Abstract
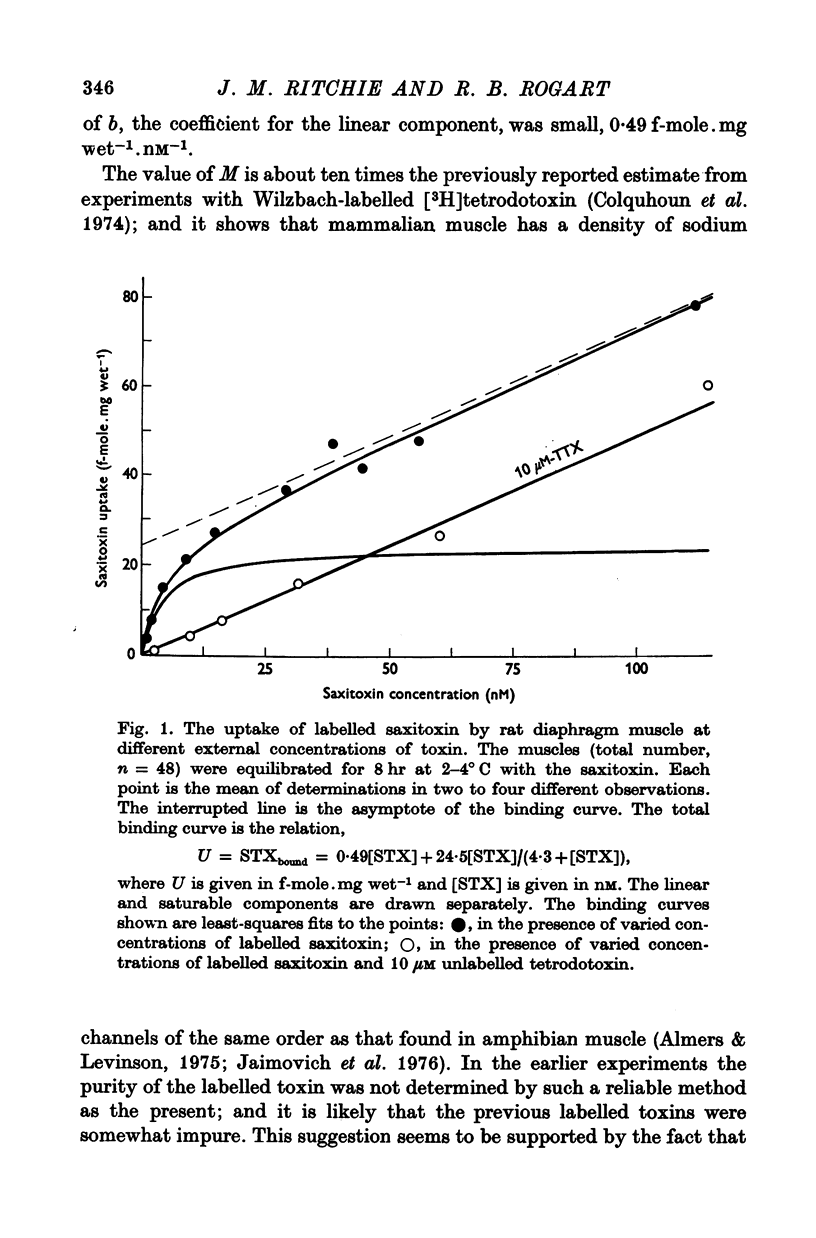
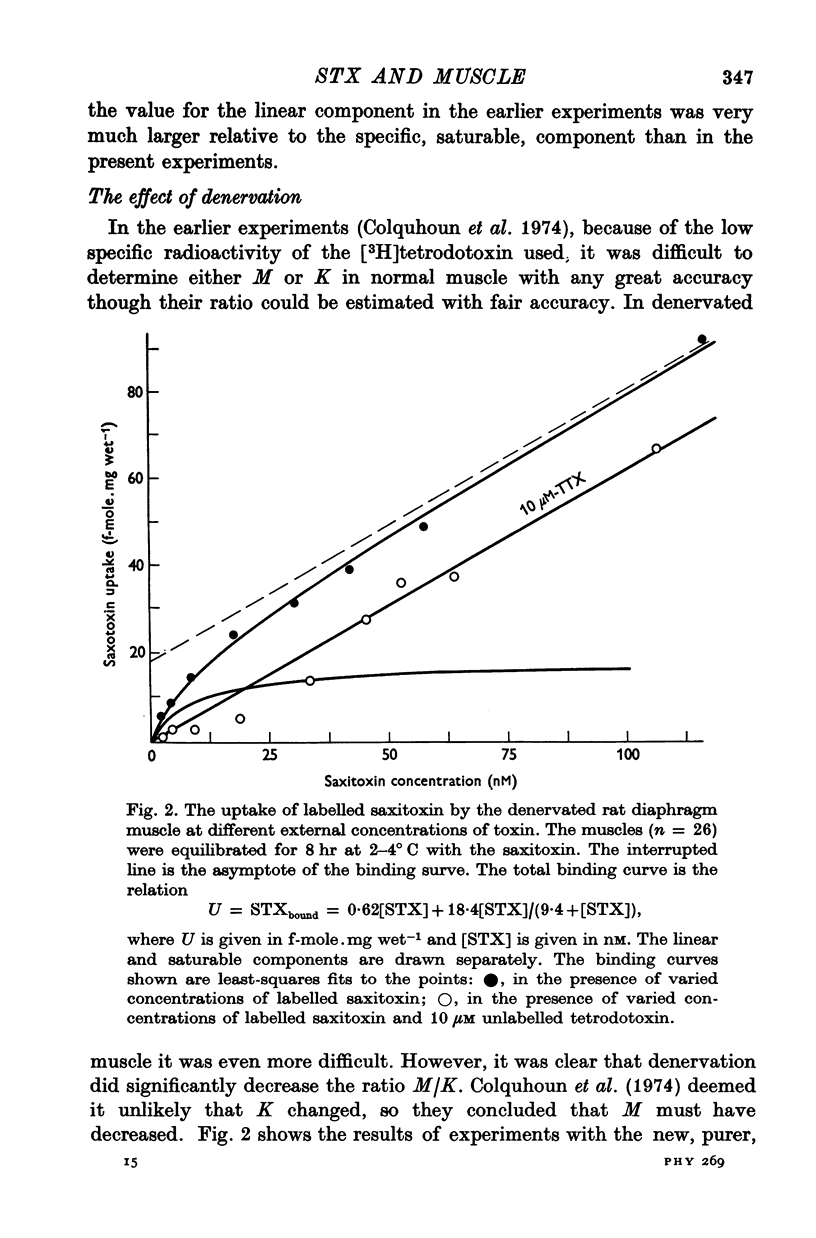
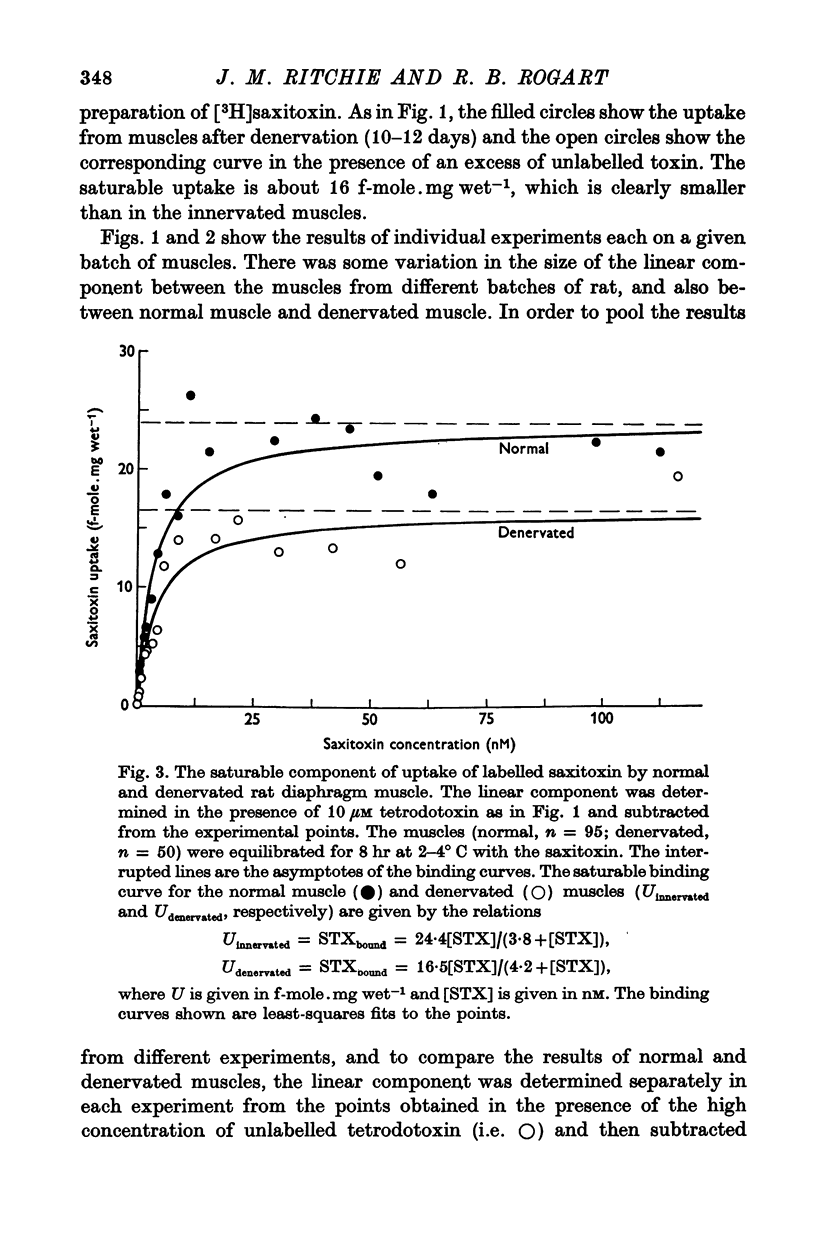
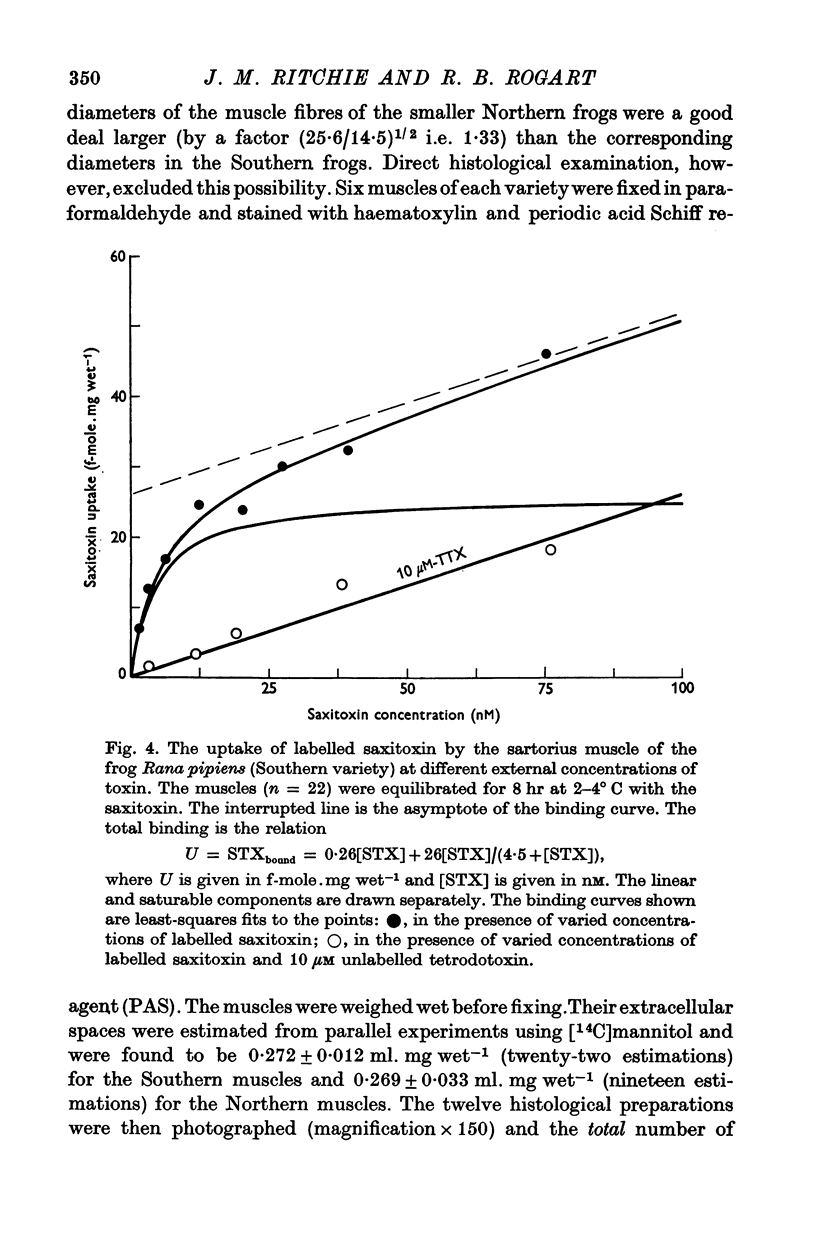
1. The binding of [3H]saxitoxin to innervated and denervated rat diaphragm muscle, and to normal frog muscle, has been measured. 2. A saturable component of saxitoxin binding, which was inhibited by tetrodotoxin, was detected in all preparations, as well as a component of non-saturable binding. The values for the maximum saturable capacity, M, and the equilibrium binding constant, K, for normal rat diaphragm muscle were: M = 24-4 f-mole.mg wet-1, and K = 3 -8 NM. 3. Denervation of rat diaphragm muscle reduced the maximum binding capacity per unit weight to 16-5 f-mole.mg-1. The value of K remained virtually unchanged at 4-2 nM. 4. It is suggested that the decrease in density per unit weight does not reflect any change in the density of sodium channels per unit area of membrane. 5. Two varieties of the same species of frog, Rana pipiens, were examined. In one variety (Southern) the value of M was 25-6 f-mole.mg-1 and the value of K was 4-3 nM. In the Northern variety the maximum binding capacity was less, M being 14-6 f-mole.mg-1; the value of K was 3-8 nM.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blankenship J. E. Tetrodotoxin: from poison to powerful tool. Perspect Biol Med. 1976 Summer;19(4):509–526. doi: 10.1353/pbm.1976.0071. