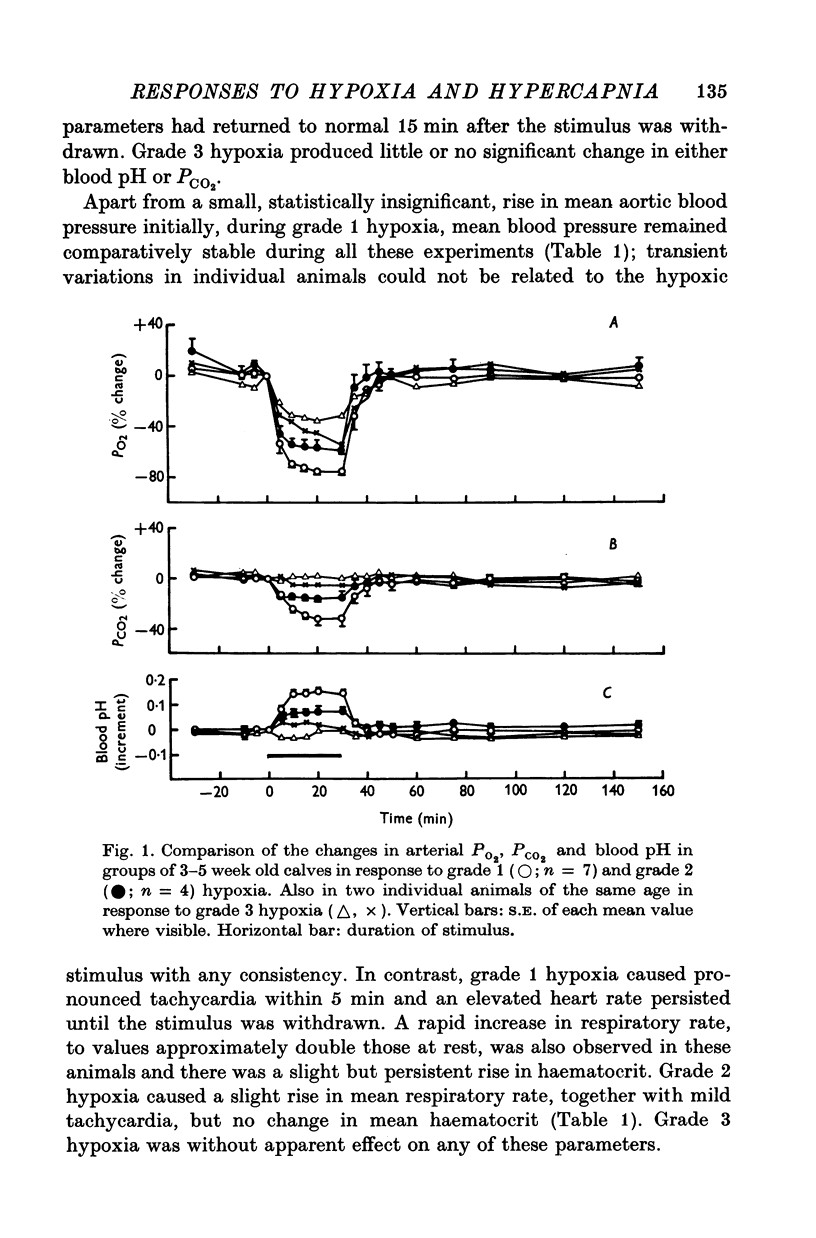
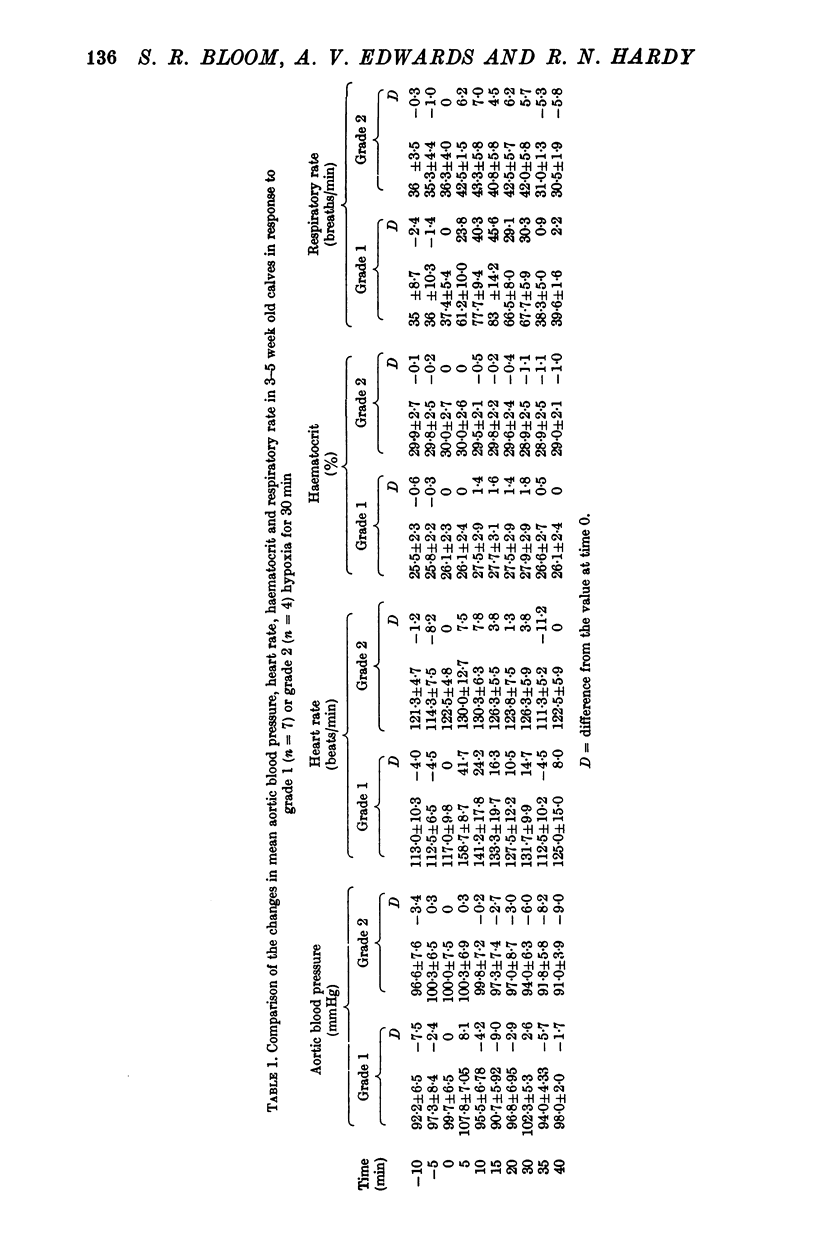
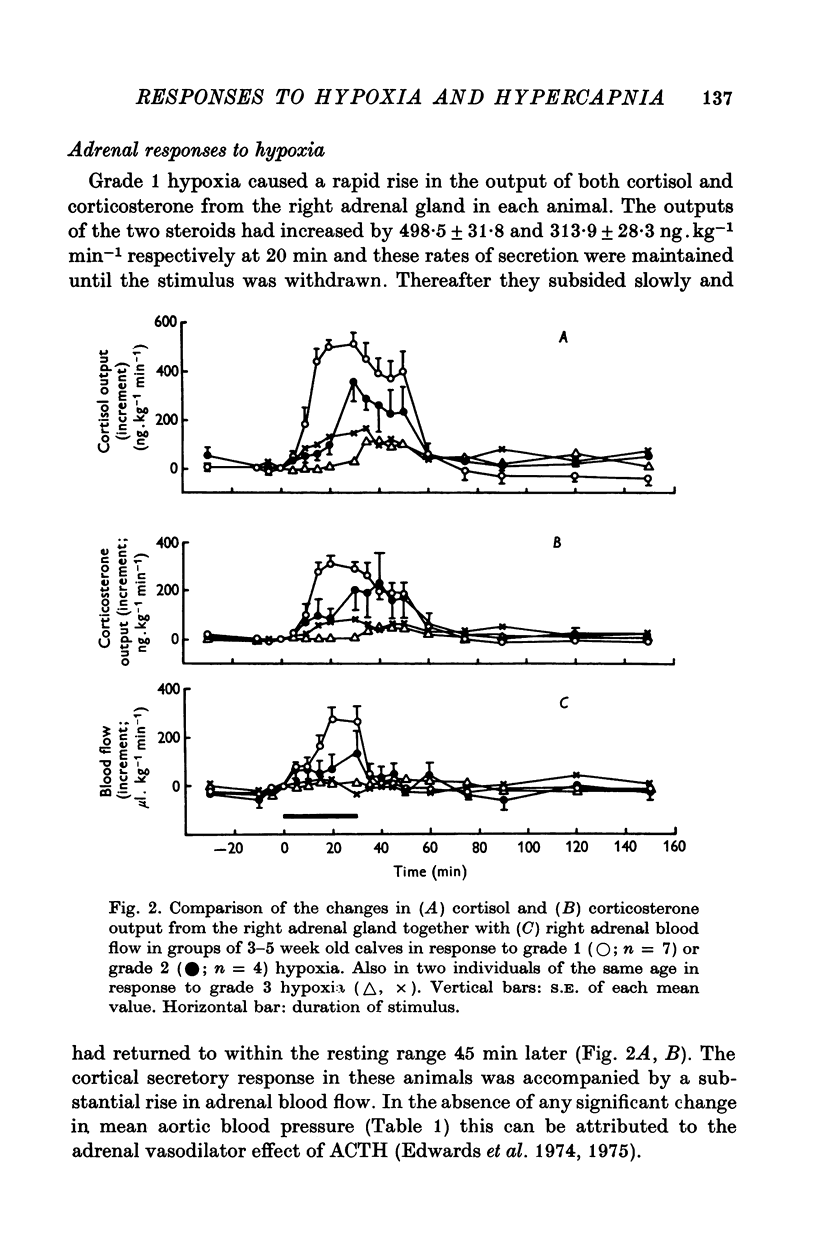
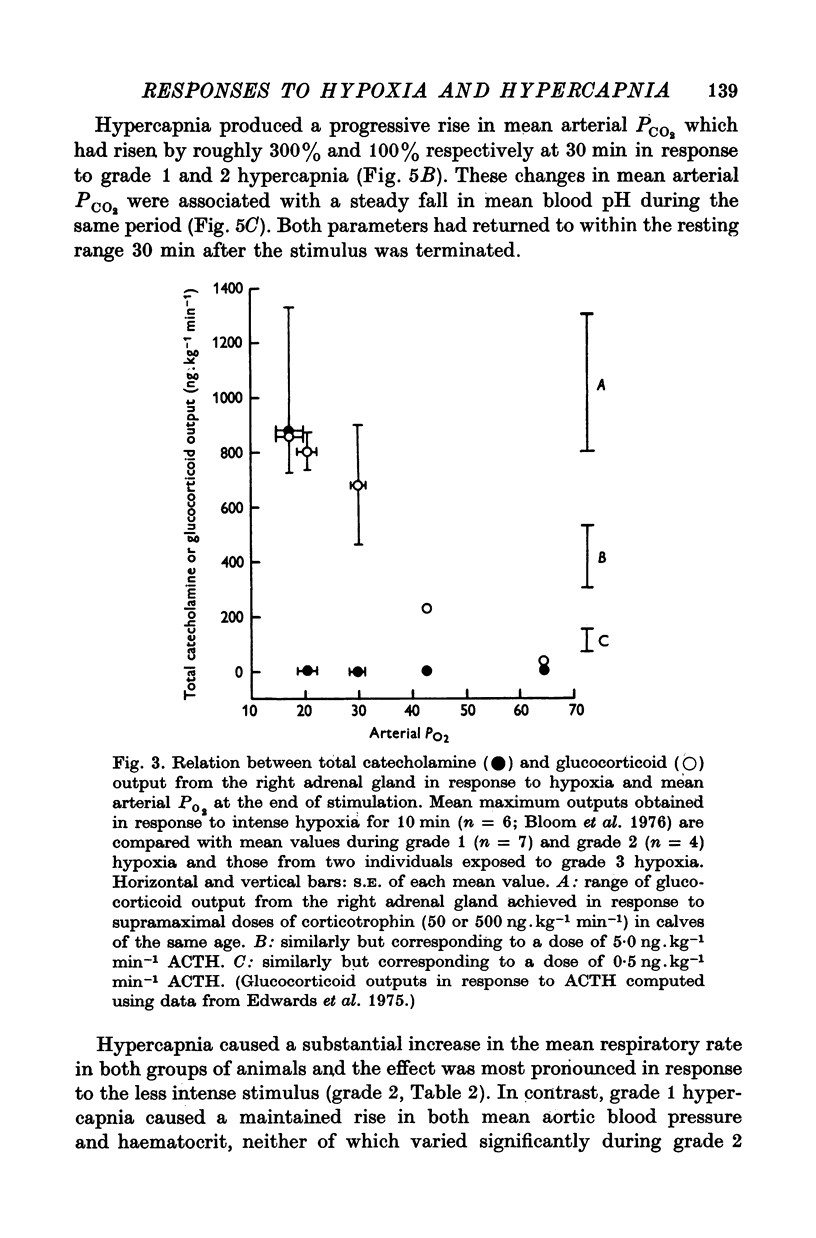
Abstract
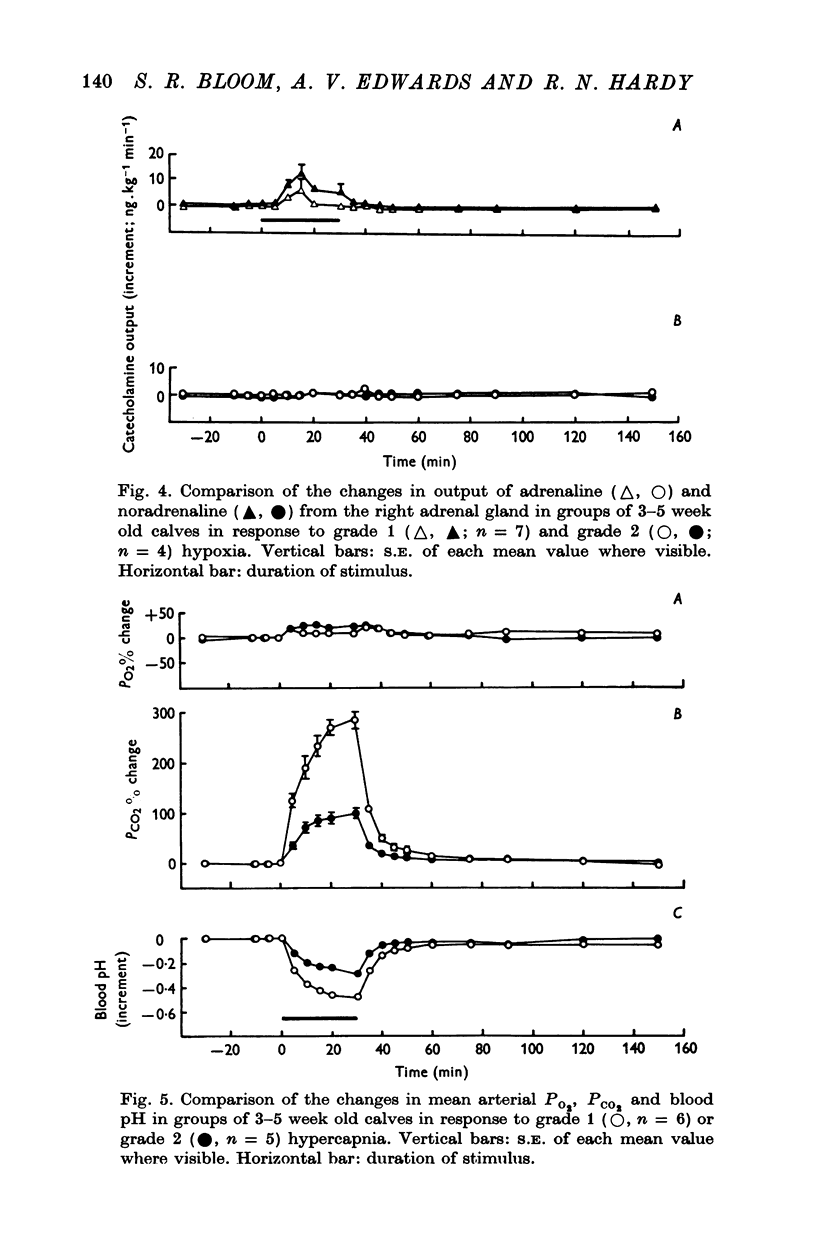
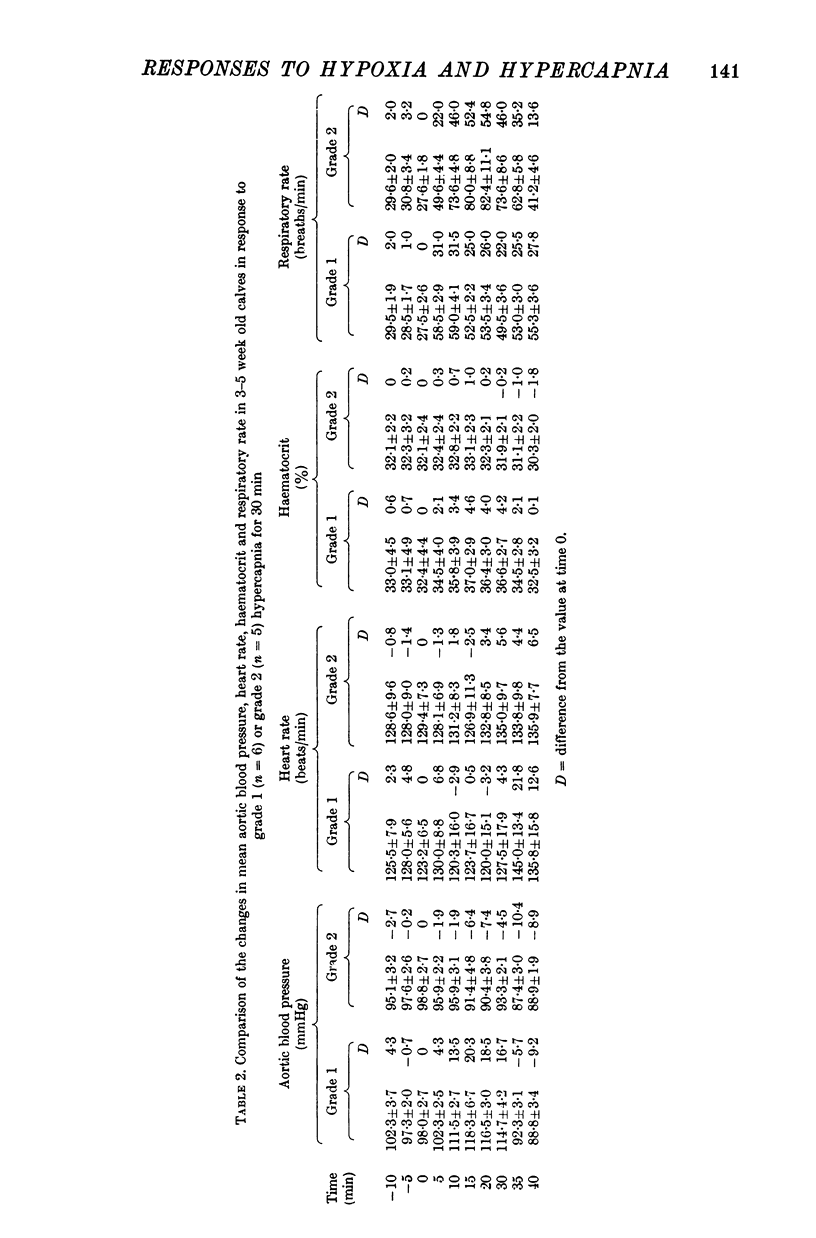
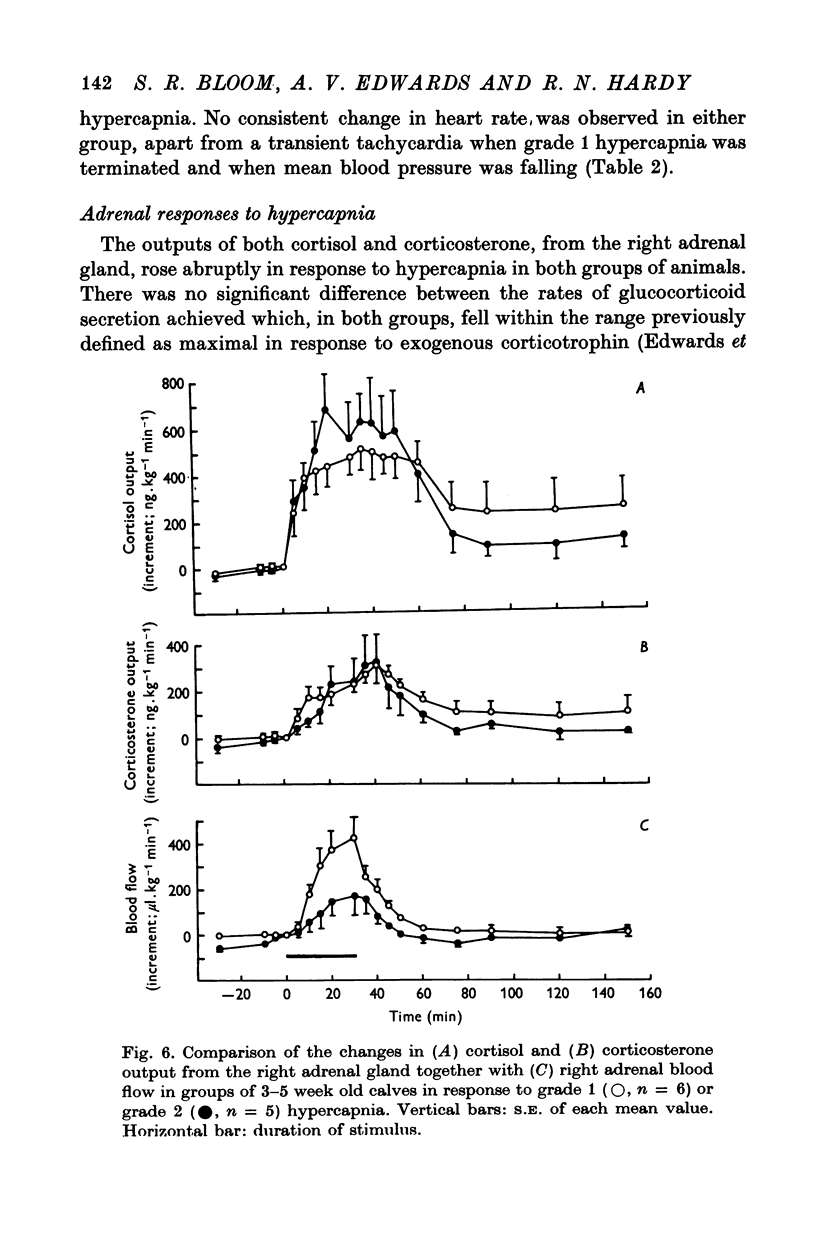
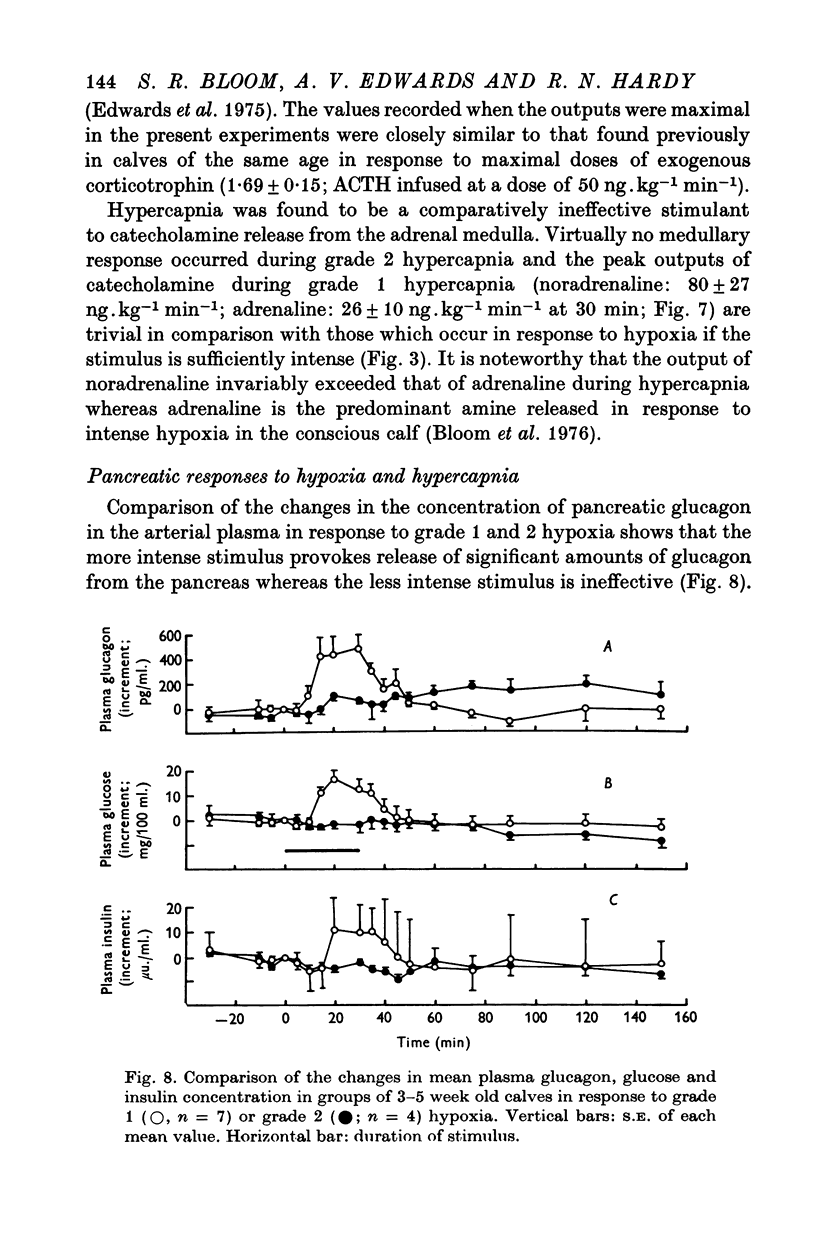
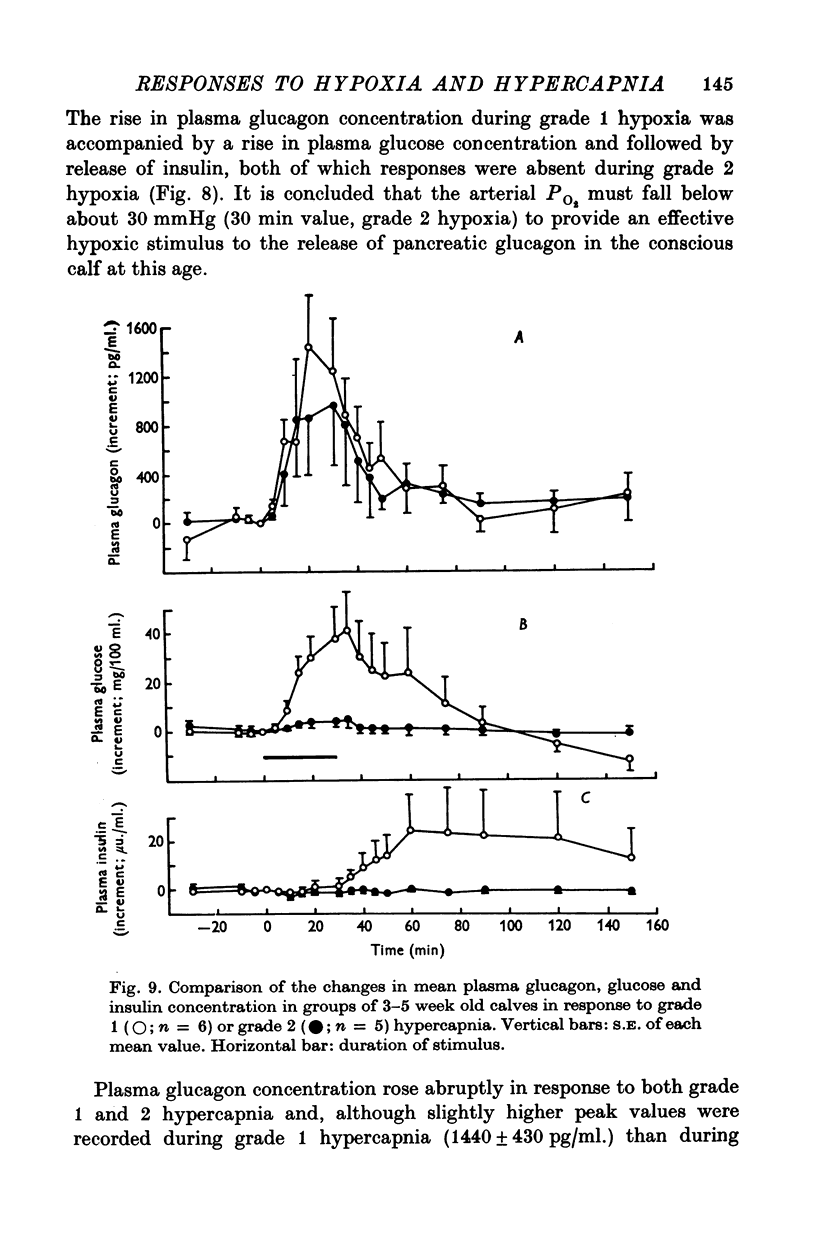
