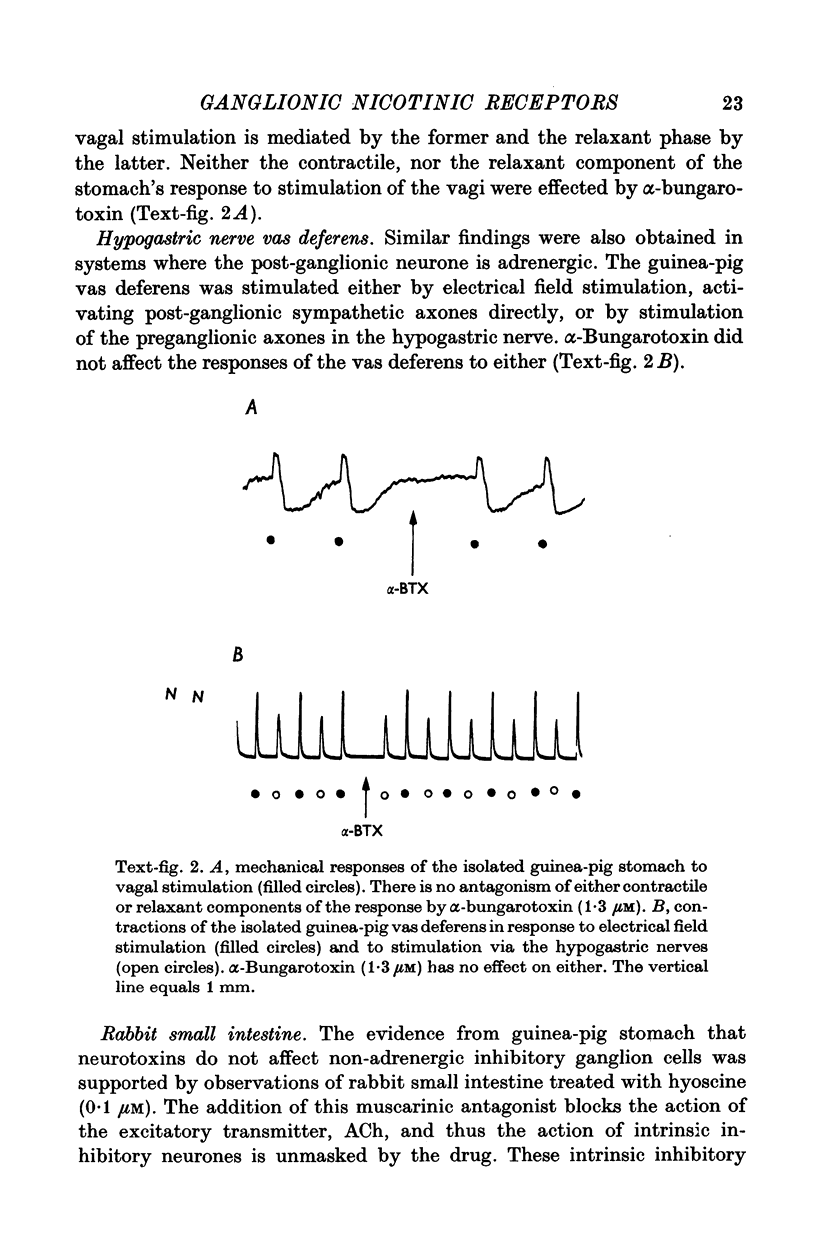
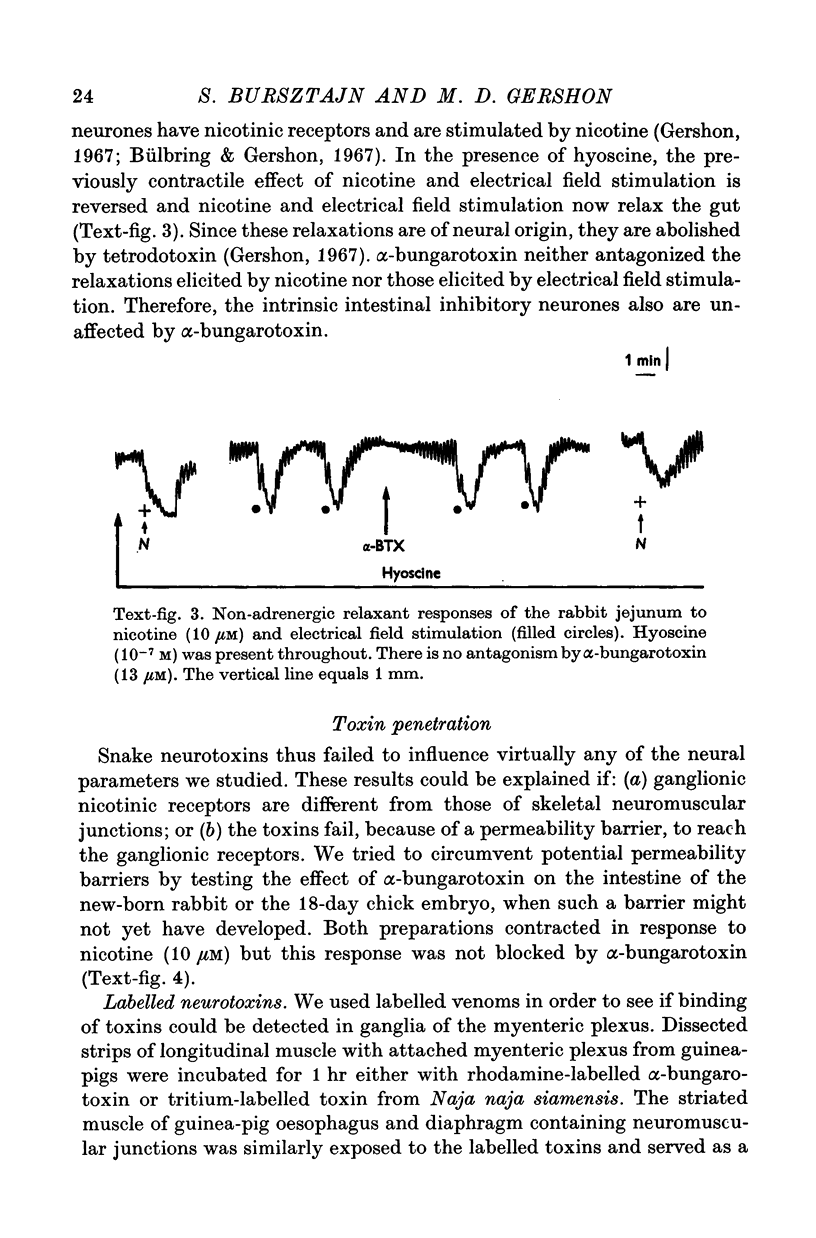
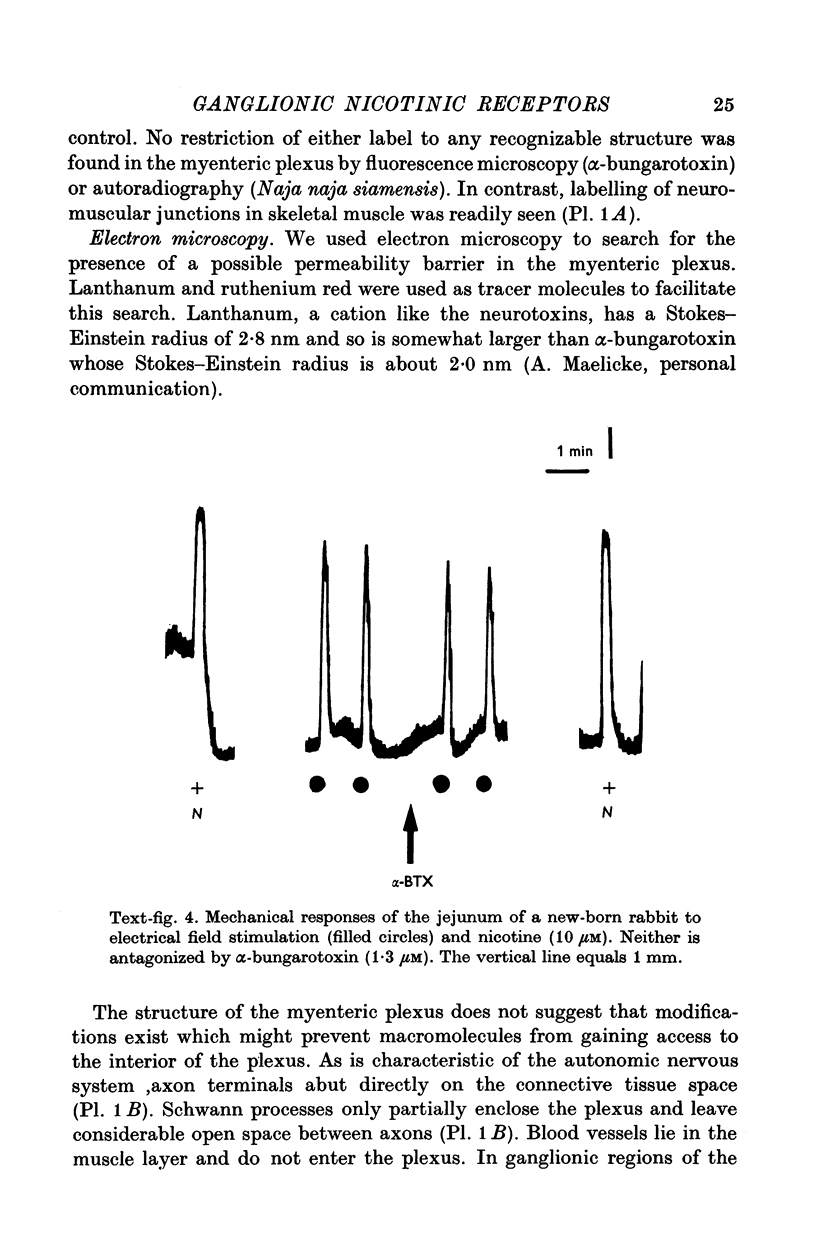
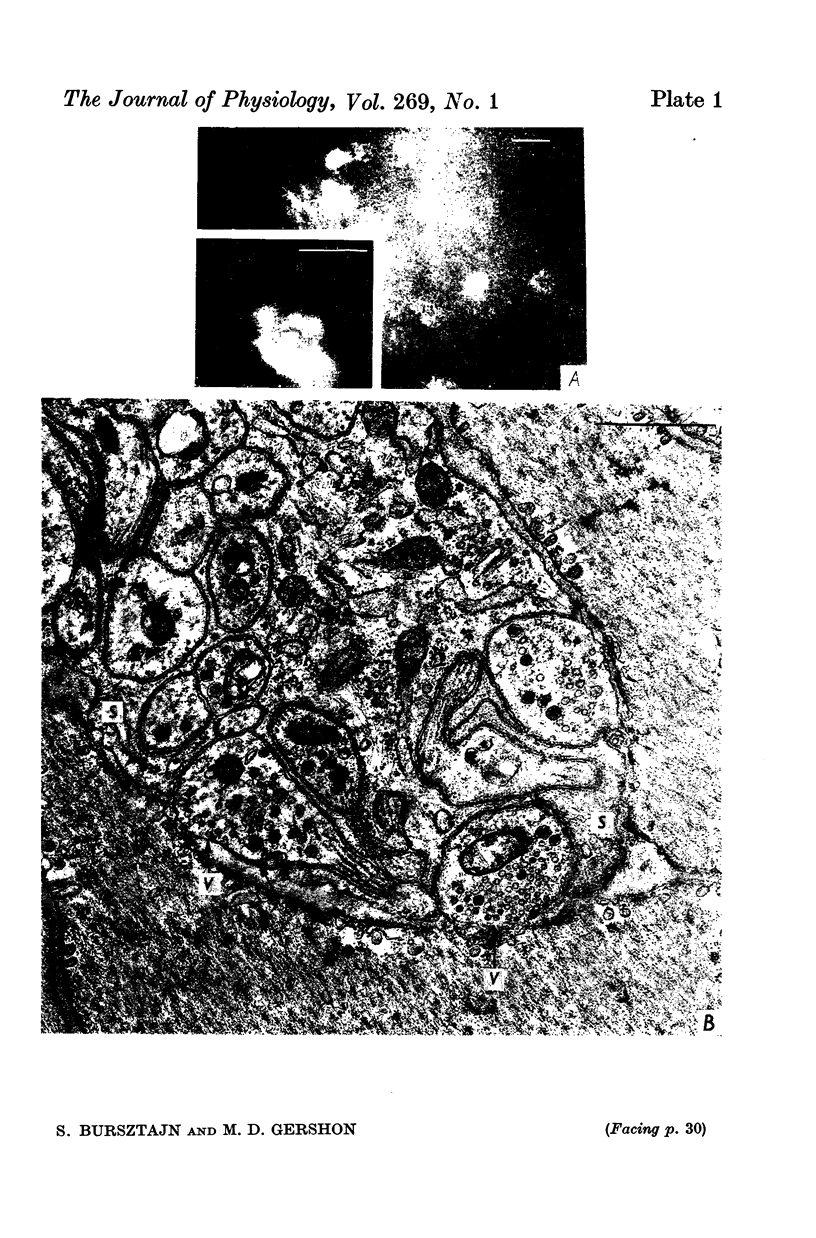
Abstract
1. We have used snake neurotoxins, alpha-bungarotoxin and venoms from Naja naja siamensis and Naja nivea, to distinguish the nicotinic receptors of ganglia from those of skeletal neuromuscular junctions. 2. These neurotoxins failed to block responses of isolated guinea-pig longitudinal muscle with adherent myenteric plexus to the nicotinic