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Henderson R., Ritchie J. M. The binding of labelled tetrodotoxin to non-myelinated nerve fibres. J Physiol. 1972 Dec;227(1):95–126. doi: 10.1113/jphysiol.1972.sp010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Rang H. P., Ritchie J. M. The binding of tetrodotoxin and alpha-bungarotoxin to normal and denervated mammalian muscle. J Physiol. 1974 Jul;240(1):199–226. doi: 10.1113/jphysiol.1974.sp010607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlij D., Grinstein S. The number of sodium ion pumping sites in skeletal muscle and its modification by insulin. J Physiol. 1976 Jul;259(1):13–31. doi: 10.1113/jphysiol.1976.sp011452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. H. Tetrodotoxin, saxitoxin, and related substances: their applications in neurobiology. Int Rev Neurobiol. 1972;15:83–166. doi: 10.1016/s0074-7742(08)60329-3. [DOI] [PubMed] [Google Scholar]
- Fambrough D. M. Acetylcholine receptors. Revised estimates of extrajunctional receptor density in denervated rat diaphragm. J Gen Physiol. 1974 Oct;64(4):468–472. doi: 10.1085/jgp.64.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grampp W., Harris J. B., Thesleff S. Inhibition of denervation changes in skeletal muscle by blockers of protein synthesis. J Physiol. 