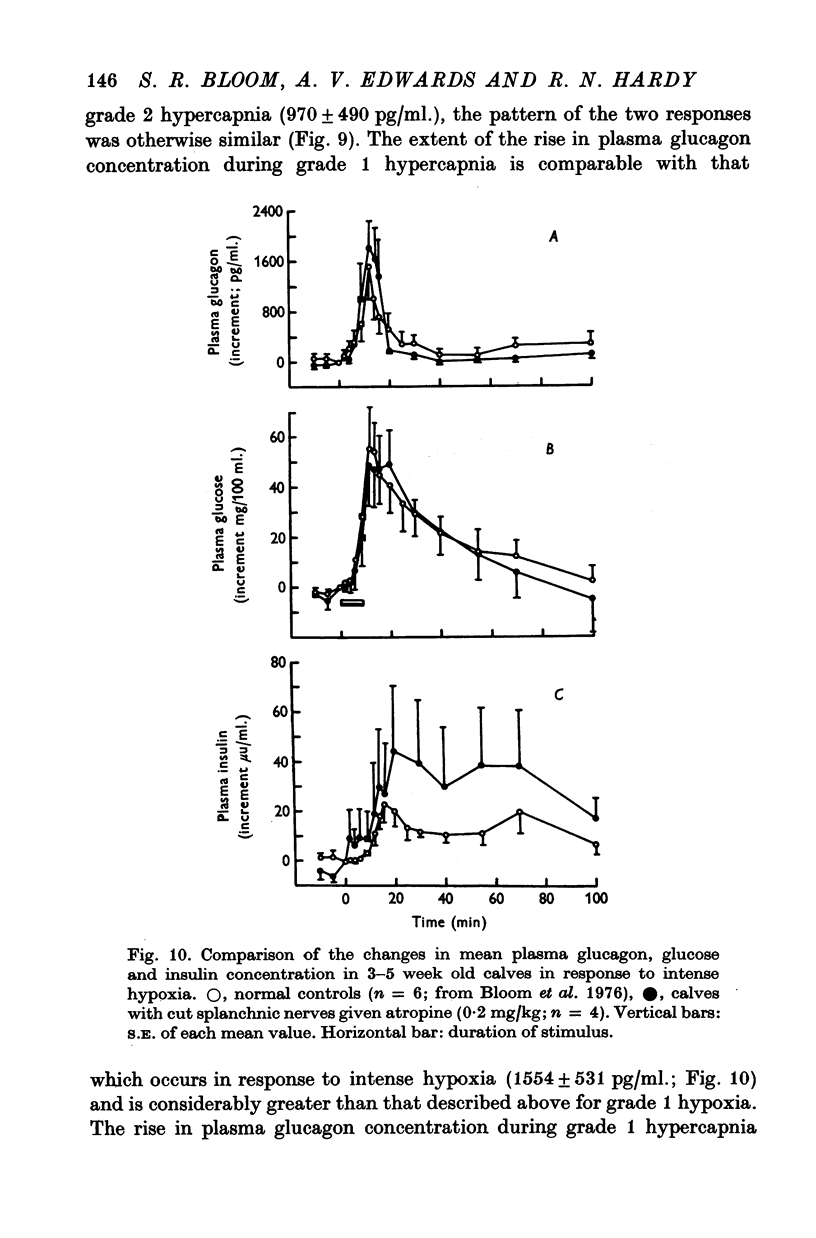
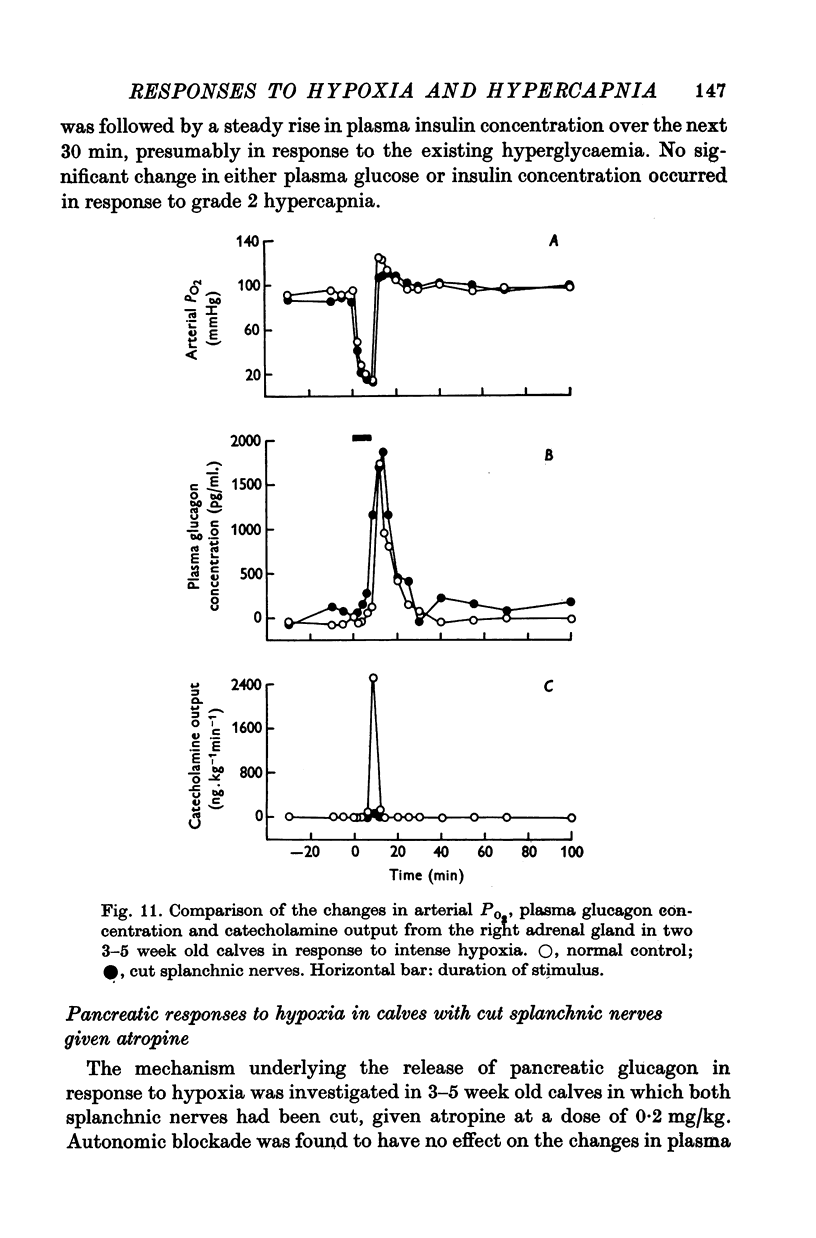
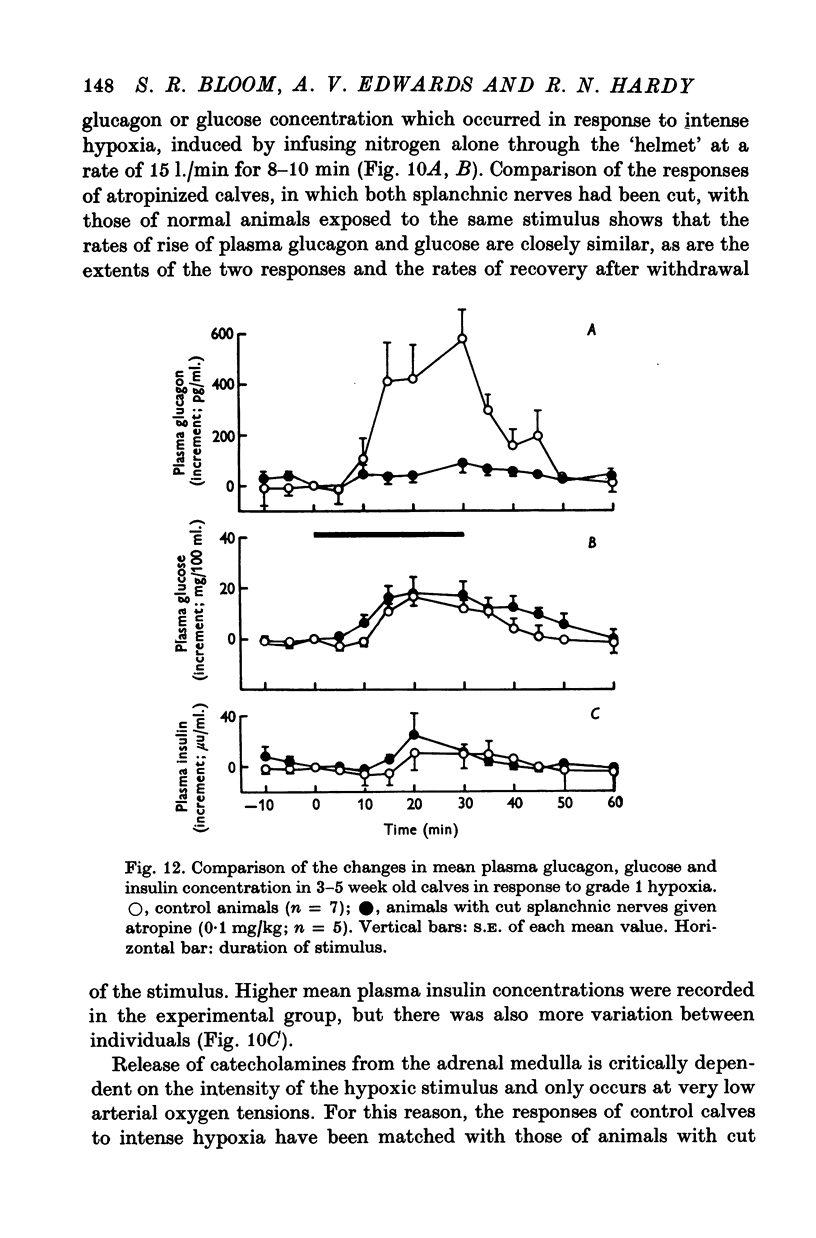
1. Adrenal and pancreatic endocrine responses to hypoxia and hypercapnia, of differing degrees of intensity, have been examined in conscious, unrestrained calves 3-5 weeks after birth. 2. The outputs of cortisol and corticosterone from the right