agonists, nicotine or dimethylphenylpiperazinium, to acetylcholine (ACh), or to electrical field stimulation. 3. The toxins failed to affect responses of the isolated guinea-pig stomach to pregnaglionic stimulation by way of the vagus nerves or of the vas deferens to preganglionic stimulation via the hypogastric nerves. 4. Snake neurotoxins did not block non-adrenergic inhibitory responses of the rabbit small intestine to nicotine or electrical field stimulation. 5. Neurotoxins were ineffective blockers against nicotinic agonists in new-born rabbit or embryonic chick intestine. 6. Attempts to increase the penetration of the toxins into tissues with dimethylsulphoxide, exposure to hypertonic solutions, or to ethylene-diaminetetracetic acid did not enable the toxins to act as nicotinic antagonists. 7. In contrast to diaphragmatic or oesophageal skeletal neuromuscular junctions no binding of rhodamine or tritium labelled toxins to structures in ganglia could be detected. 8. No potential permeability barriers were found by electron microscopy of the ganglia of the guinea-pig myenteric plexus. 9. The tracers, lanthanum ion and ruthenium red, readily penetrated into all regions of the myenteric plexus including synaptic gaps. 10. It is concluded that the failure of snake neurotoxins to act as nicotinic antagonists or to bind to ganglia is not due to their inability to reach ganglionic nicotinic receptors. Therefore, it is likely that ganglionic nicotinic receptors are different from those of the skeletal neuromuscular junction.
