Abstract
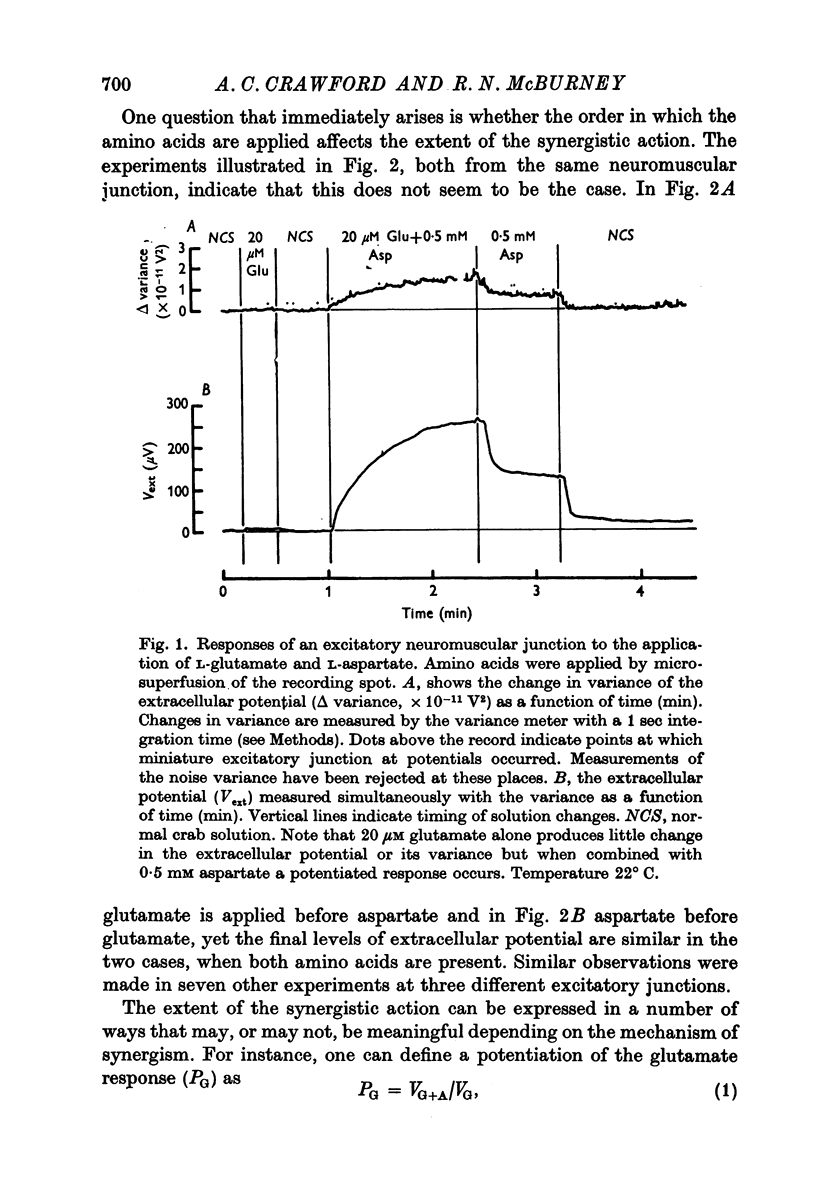
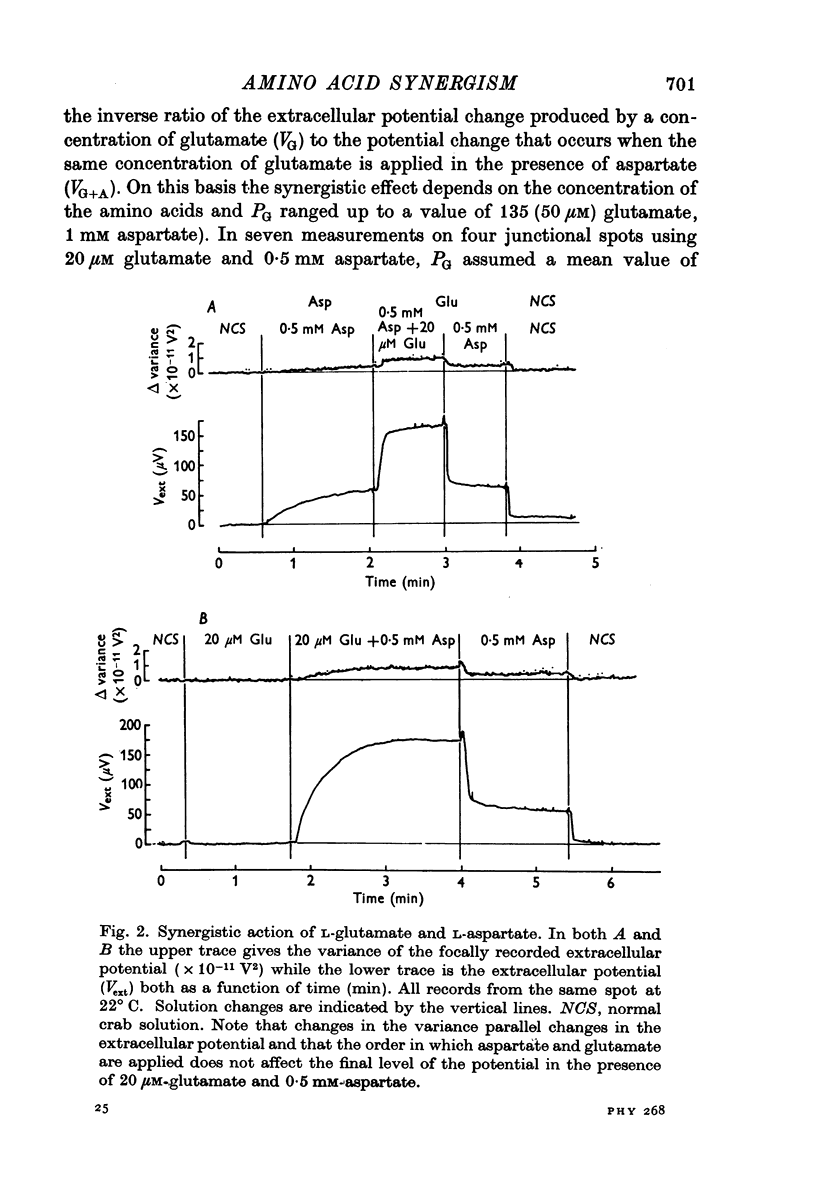
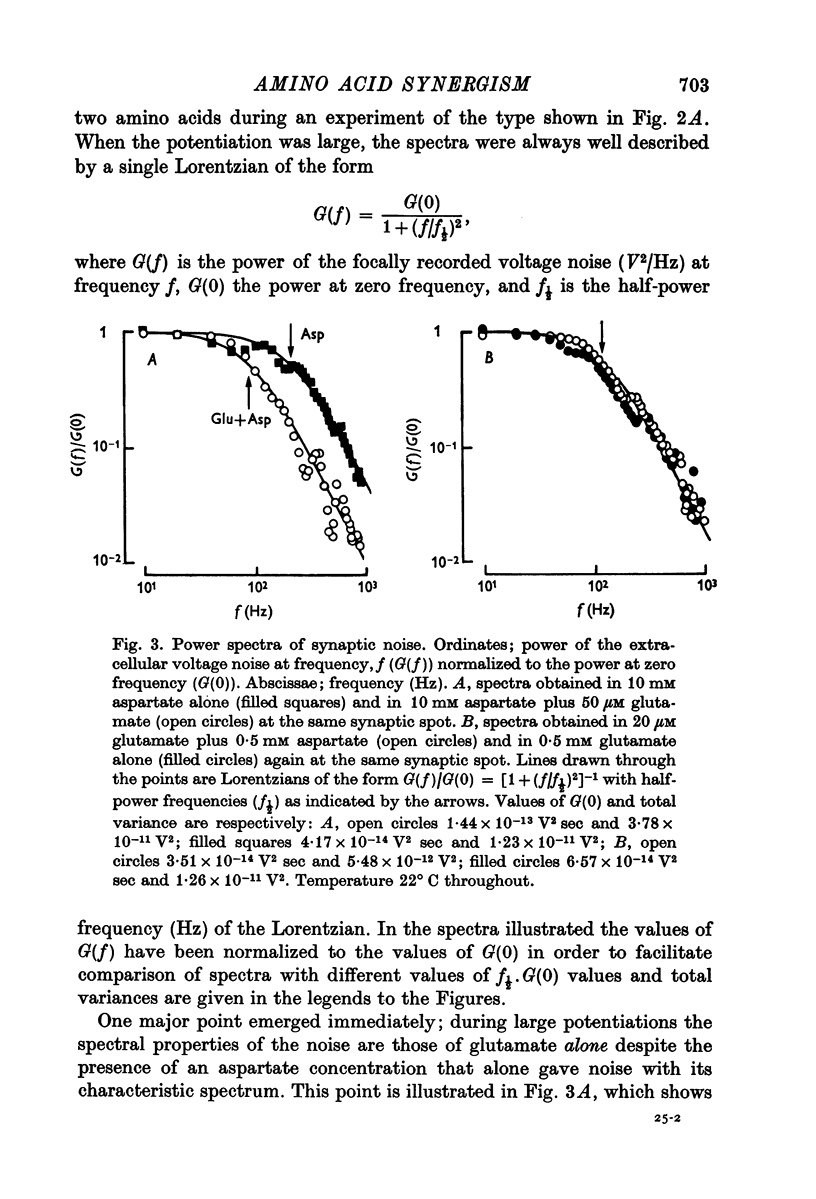
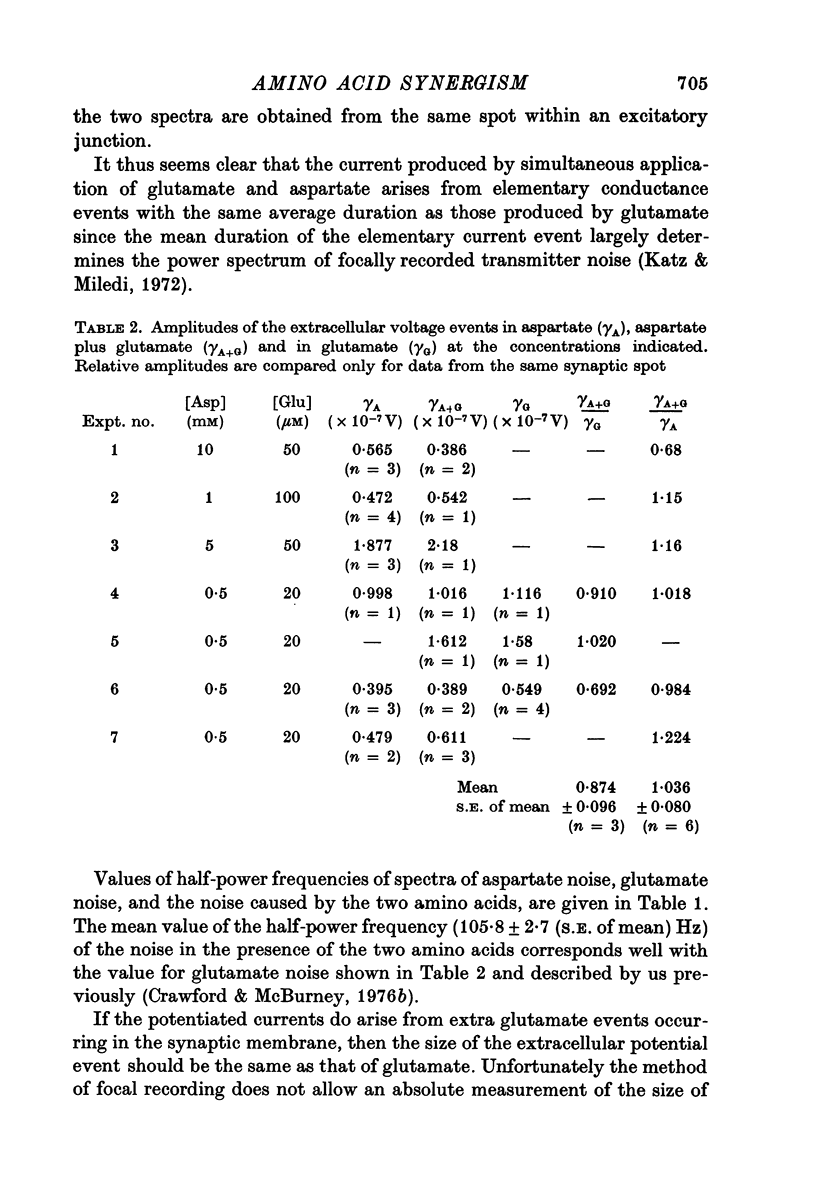
1. When L-glutamate and L-aspartate are simultaneously applied to the excitatory neuromuscular junctions of Maia squinado, they produce an increase in the conductance of the post-synaptic membrane much larger than the sum of conductance effects produced by the individual amino acids alone. 2. An examination of the synaptic noise occurring during this synergistic action reveals that the elementary conductnace events produced by aspartate are suppressed and that normal elementary conductance events produced by glutamate are occurring