adrenal gland were found to vary inversely with arterial Po2 between 17 and 55 mmHg. Significant increase in mean adrenal blood flow was not observed at arterial oxygen tensions above about 30 mmHg. 3. Release of physiologically effective amounts of catecholamines from the adrenal medulla occurred only in response to intense hypoxia (arterial Po2 17-1 +/- 2-8 mmHg) and was effectively abolished by section of both splanchnic nerves. Release of pancreatic glucagon in response to such intense hypoxia was unaffected by section of both splanchnic nerves and administration of atropine. In contrast, the rise in plasma pancreatic glucagon concentration during less intense hypoxia was abolished by autonomic blockade. 4. Hypercapnia produced by inhalation of either 5% or 10% CO2 for 30 min stimulated maximal release of adrenal glucocorticoids and caused a substantial rise in plasma glucagon concentration. In contrast, the adrenal medulla was found to be extremely resistant to hypercapnia. Significant release of catecholamines was only observed during intense hypercapnia (inhalation of 10% CO2) and noradrenaline was invariably found to be the predominant amine. 5. The results of these experiments show how endocrine responses to hypoxia and hypercapnia are graded in the conscious calf. Of the mechanisms we have examined the pituitary-adrenal cortical axis is the most sensitive and the adrenal medulla the most resistant, while the pancreatic alpha cell occupies an intermediate position.