Full text
PDF









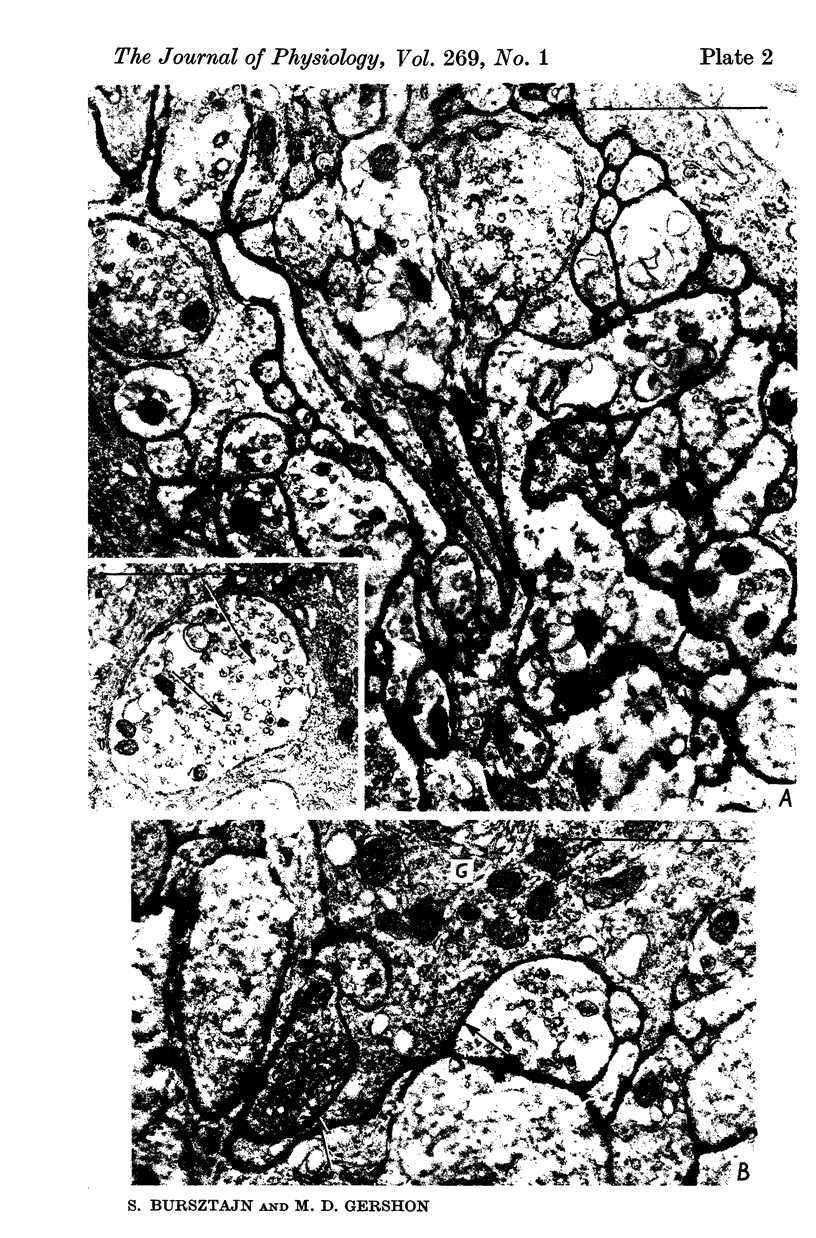

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. J., Cohen M. W. Fluorescent staining of acetylcholine receptors in vertebrate skeletal muscle. J Physiol. 1974 Mar;237(2):385–400. doi: 10.1113/jphysiol.1974.sp010487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacq Z. M., Brown G. L. Pharmacological experiments on mammalian voluntary muscle, in relation to the theory of chemical transmission. J Physiol. 1937 Feb 19;89(1):45–60. doi: 10.1113/jphysiol.1937.sp003461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard E. A., Wieckowski J., Chiu T. H. Cholinergic receptor molecules and cholinesterase molecules at mouse skeletal muscle junctions. Nature. 1971 Nov 26;234(5326):207–209. doi: 10.1038/234207a0. [DOI] [PubMed] [Google Scholar]
- Berg D. K., Hall Z. W. Increased extrajunctional acetylcholine sensitivity produced by chronic acetylcholine sensitivity produced by chronic post-synaptic neuromuscular blockade. J Physiol. 1975 Jan;244(3):659–676. doi: 10.1113/jphysiol.1975.sp010818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. K., Kelly R. B., Sargent P. B., Williamson P., Hall Z. W. Binding of -bungarotoxin to acetylcholine receptors in mammalian muscle (snake venom-denervated muscle-neonatal muscle-rat diaphragm-SDS-polyacrylamide gel electrophoresis). Proc Natl Acad Sci U S A. 1972 Jan;69(1):147–151. doi: 10.1073/pnas.69.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., Gershon M. D. 5-hydroxytryptamine participation in the vagal inhibitory innervation of the stomach. J Physiol. 1967 Oct;192(3):823–846. doi: 10.1113/jphysiol.1967.sp008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG C. C., LEE C. Y. ISOLATION OF NEUROTOXINS FROM THE VENOM OF BUNGARUS MULTICINCTUS AND THEIR MODES OF NEUROMUSCULAR BLOCKING ACTION. Arch Int Pharmacodyn Ther. 1963 Jul 1;144:241–257. [PubMed] [Google Scholar]
- Campbell G. The inhibitory nerve fibres in the vagal supply to the guinea-pig stomach. J Physiol. 1966 Aug;185(3):600–612. doi: 10.1113/jphysiol.1966.sp008004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P., Kasai M., Lee C. Y. Use of a snake venom toxin to characterize the cholinergic receptor protein. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1241–1247. doi: 10.1073/pnas.67.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D., Reich E. Neurotoxin from venom of the cobra, Naja naja siamensis. Purification and radioactive labeling. J Biol Chem. 1972 May 25;247(10):3008–3013. [PubMed] [Google Scholar]
- Daniels M. P., Vogel Z. Immunoperoxidase staining of alpha-bungarotoxin binding sites in muscle endplates shows distribution of acetylcholine receptors. Nature. 1975 Mar 27;254(5498):339–341. doi: 10.1038/254339a0. [DOI] [PubMed] [Google Scholar]
- Eaker D., Harris J. B., Thesleff S. Action of a cobra neurotoxin on denervated rat skeletal muscle. Eur J Pharmacol. 1971 Jul;15(2):254–256. doi: 10.1016/0014-2999(71)90182-8. [DOI] [PubMed] [Google Scholar]
- Earl J. E., Excell B. J. The effects of toxic components of Naja nivea (Cape cobra) venom on neuromuscular transmission and muscle membrane permeability. Comp Biochem Physiol A Comp Physiol. 1972 Mar;41(3):597–615. doi: 10.1016/0300-9629(72)90015-1. [DOI] [PubMed] [Google Scholar]
