Abstract
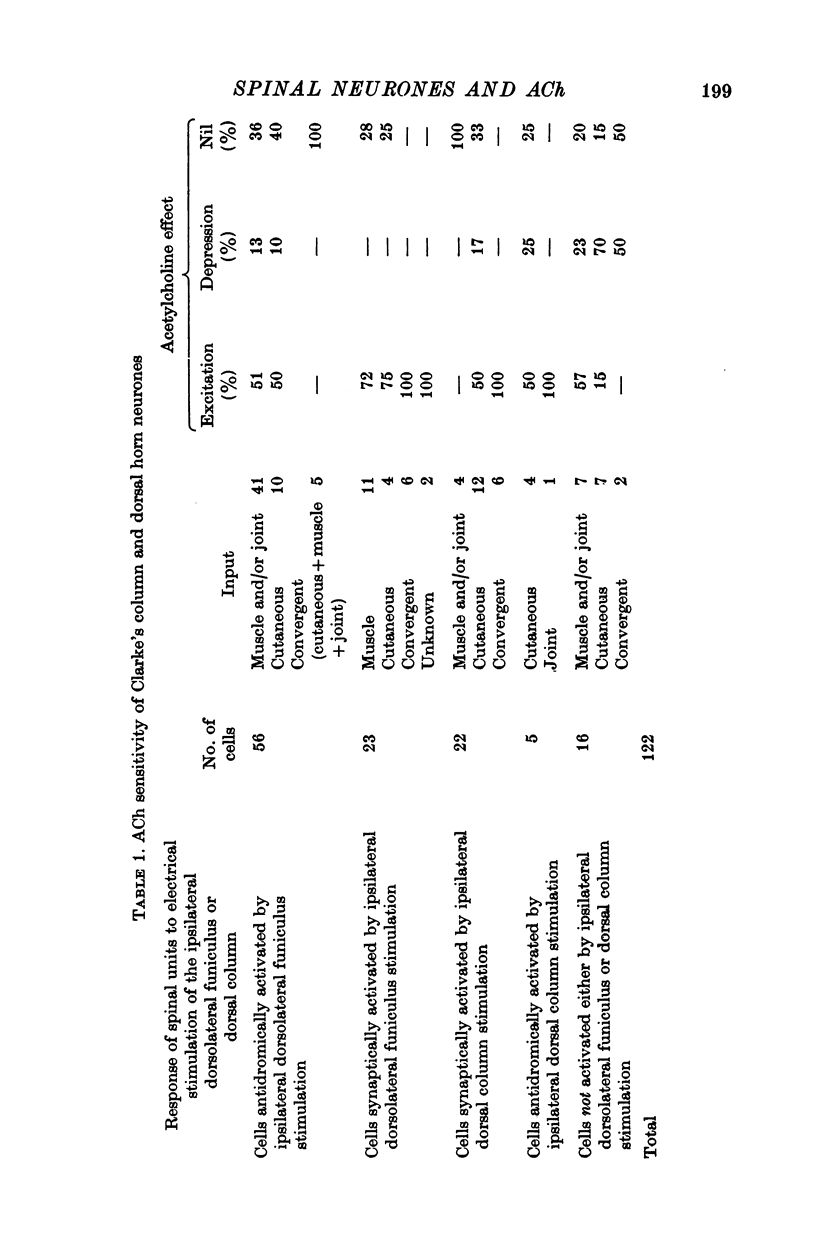
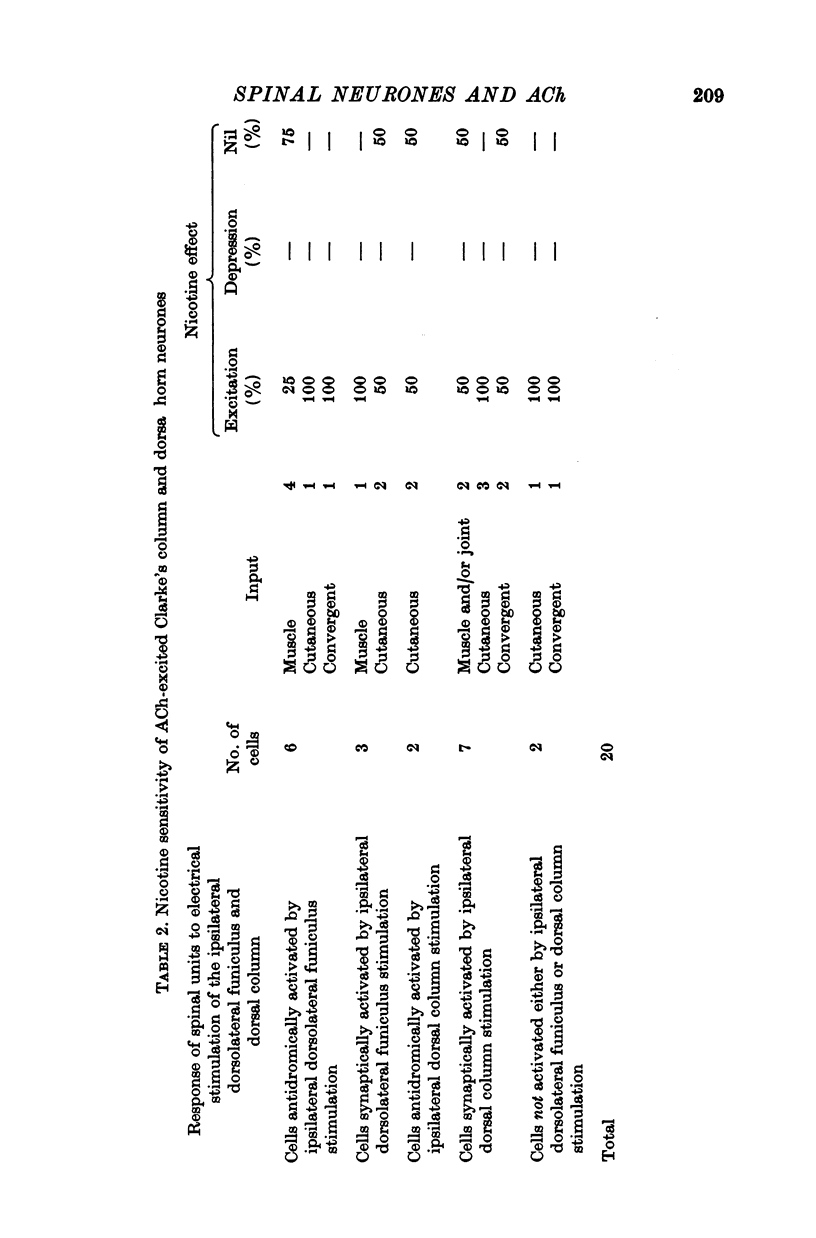
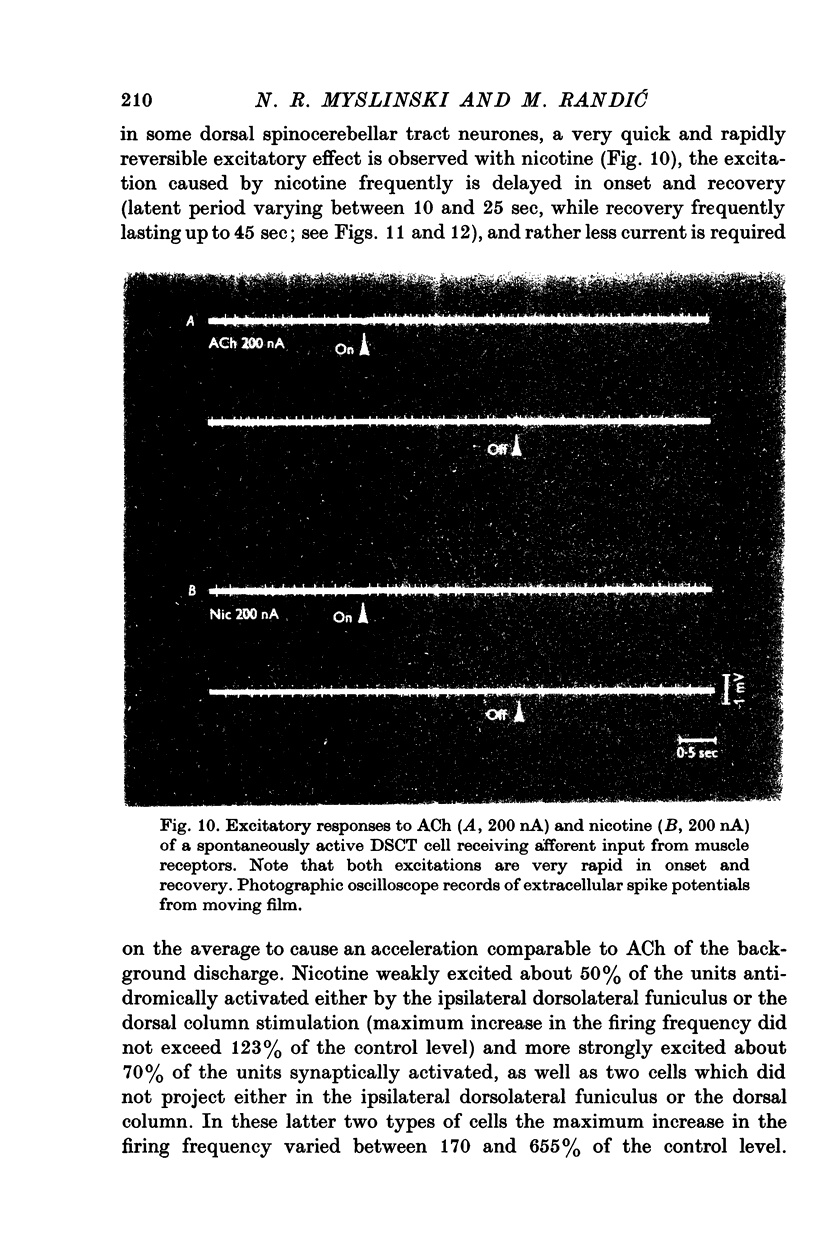
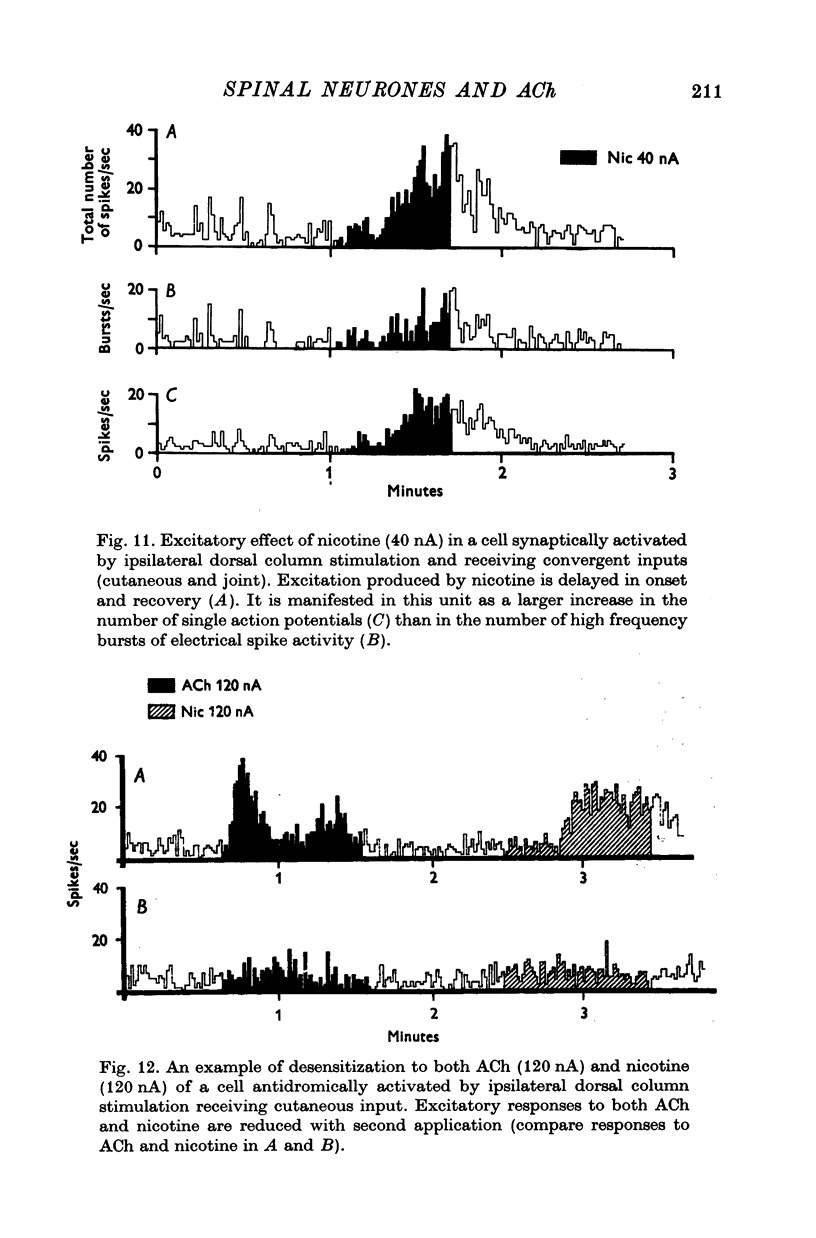
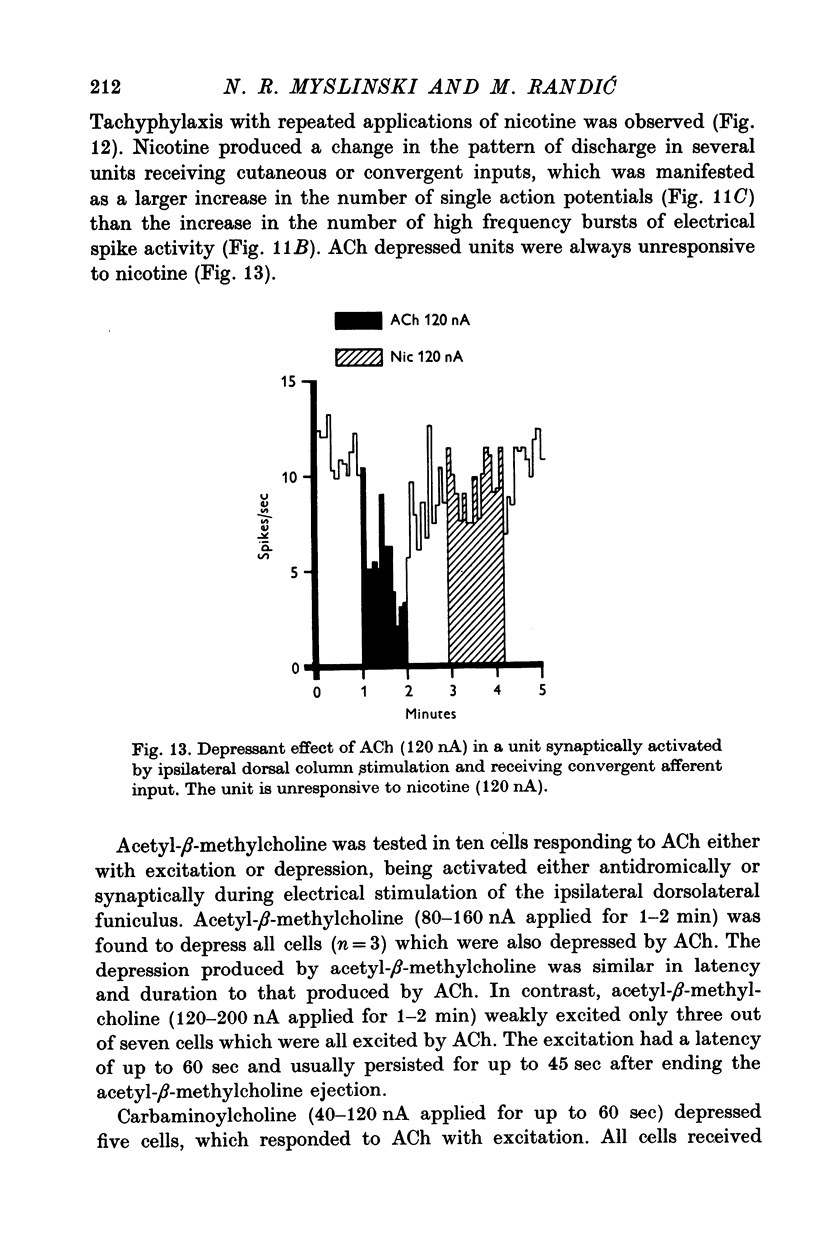
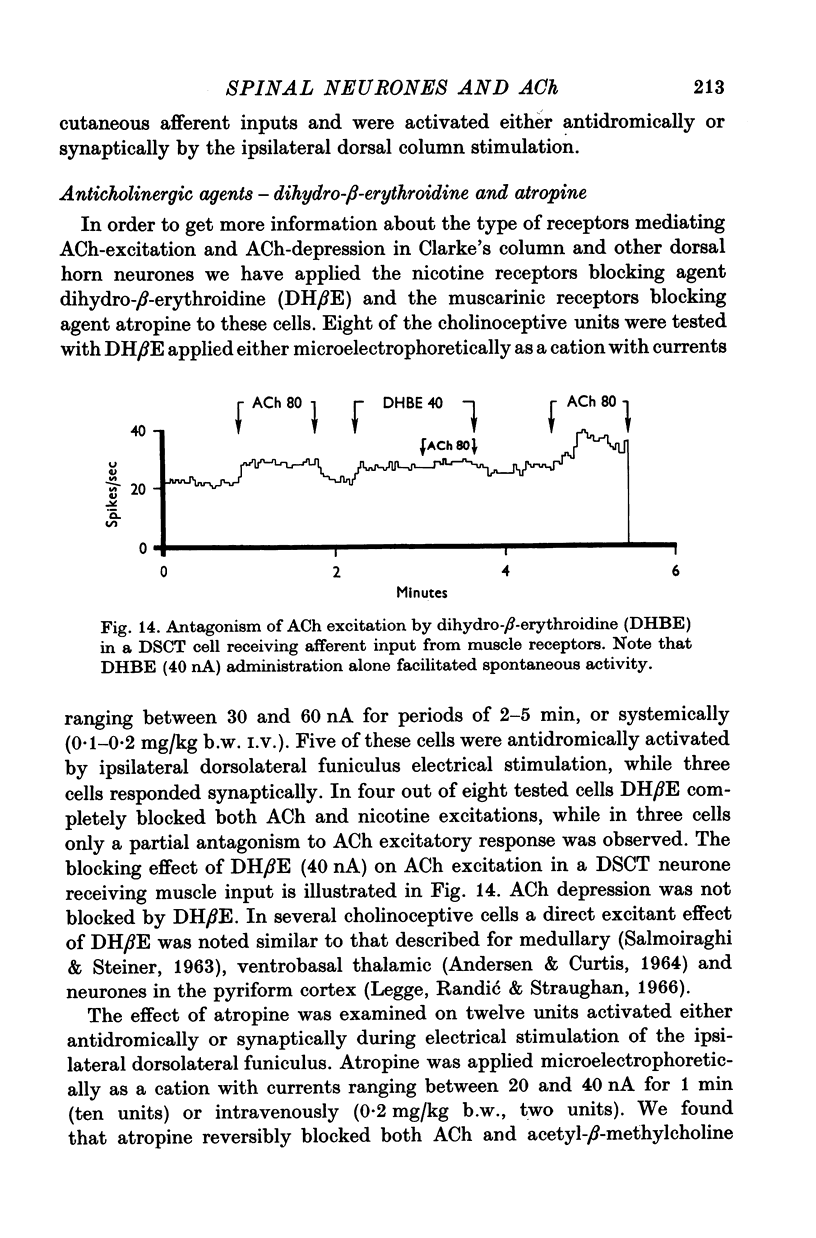
1. The responses of identified cells in the cat Clarke's column and dorsal horn to micro-electrophoretically applied cholinomimetics and anti-cholinergic substances have been investigated. 2. Both antidromically identified (DSCT neurones) and synaptically activated neurones from the region of the Clarke's column of the spinal cord were excited by ACh. However, the proportion of ACh excited cells was greater in units synaptically activated by ipsilateral dorsolateral funiculus stimulation (78%) than in DSCT neurones (50%). In addition, about 55% of neurones activated either antidromically or synaptically by ipsilateral dorsal column stimulation were excited by ACh. 3. In contrast to a relatively weak excitatory potency on the DSCT neurones (maximum firing frequency did not exceed 130% of the control activated by ipsilateral dorsolateral funiculus stimulation (maximum firing frequency reached 430% of the control level). 4. ACh has a relatively quick and rapidly reversible excitatory effect on Clarke's column neurones and some types of dorsal horn interneurones, which can be obtained also with nicotine. However, the action of nicotine is frequently delayed in onset and recovery. This excitatory action of ACh can be blocked or markedly depressed by dihydro-beta-erythroidine. These results and those obtained with acetyl-beta-methylcholine and atropine seem to suggest that the receptors mediating excitation of the cholinoceptive spinal cells activated either antidromically or synaptically by ipsilateral dorsolateral funiculus stimulation besides predominantly nicotinic have also weak muscarinic properties. 5. Desensitization with repeated applications of ACh and nicotine has been observed in both DSCT neurones and units antidromically activated by ipsilateral dorsal column stimulation. 6. About 11% of units antidromically activated by ipsilateral dorsolateral funiculus stimulation were depressed by ACh. In addition, the depressant effect of ACh was more frequently encountered in the cells unresponsive either to the dorsolateral funiculus or dorsal column stimulation. ACh depression was also seen in units activated either antidromically or synaptically by ipsilateral dorsal column stimulation. In contrast, none of the units synaptically activated by the ipsilateral dorsolateral funiculus stimulation were depressed by ACh. The same was true for spinal neurones receiving convergent peripheral inputs activated either antidromically or synaptically by ipsilateral dorsolateral or dorsal column stimulation. 7. The findings that ACh depression of all tested DSCT neurones is blocked by atropine and readily evoked by acetyl-beta-methylcholine indicates that receptors mediating the effect are of muscarinic type.