Full text
PDF




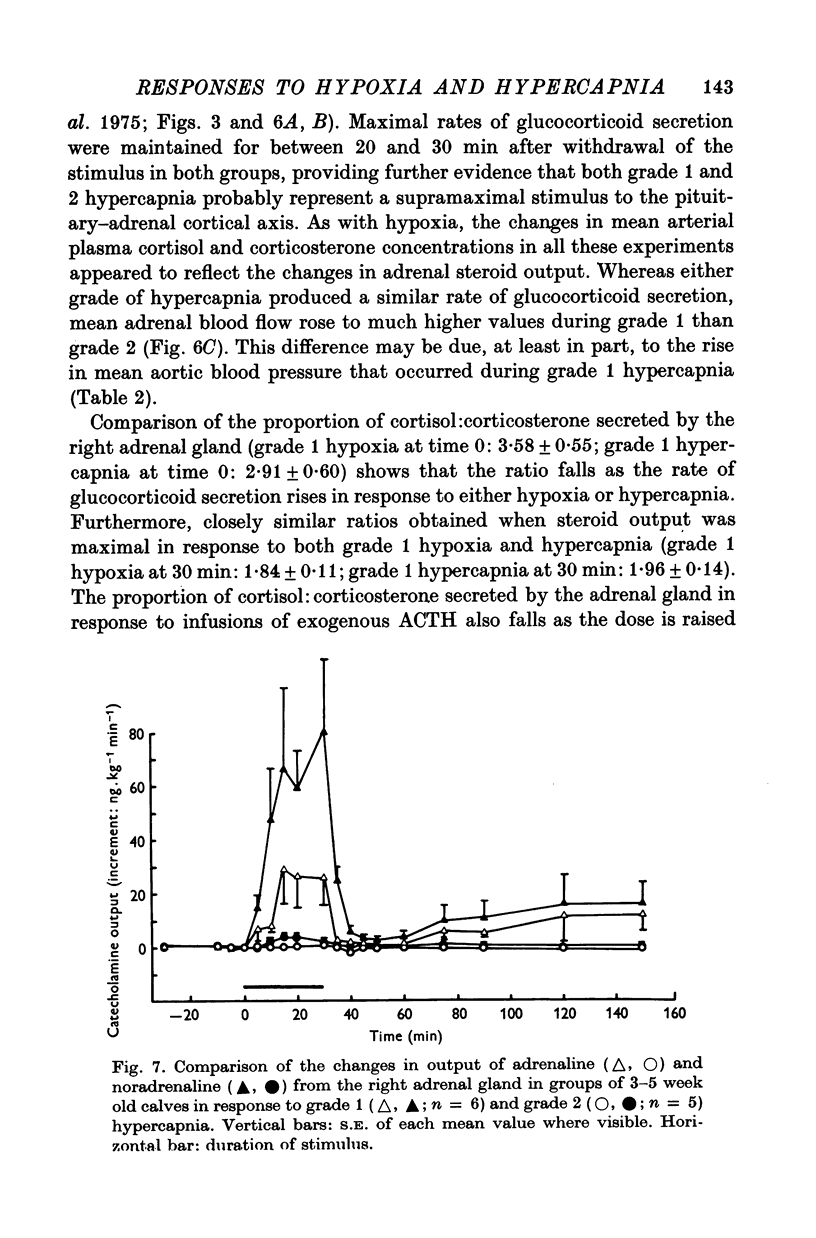






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano J. D., Ekins R. P., Maritz G., Turner R. C. A sensitive, precise radioimmunoassay of serum insulin relying on charcoal separation of bound and free hormone moieties. Acta Endocrinol (Copenh) 1972 Jul;70(3):487–509. doi: 10.1530/acta.0.0700487. [DOI] [PubMed] [Google Scholar]
- Assan R., Slusher N. Structure-function and structure-immunoreactivity relationships of the glucagon molecule and related synthetic peptides. Diabetes. 1972 Aug;21(8):843–855. doi: 10.2337/diab.21.8.843. [DOI] [PubMed] [Google Scholar]
- Bloom S. R., Edwards A. V., Hardy R. N., Malinowska K. W., Silver M. Endocrine responses to insulin hypoglycaemia in the young calf. J Physiol. 1975 Jan;244(3):783–803. doi: 10.1113/jphysiol.1975.sp010826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom S. R., Edwards A. V., Hardy R. N., Malinowska K., Silver M. Cardiovascular and endocrine responses to feeding in the young calf. J Physiol. 1975 Dec;253(1):135–155. doi: 10.1113/jphysiol.1975.sp011184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom S. R., Edwards A. V., Hardy R. N., Silver M. Adrenal and pancreatic endocrine responses to hypoxia in the conscious calf. J Physiol. 1976 Oct;261(2):271–283. doi: 10.1113/jphysiol.1976.sp011558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom S. R., Edwards A. V., Vaughan N. J. The role of the sympathetic innervation in the control of plasma glucagon concentration in the calf. J Physiol. 1973 Sep;233(2):457–466. doi: 10.1113/jphysiol.1973.sp010317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddy K., Jones C. T., Mantell C., Ratcliffe J. G., Robinson J. S. Changes in plasma ACTH and corticosteroid of the maternal and fetal sheep during hypoxia. Endocrinology. 1974 Feb;94(2):588–591. doi: 10.1210/endo-94-2-588. [DOI] [PubMed] [Google Scholar]
- COMLINE R. S., SILVER M. The release of adrenaline and noradrenaline from the adrenal glands of the foetal sheep. J Physiol. 1961 May;156:424–444. doi: 10.1113/jphysiol.1961.sp006685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comline R. S., Silver M. Development of activity in the adrenal medulla of the foetus and new-born animal. Br Med Bull. 1966 Jan;22(1):16–20. doi: 10.1093/oxfordjournals.bmb.a070430. [DOI] [PubMed] [Google Scholar]
- Comline R. S., Silver M. The development of the adrenal medulla of the foetal and new-born calf. J Physiol. 1966 Mar;183(2):305–340. doi: 10.1113/jphysiol.1966.sp007868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley J. A., Henderson C. G., Moffat L. E., Ungar A., Waite J., West C. P. Proceedings: The release of catecholamines from perfused canine adrenal glands by corticosteroids. J Physiol. 1976 Jan;254(1):30P–31P. [PubMed] [Google Scholar]
- Critchley J. A., Ungar A. Proceedings: Do the anterior pituitary and adrenal cortex participate in the reflex response of the adrenal medulla to arterial hypoxia? J Physiol. 1974 May;239(1):16P–17P. [PMC free article] [PubMed] [Google Scholar]
- Critchley J. A., Ungar A., Welburn P. J. Proceedings: The release of adrenaline and noradrenaline by the adrenal glands of cats and dogs in reflexes arising from the carotid chemoreceptors and baroreceptors. J Physiol. 1973 Oct;234(2):111P–112P. [PubMed] [Google Scholar]
