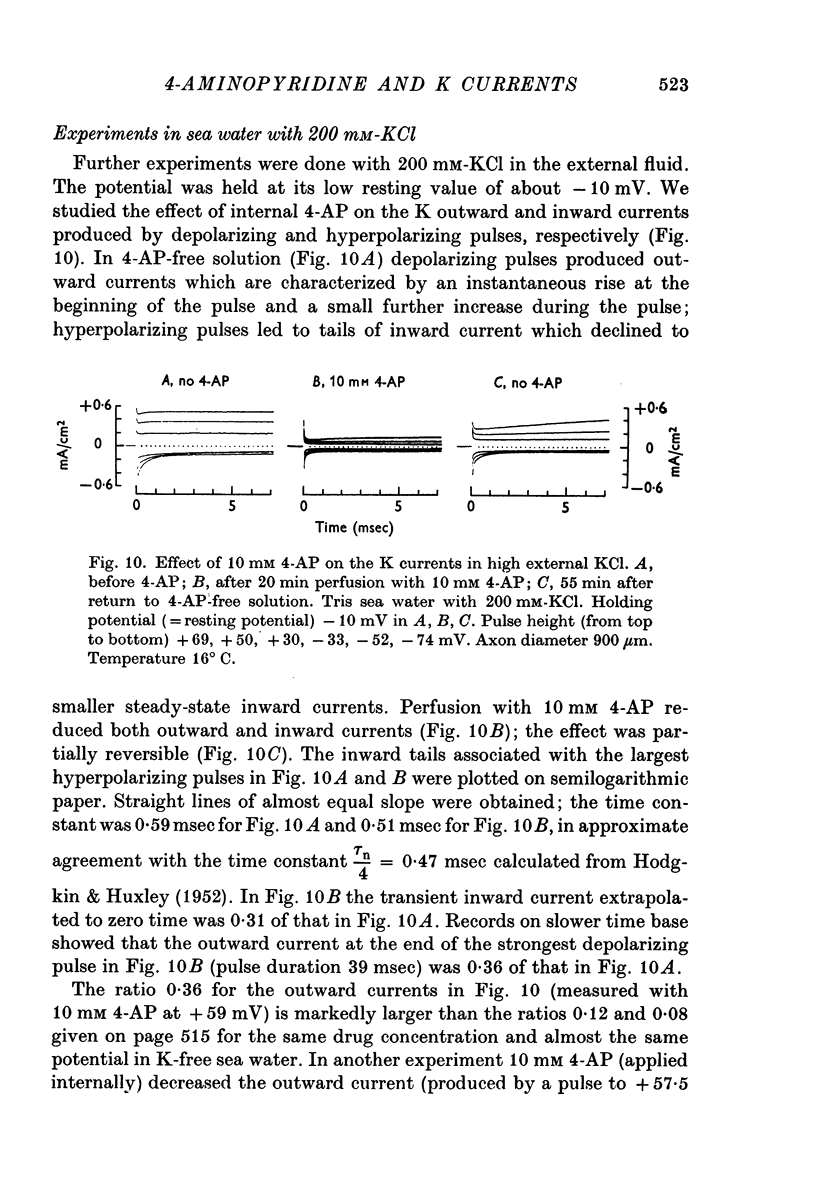
5. In K-rich sea water 4-AP reduces both the K outward current and the K inward current; the blocking effect on the K outward current is smaller than in K-free sea water.