at an enormously increased rate. 3. It is suggested that aspartate causes this potentiation by inhibiting a system for transmitter inactivation in the region of the post-synaptic receptors and that this system, under normal conditions, prevents the access of externally applied glutamate to the synaptic receptors.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Potashner S. J. The dependence of glutamate uptake by crab nerve on external Na + and K + . Biochim Biophys Acta. 1971 Dec 3;249(2):616–622. doi: 10.1016/0005-2736(71)90142-8. [DOI] [PubMed] [Google Scholar]
- Crawford A. C., McBurney R. N. On the elementary conductance event produced by L-glutamate and quanta of the natural transmitter at the neuromuscular junctions of Maia squinado. J Physiol. 1976 Jun;258(1):205–225. doi: 10.1113/jphysiol.1976.sp011415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A. C., McBurney R. N. The post-synaptic action of some putative excitatory transmitter substances. Proc R Soc Lond B Biol Sci. 1976 Mar 16;192(1109):481–489. doi: 10.1098/rspb.1976.0026. [DOI] [PubMed] [Google Scholar]
- Jahromi S. S., Atwood H. L. Three-dimensional ultrastructure of the crayfish neuromuscular apparatus. J Cell Biol. 1974 Nov;63(2 Pt 1):599–613. doi: 10.1083/jcb.63.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973 Jun;231(3):549–574. doi: 10.1113/jphysiol.1973.sp010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank R. P., Freeman A. R. Cooperative interaction of glutamate and aspartate with receptors in the neuromuscular excitatory membrane in walking limbs of the lobster. J Neurobiol. 1975 May;6(3):289–303. doi: 10.1002/neu.480060305. [DOI] [PubMed] [Google Scholar]
- Shank R. P., Freeman A. R., McBride W. J., Aprison M. H. Glutamate and aspartate as mediators of neuromuscular excitation in the lobster. Comp Biochem Physiol C. 1975 Jan 1;50(1):127–131. [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. THE EFFECT ON CRAYFISH MUSCLE OF IONTOPHORETICALLY APPLIED GLUTAMATE. J Physiol. 1964 Mar;170:296–317. doi: 10.1113/jphysiol.1964.sp007332. [DOI] [PMC free article] [PubMed] [Google Scholar]