- Edwards A. V., Hardy R. N., Malinowska K. W. The effects of infusions of synthetic adrenocorticotrophin in the conscious calf. J Physiol. 1974 Jun;239(3):477–498. doi: 10.1113/jphysiol.1974.sp010579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A. V., Hardy R. N., Malinowska K. W. The sensitivity of adrenal responses to synthetic adrenocorticotrophin in the conscious unrestrained calf. J Physiol. 1975 Mar;245(3):639–653. doi: 10.1113/jphysiol.1975.sp010866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A. V., Silver M. The glycogenolytic response to stimulation of the splanchnic nerves in adrenalectomized calves. J Physiol. 1970 Nov;211(1):109–124. doi: 10.1113/jphysiol.1970.sp009269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A. V. The glycogenolytic response to stimulation of the splanchnic nerves in adrenalectomized calves, sheep, dogs, cats and pigs. J Physiol. 1971 Mar;213(3):741–759. doi: 10.1113/jphysiol.1971.sp009412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRAI K., ATKINS G., MAROTTA S. F. 17-HYDROXYCORTICOSTEROID SECRETION DURING HYPOXIA IN ANESTHETIZED DOGS. Aerosp Med. 1963 Sep;34:814–816. [PubMed] [Google Scholar]
- Lau C. Effects of O 2 -CO 2 changes on hypothalamoypophyseal-adrenocortical activation. Am J Physiol. 1971 Aug;221(2):607–612. doi: 10.1152/ajplegacy.1971.221.2.607. [DOI] [PubMed] [Google Scholar]
- Lau C. Role of respiratory chemoreceptors in adrenocortical activation. Am J Physiol. 1971 Aug;221(2):602–606. doi: 10.1152/ajplegacy.1971.221.2.602. [DOI] [PubMed] [Google Scholar]
- MARKS B. H., BHATTACHARYA A. N., VERNIKOS-DANELLIS J. EFFECT OF HYPOXIA ON SECRETION OF ACTH IN THE RAT. Am J Physiol. 1965 May;208:1021–1025. doi: 10.1152/ajplegacy.1965.208.5.1021. [DOI] [PubMed] [Google Scholar]
- MILLAR R. A. Plasma adrenaline and noradrenaline during diffusion respiration. J Physiol. 1960 Jan;150:79–90. doi: 10.1113/jphysiol.1960.sp006374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinowska K. W., Hardy R. N., Nathanielsz P. W. Neonatal adrenocortical function and its possible relation to the uptake of macromolecules by the small intestine of the guinea-pig and rabbit. J Endocrinol. 1972 Nov;55(2):397–404. doi: 10.1677/joe.0.0550397. [DOI] [PubMed] [Google Scholar]
- Moncloa F., Donayre J., Sobrevilla L. A., Guerra-García R. Endocrine studies at high altitude. II. Adrenal cortical function in sea level natives exposed to high altitudes (4300 metersfor two weeks. J Clin Endocrinol Metab. 1965 Dec;25(12):1640–1642. doi: 10.1210/jcem-25-12-1640. [DOI] [PubMed] [Google Scholar]
- SARCIONE E. J., BACK N., SOKAL J. E., MEHLMAN B., KNOBLOCK E. Elevation of plasma epinephrine levels produced by glucagon in vivo. Endocrinology. 1963 Apr;72:523–526. doi: 10.1210/endo-72-4-523. [DOI] [PubMed] [Google Scholar]
- SCIAN L. F., WESTERMANN C. D., VERDESCA A. S., HILTON J. G. Adrenocortical and medullary effects of glucagon. Am J Physiol. 1960 Nov;199:867–870. doi: 10.1152/ajplegacy.1960.199.5.867. [DOI] [PubMed] [Google Scholar]
- TENNEY S. M. Sympatho-adrenal stimulation by carbon dioxide and the inhibitory effect of carbonic acid on epinephrine response. Am J Physiol. 1956 Nov;187(2):341–346. doi: 10.1152/ajplegacy.1956.187.2.341. [DOI] [PubMed] [Google Scholar]
- VON EULER U. S., FLODING I. A fluorimetric micromethod for differential estimation of adrenaline and noradrenaline. Acta Physiol Scand Suppl. 1955;33(118):45–56. [PubMed] [Google Scholar]
- VON EULER U. S., FOLKOW B. Einfluss verschiedener afferenter Nervenreize auf die Zusammensetzung des Nebennierenmarkinkretes bei der Katze. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1953;219(3):242–247. [PubMed] [Google Scholar]