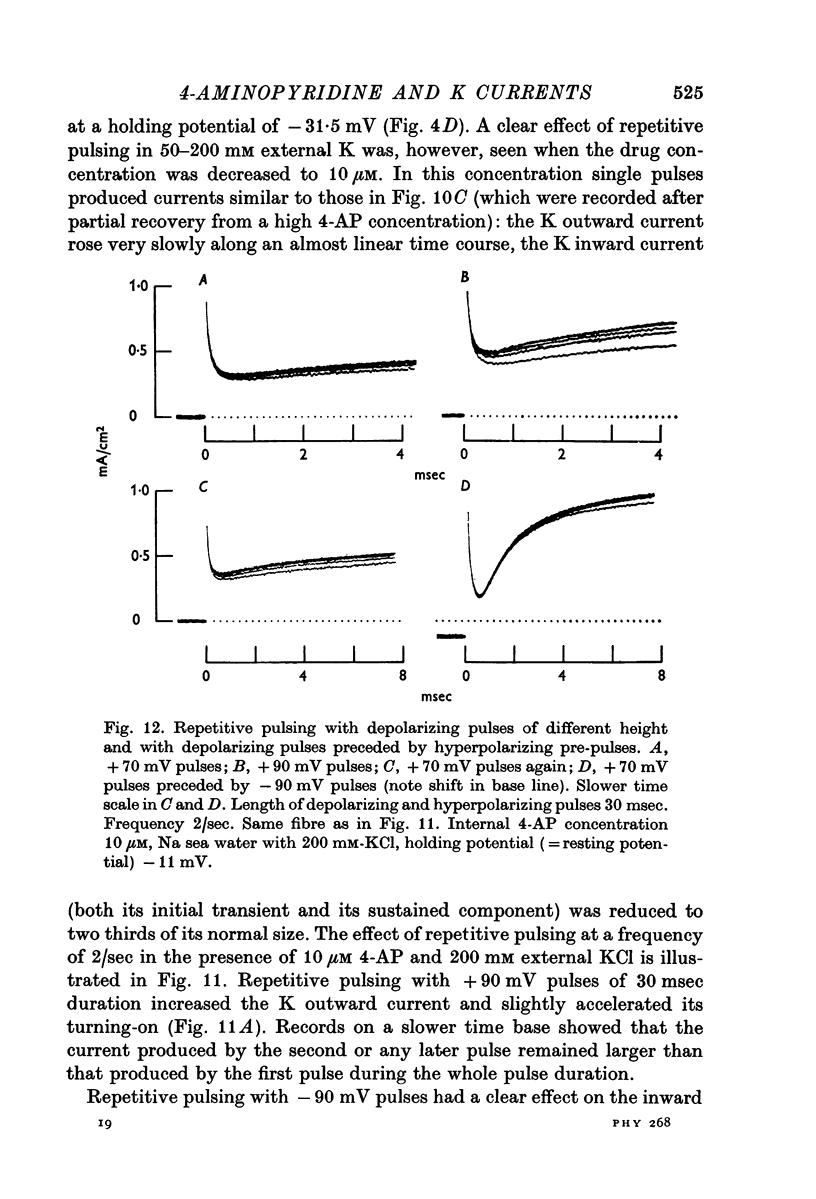
6. The K outward current in fibres treated with 10 μM 4-AP and immersed in K-rich sea water is increased and accelerated by repetitive depolarizing pulses. The effect of repetitive pulsing is not dependent on the size of the K outward current (which can be increased by removing K inactivation).
7. The effect of repetitive pulsing and the voltage dependence of the 4-AP block can be explained by the hypothesis that 4-AP molecules are displaced from their blocking sites during the pulse and slowly rebound afterwards. Removal of the 4-AP block by a depolarizing pulse seems to be a direct effect of the potential during the pulse and not related to K current.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG C. M., BINSTOCK L. ANOMALOUS RECTIFICATION IN THE SQUID GIANT AXON INJECTED WITH TETRAETHYLAMMONIUM CHLORIDE. J Gen Physiol. 1965 May;48:859–872. doi: 10.1085/jgp.48.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Ionic pores, gates, and gating currents. Q Rev Biophys. 1974 May;7(2):179–210. doi: 10.1017/s0033583500001402. [DOI] [PubMed] [Google Scholar]
- BAKER P. F., HODGKIN A. L., SHAW T. I. Replacement of the axoplasm of giant nerve fibres with artificial solutions. J Physiol. 1962 Nov;164:330–354. doi: 10.1113/jphysiol.1962.sp007025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Voltage clamp experiments on internally perfused giant axons. J Physiol. 1965 Oct;180(4):788–820. doi: 10.1113/jphysiol.1965.sp007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenstein G., Gilbert D. L. Slow changes of potassium permeability in the squid giant axon. Biophys J. 1966 Sep;6(5):553–566. doi: 10.1016/S0006-3495(66)86677-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956 Feb 28;131(2):341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves H., Pichon Y. Proceedings: Effects of 4-aminopyridine on the potassium current in internally perfused giant axons of the squid. J Physiol. 1975 Sep;251(1):60P–62P. [PubMed] [Google Scholar]
- Pelhate M., Pichon Y. Proceedings: Selective inhibition of potassium current in the giant axon of the cockroach. J Physiol. 1974 Oct;242(2):90P–91P. [PubMed] [Google Scholar]
- Sheridan R. E., Lester H. A. Relaxation measurements on the acetylcholine receptor. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3496–3500. doi: 10.1073/pnas.72.9.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbricht W., Wagner H. H. Block of potassium channels of the nodal membrane by 4-aminopyridine and its partial removal on depolarization. Pflugers Arch. 1976 Nov 30;367(1):77–87. doi: 10.1007/BF00583659. [DOI] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J. Z., Oxford G. S., Wu C. H., Narahashi T. Dynamics of aminopyridine block of potassium channels in squid axon membrane. J Gen Physiol. 1976 Nov;68(5):519–535. doi: 10.1085/jgp.68.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J. Z., Oxford G. S., Wu C. H., Narahashi T. Interactions of aminopyridines with potassium channels of squid axon membranes. Biophys J. 1976 Jan;16(1):77–81. doi: 10.1016/S0006-3495(76)85663-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