Full text
PDF




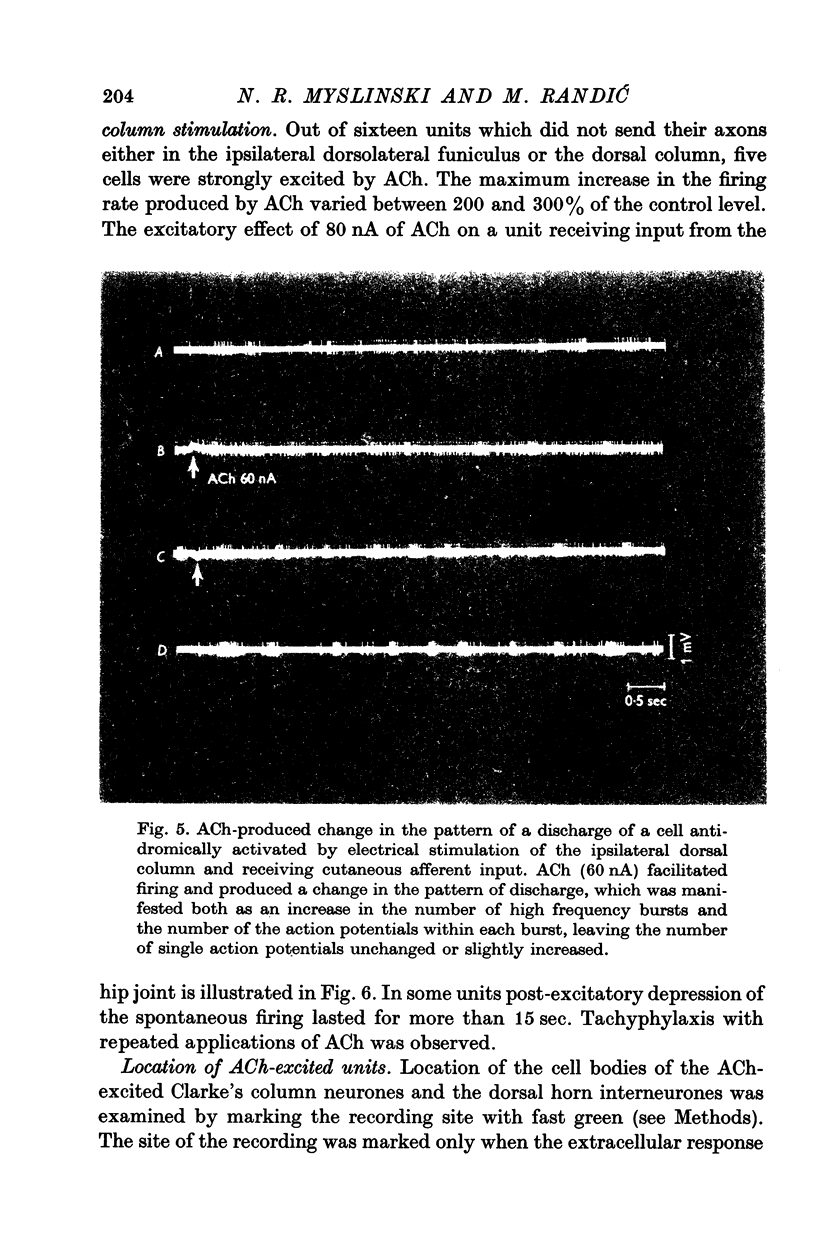
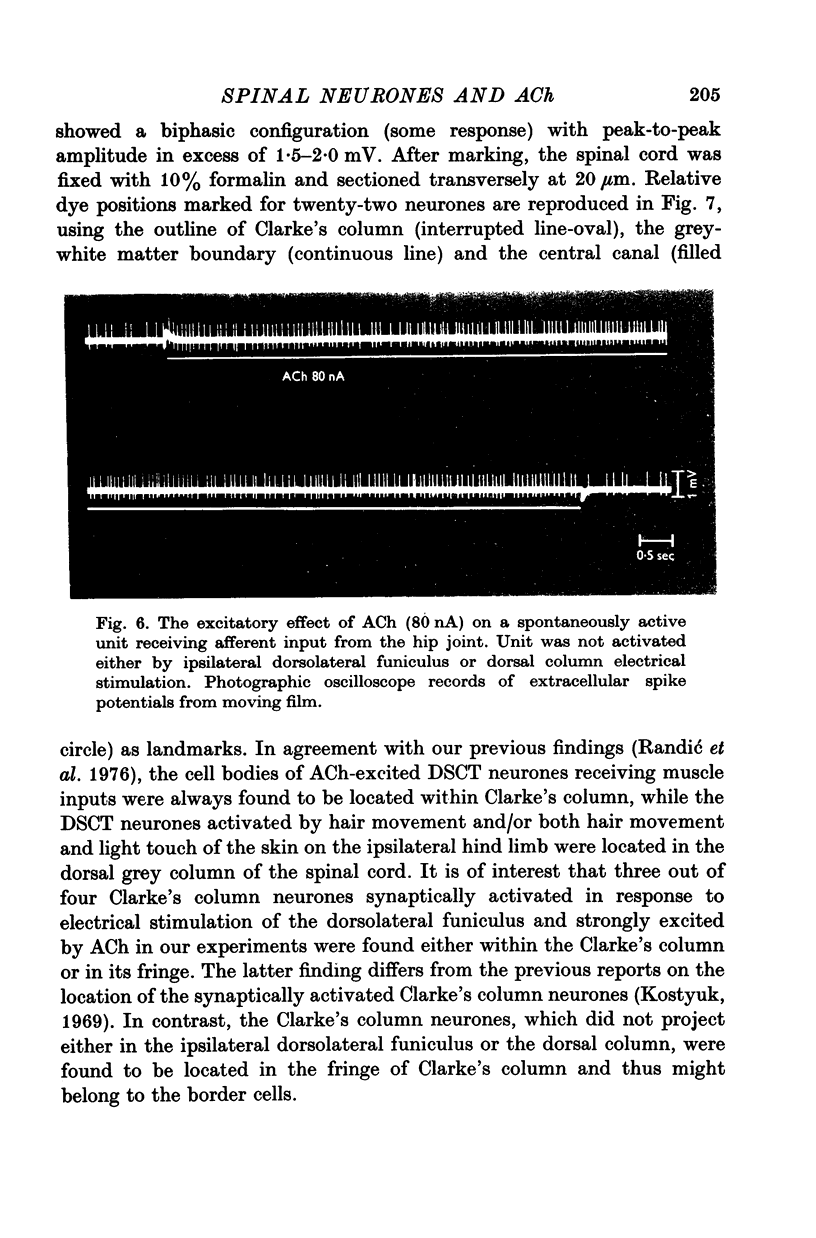
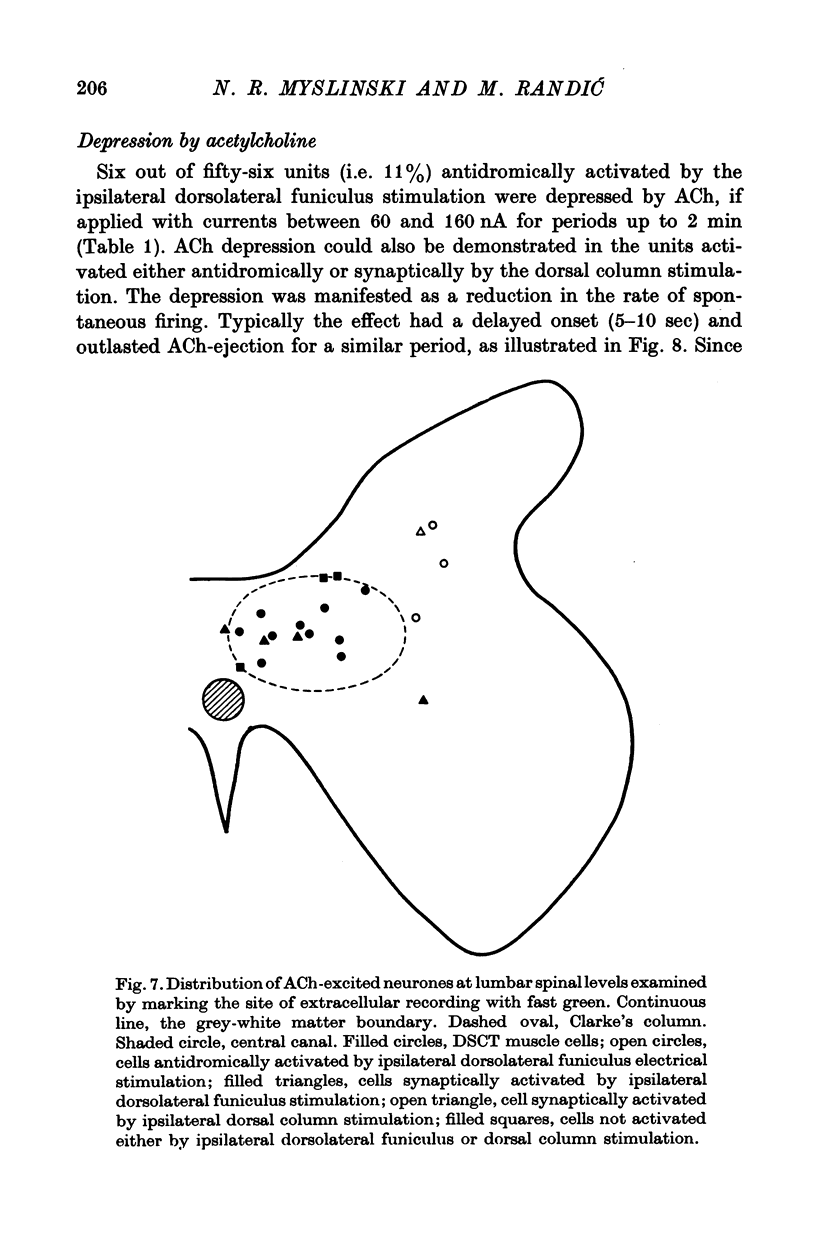
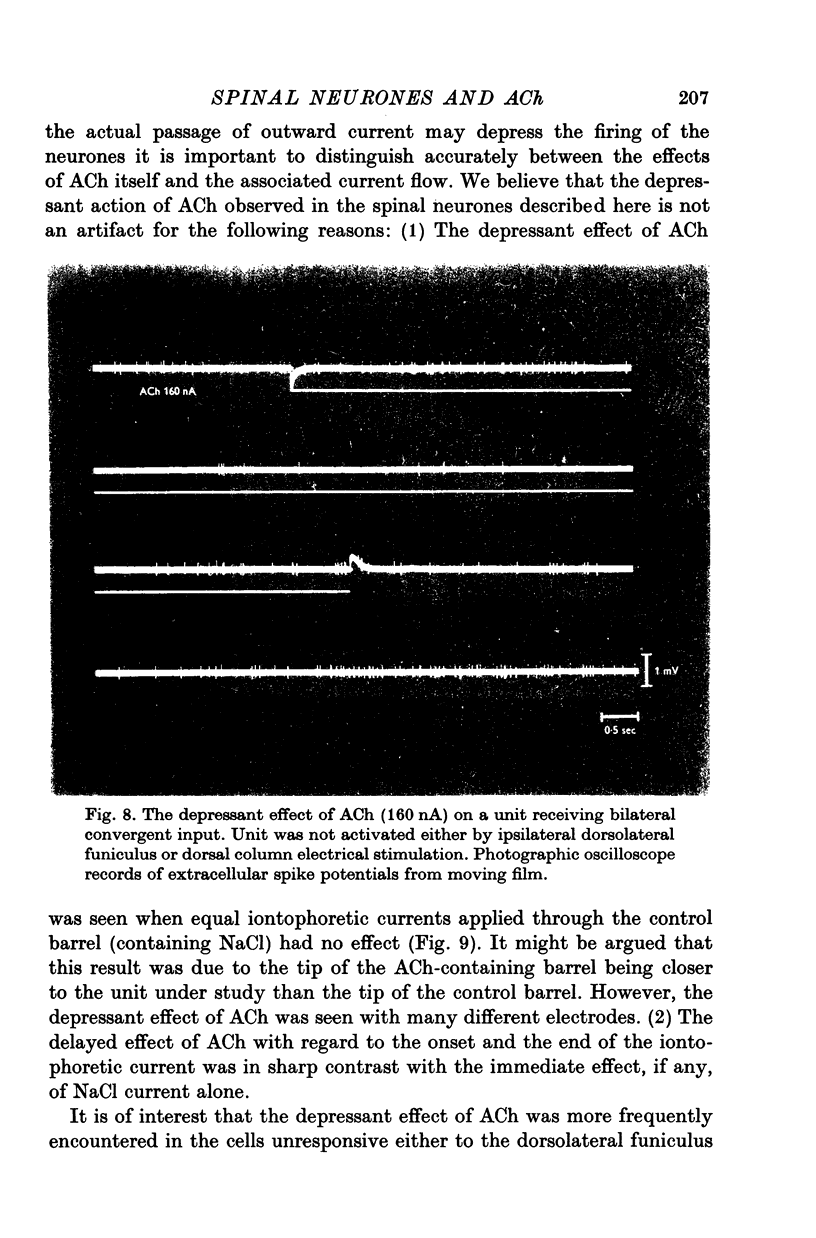






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., CURTIS D. R. THE PHARMACOLOGY OF THE SYNAPTIC AND ACETYLCHOLINE-INDUCED EXCITATION OF VENTROBASAL THALAMIC NEURONES. Acta Physiol Scand. 1964 May-Jun;61:100–120. doi: 10.1111/j.1748-1716.1964.tb02946.x. [DOI] [PubMed] [Google Scholar]
- Barker J. L., Crayton J. W., Nicoll R. A. Noradrenaline and acetylcholine responses of supraoptic neurosecretory cells. J Physiol. 1971 Oct;218(1):19–32. doi: 10.1113/jphysiol.1971.sp009602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley P. B., Dray A. Short-latency excitation of brain stem neurones in the rat by acetylcholine. Br J Pharmacol. 1972 Jun;45(2):372–374. doi: 10.1111/j.1476-5381.1972.tb08091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess P. R., Petit D., Warren R. M. Receptor types in cat hairy skin supplied by myelinated fibers. J Neurophysiol. 1968 Nov;31(6):833–848. doi: 10.1152/jn.1968.31.6.833. [DOI] [PubMed] [Google Scholar]
- CURTIS D. R., DAVIS R. The excitation of lateral geniculate neurones by quaternary ammonium derivatives. J Physiol. 1963 Jan;165:62–82. doi: 10.1113/jphysiol.1963.sp007042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D. R., ECCLES R. M. The excitation of Renshaw cells by pharmacological agents applied electrophoretically. J Physiol. 1958 May 28;141(3):435–445. doi: 10.1113/jphysiol.1958.sp005987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D. R., PHILLIS J. W., WATKINS J. C. Cholinergic and non-cholinergic transmission in the mammalian spinal cord. J Physiol. 1961 Sep;158:296–323. doi: 10.1113/jphysiol.1961.sp006770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Ryall R. W. The excitation of Renshaw cells by cholinomimetics. Exp Brain Res. 1966;2(1):49–65. doi: 10.1007/BF00234360. [DOI] [PubMed] [Google Scholar]