1972 Mar;221(3):743–754. doi: 10.1113/jphysiol.1972.sp009780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. B., Marshall M. W. Tetrodotoxin-resistant action potentials in newborn rat muscle. Nat New Biol. 1973 Jun 6;243(127):191–192. doi: 10.1038/newbio243191a0. [DOI] [PubMed] [Google Scholar]
- Henderson R., Ritchie J. M., Strichartz G. R. The binding of labelled saxitoxin to the sodium channels in nerve membranes. J Physiol. 1973 Dec;235(3):783–804. doi: 10.1113/jphysiol.1973.sp010417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Campbell D. T. An improved vaseline gap voltage clamp for skeletal muscle fibers. J Gen Physiol. 1976 Mar;67(3):265–293. doi: 10.1085/jgp.67.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ildefonse M., Roy G. Kinetic properties of the sodium current in striated muscle fibres on the basis of the Hodgkin-Huxley theory. J Physiol. 1972 Dec;227(2):419–431. doi: 10.1113/jphysiol.1972.sp010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaimovich E., Venosa R. A., Shrager P., Horowicz P. Density and distribution of tetrodotoxin receptors in normal and detubulated frog sartorius muscle. J Gen Physiol. 1976 Apr;67(4):399–416. doi: 10.1085/jgp.67.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C. Y. Tetrodotoxin, saxitoxin and their significance in the study of excitation phenomena. Pharmacol Rev. 1966 Jun;18(2):997–1049. [PubMed] [Google Scholar]