- Fambrough D. M., Hartzell H. C. Acetylcholine receptors: number and distribution at neuromuscular junctions in rat diaphragm. Science. 1972 Apr 14;176(4031):189–191. doi: 10.1126/science.176.4031.189. [DOI] [PubMed] [Google Scholar]
- Fertuck H. C., Salpeter M. M. Localization of acetylcholine receptor by 125I-labeled alpha-bungarotoxin binding at mouse motor endplates. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1376–1378. doi: 10.1073/pnas.71.4.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertuck H. C., Woodward W., Salpeter M. M. In vivo recovery of muscle contraction after alpha-bungarotoxin binding. J Cell Biol. 1975 Jul;66(1):209–213. doi: 10.1083/jcb.66.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkleman B. On the nature of inhibition in the intestine. J Physiol. 1930 Sep 18;70(2):145–157. doi: 10.1113/jphysiol.1930.sp002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon M. D. Effects of tetrodotoxin on innervated smooth muscle preparations. Br J Pharmacol Chemother. 1967 Mar;29(3):259–279. doi: 10.1111/j.1476-5381.1967.tb01958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon M. D., Ross L. L. Location of sites of 5-hydroxytryptamine storage and metabolism by radioautography. J Physiol. 1966 Oct;186(2):477–492. doi: 10.1113/jphysiol.1966.sp008047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. A., Sytkowski A. J., Vogel Z., Nirenberg M. W. -Bungarotoxin used as a probe for acetylcholine receptors of cultured neurones. Nature. 1973 May 18;243(5403):163–166. doi: 10.1038/243163a0. [DOI] [PubMed] [Google Scholar]
- HUKOVIC S. Responses of the isolated sympathetic nerveductus deferens preparation of the guinea-pig. Br J Pharmacol Chemother. 1961 Apr;16:188–194. doi: 10.1111/j.1476-5381.1961.tb00312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnovsky M. J. The ultrastructural basis of capillary permeability studied with peroxidase as a tracer. J Cell Biol. 1967 Oct;35(1):213–236. doi: 10.1083/jcb.35.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J., Sealock R., Bon C. Effects of alpha-toxins from Bungarus multicinctus and Bungarus caeruleus on cholinergic responses in Aplysia neurons. Brain Res. 1976 May 14;107(3):527–540. doi: 10.1016/0006-8993(76)90142-6. [DOI] [PubMed] [Google Scholar]
- Langley J. N., Magnus R. Some observations of the movements of the intestine before and after degenerative section of the mesenteric nerves. J Physiol. 1905 Sep 8;33(1):34–51. doi: 10.1113/jphysiol.1905.sp001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. Y., Chang C. C. Modes of actions of purified toxins from elapid venoms on neuromuscular transmission. Mem Inst Butantan. 1966;33(2):555–572. [PubMed] [Google Scholar]
- Lee C. Y., Chang S. L., Kau S. T., Luh S. H. Chromatographic separation of the venom of Bungarus multicinctus and characterization of its components. J Chromatogr. 1972 Oct 5;72(1):71–82. doi: 10.1016/0021-9673(72)80009-8. [DOI] [PubMed] [Google Scholar]