- Dreifuss J. J., Kelly J. S. The activity of identified supraoptic neurones and their response to acetylcholine applied by iontophoresis. J Physiol. 1972 Jan;220(1):105–118. doi: 10.1113/jphysiol.1972.sp009697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., KOKETSU K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J Physiol. 1954 Dec 10;126(3):524–562. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg I., Ryall R. W. The inhibitory action of noradrenaline and other monoamines on spinal neurones. J Physiol. 1966 Jul;185(2):298–322. doi: 10.1113/jphysiol.1966.sp007988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELDBERG W., GRAY J. A. B., PERRY W. L. M. Effects of close arterial injections of acetylcholine on the activity of the cervical spinal cord of the cat. J Physiol. 1953 Mar;119(4):428–438. doi: 10.1113/jphysiol.1953.sp004857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERNANDEZ DE MOLINA A., GRAY J. A., PALMER J. F. Effects of acetylcholine on the activity of the lumbosacral cord of the cat. J Physiol. 1958 Apr 3;141(1):169–176. doi: 10.1113/jphysiol.1958.sp005964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldberg W., Vogt M. Acetylcholine synthesis in different regions of the central nervous system. J Physiol. 1948 Jun 25;107(3):372–381. doi: 10.1113/jphysiol.1948.sp004282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRNJEVIC K., PHILLIS J. W. Iontophoretic studies of neurones in the mammalian cerebral cortex. J Physiol. 1963 Feb;165:274–304. doi: 10.1113/jphysiol.1963.sp007057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K., Pumain R., Renaud L. The mechanism of excitation by acetylcholine in the cerebral cortex. J Physiol. 1971 May;215(1):247–268. doi: 10.1113/jphysiol.1971.sp009467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kása P., Mann S. P., Hebb C. Localization of choline acetyltransferase. Ultrastructural localization in spinal neurones. Nature. 1970 May 30;226(5248):814–816. doi: 10.1038/226814a0. [DOI] [PubMed] [Google Scholar]