- Marshall M. W., Ward M. R. Anode break excitation in denervated rat skeletal muscle fibres. J Physiol. 1974 Jan;236(2):413–420. doi: 10.1113/jphysiol.1974.sp010443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. Electron-microscopic structure of denervated skeletal muscle. Proc R Soc Lond B Biol Sci. 1969 Nov 18;174(1035):253–269. doi: 10.1098/rspb.1969.0091. [DOI] [PubMed] [Google Scholar]
- Redfern P., Thesleff S. Action potential generation in denervated rat skeletal muscle. I. Quantitative aspects. Acta Physiol Scand. 1971 Apr;81(4):557–564. doi: 10.1111/j.1748-1716.1971.tb04932.x. [DOI] [PubMed] [Google Scholar]
- Redfern P., Thesleff S. Action potential generation in denervated rat skeletal muscle. II. The action of tetrodotoxin. Acta Physiol Scand. 1971 May;82(1):70–78. doi: 10.1111/j.1748-1716.1971.tb04943.x. [DOI] [PubMed] [Google Scholar]
- Ritchie J. M., Rogart R. B. The binding of labelled saxitoxin to normal and denervated muscle [proceedings]. J Physiol. 1976 Dec;263(1):129P–130P. [PubMed] [Google Scholar]
- SOLA O. M., MARTIN A. W. Denervation hypertrophy and atrophy of the hemidiaphragm of the rat. Am J Physiol. 1953 Feb;172(2):324–332. doi: 10.1152/ajplegacy.1953.172.2.324. [DOI] [PubMed] [Google Scholar]