- Lee C. Y., Tseng L. F., Chiu T. H. Influence of denervation on localization of neurotoxins from clapid venoms in rat diaphragm. Nature. 1967 Sep 9;215(5106):1177–1178. doi: 10.1038/2151177a0. [DOI] [PubMed] [Google Scholar]
- Lester H. A. Postsynaptic action of cobra toxin at the myoneural junction. Nature. 1970 Aug 15;227(5259):727–728. doi: 10.1038/227727a0. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- Miledi R., Molinoff P., Potter L. T. Isolation of the cholinergic receptor protein of Torpedo electric tissue. Nature. 1971 Feb 19;229(5286):554–557. doi: 10.1038/229554a0. [DOI] [PubMed] [Google Scholar]
- Moore W. J., Loy N. J. Irreversible binding of a krait neurotoxin to membrane proteins from eel electroplax and hog brain. Biochem Biophys Res Commun. 1972 Mar 24;46(6):2093–2099. doi: 10.1016/0006-291x(72)90764-4. [DOI] [PubMed] [Google Scholar]
- Nurse C. A., O'Lague P. H. Formation of cholinergic synapses between dissociated sympathetic neurons and skeletal myotubes of the rat in cell culture. Proc Natl Acad Sci U S A. 1975 May;72(5):1955–1959. doi: 10.1073/pnas.72.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATON W. D. M., PERRY W. L. M. The relationship between depolarization and block in the cat's superior cervical ganglion. J Physiol. 1953 Jan;119(1):43–57. doi: 10.1113/jphysiol.1953.sp004827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATON W. D. M., ZAIMIS E. J. The pharmacological actions of polymethylene bistrimethyl-ammonium salts. Br J Pharmacol Chemother. 1949 Dec;4(4):381–400. doi: 10.1111/j.1476-5381.1949.tb00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATON W. D. The response of the guineapig ileum to electrical stimulation by coaxial electrodes. J Physiol. 1955 Feb 28;127(2):40–1P. [PubMed] [Google Scholar]
- PATON W. D., VANE J. R. Analysis of he responses of the isolated stomach to electrical stimulation and to drugs. J Physiol. 1963 Jan;165:10–46. doi: 10.1113/jphysiol.1963.sp007040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton W. D., Savini E. C. The action of nicotine on the motor endplate in the cat. Br J Pharmacol Chemother. 1968 Feb;32(2):360–380. doi: 10.1111/j.1476-5381.1968.tb00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton W. D., Vizi E. S. The inhibitory action of noradrenaline and adrenaline on acetylcholine output by guinea-pig ileum longitudinal muscle strip. Br J Pharmacol. 1969 Jan;35(1):10–28. doi: 10.1111/j.1476-5381.1969.tb07964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton W. D., Zar M. A. The origin of acetylcholine released from guinea-pig intestine and longitudinal muscle strips. J Physiol. 1968 Jan;194(1):13–33. doi: 10.1113/jphysiol.1968.sp008392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees R. P., Bunge M. B., Bunge R. P. Morphological changes in the neuritic growth cone and target neuron during synaptic junction development in culture. J Cell Biol. 1976 Feb;68(2):240–263. doi: 10.1083/jcb.68.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer L. A., Eldefrawi M. E. Identification of the nicotinic and muscarinic acetylcholine receptors in subcellular fractions of mouse brain. Neuropharmacology. 1974 Jan;13(1):53–63. doi: 10.1016/0028-3908(74)90007-0. [DOI] [PubMed] [Google Scholar]