- Legge K. F., Randic M., Straughan D. W. The pharmacology of neurones in the pyriform cortex. Br J Pharmacol Chemother. 1966 Jan;26(1):87–107. doi: 10.1111/j.1476-5381.1966.tb01814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintosh F. C. The distribution of acetylcholine in the peripheral and the central nervous system. J Physiol. 1941 Jun 30;99(4):436–442. doi: 10.1113/jphysiol.1941.sp003913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance I., Phillis J. W. Cholinergic mechanisms in the cerebellar cortex. Int J Neuropharmacol. 1968 Sep;7(5):447–462. doi: 10.1016/0028-3908(68)90044-0. [DOI] [PubMed] [Google Scholar]
- Odutola A. B. The organization of cholinesterase-containing systems of the monkey spinal cord. Brain Res. 1972 Apr 28;39(2):353–368. doi: 10.1016/0006-8993(72)90441-6. [DOI] [PubMed] [Google Scholar]
- Phillis J. W., Tebecis A. K. The responses of thalamic neurons to iontophoretically applied monoamines. J Physiol. 1967 Oct;192(3):715–745. doi: 10.1113/jphysiol.1967.sp008327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillis J. W., Tebecis A. K., York D. H. A study of cholinoceptive cells in the lateral geniculate nucleus. J Physiol. 1967 Oct;192(3):695–713. doi: 10.1113/jphysiol.1967.sp008326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillis J. W. The pharmacology of thalamic and geniculate neurons. Int Rev Neurobiol. 1971;14:1–48. doi: 10.1016/s0074-7742(08)60182-8. [DOI] [PubMed] [Google Scholar]
- Randić M., Myslinski N. R., Gordon J. H. Spinal localization of neurons receiving inputs from cutaneous afferents in the cat hindlimb. Brain Res. 1976 Apr 9;105(3):573–577. doi: 10.1016/0006-8993(76)90606-5. [DOI] [PubMed] [Google Scholar]
- Roberts M. H., Straughan D. W. Excitation and depression of cortical neurones by 5-hydroxytryptamine. J Physiol. 1967 Nov;193(2):269–294. doi: 10.1113/jphysiol.1967.sp008357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessmann U., Friede R. L. The segmental distribution of acetyl cholinesterase in the cat spinal cord. J Anat. 1967 Jan;101(Pt 1):27–32. [PMC free article] [PubMed] [Google Scholar]
- SALMOIRAGHI G. C., STEINER F. A. Acetylcholine sensitivity of cat's medullary neurons. J Neurophysiol. 1963 Jul;26:581–597. doi: 10.1152/jn.1963.26.4.581. [DOI] [PubMed] [Google Scholar]
- Salmoiraghi G. C., Stefanis C. N. A critique of iontophoretic studies of central nervous system neurons. Int Rev Neurobiol. 1967;10:1–30. doi: 10.1016/s0074-7742(08)60149-x. [DOI] [PubMed] [Google Scholar]
- Silver A., Wolstencroft J. H. Cholinesterases and choline acetylase in the spinal cord of the cat. J Physiol. 1970 Sep;210(2):92P–93P. [PubMed] [Google Scholar]
- Silver A., Wolstencroft J. H. The distribution of cholinesterases in relation to the structure of the spinal cord in the cat. Brain Res. 1971 Nov;34(2):205–227. doi: 10.1016/0006-8993(71)90277-0. [DOI] [PubMed] [Google Scholar]
- Stone T. W. Cholinergic mechanisms in the rat somatosensory cerebral cortex. J Physiol. 1972 Sep;225(2):485–499. doi: 10.1113/jphysiol.1972.sp009951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weight F. F., Salmoiraghi G. C. Responses of spinal cord interneurons to acetylcholine, norepinephrine and serotonin administered by microelectrophoresis. J Pharmacol Exp Ther. 1966 Sep;153(3):420–427. [PubMed] [Google Scholar]
- Zieglgänsberger W., Reiter C. A cholinergic mechanism in the spinal cord of cats. Neuropharmacology. 1974 Jun;13(6):519–527. doi: 10.1016/0028-3908(74)90141-5. [DOI] [PubMed] [Google Scholar]


