Abstract
1. The increment-threshold for a small test spot in the peripheral visual field was measured against backgrounds that were red or blue.
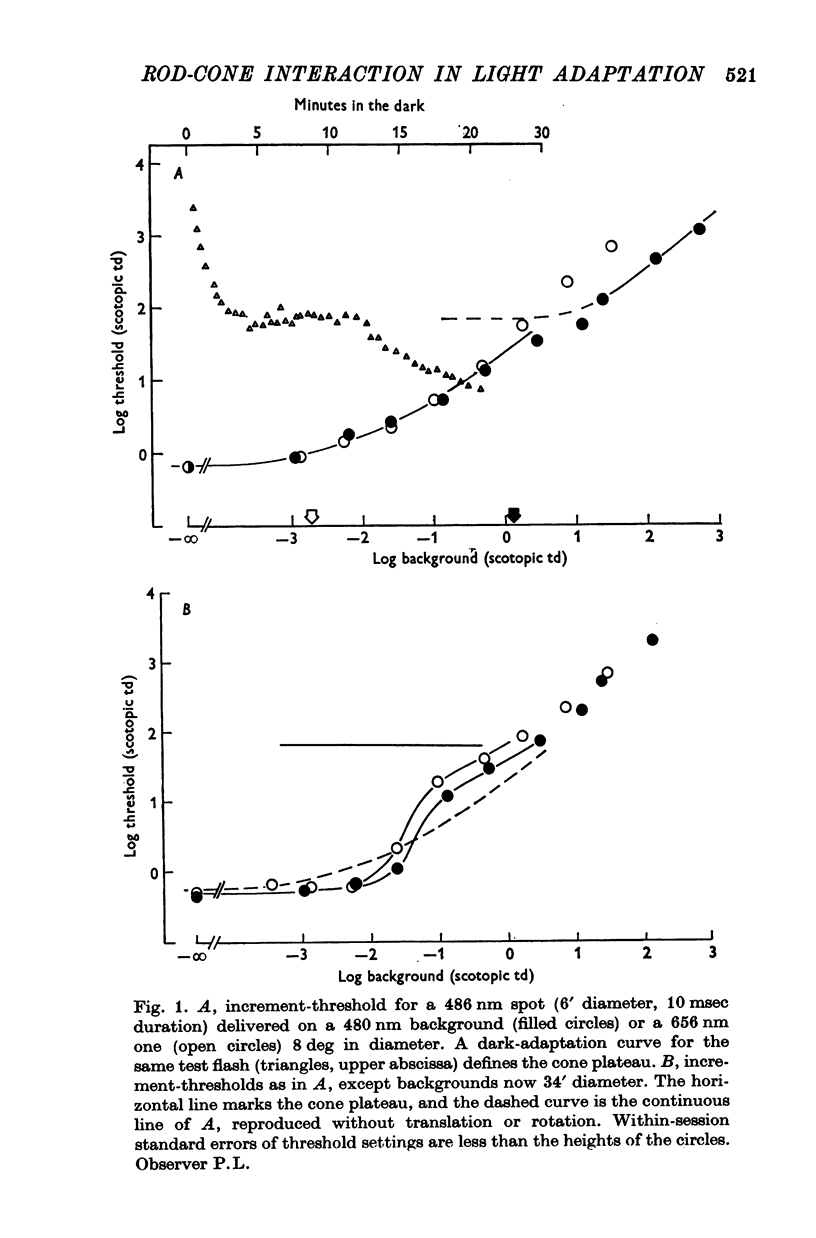
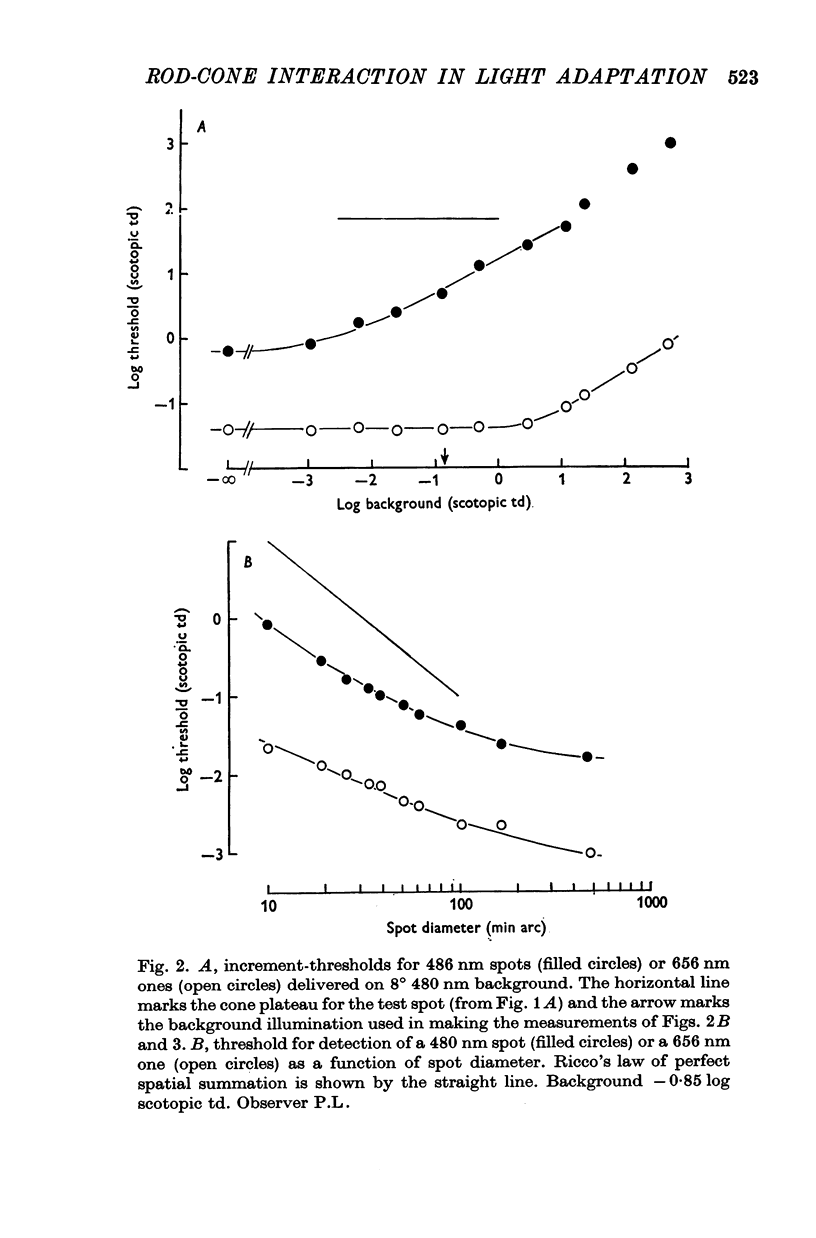
2. When the background was a large uniform field, threshold over most of the scotopic range depended exactly upon the background's effect upon rods. This confirms Flamant & Stiles (1948). But when the background was small, threshold was elevated more by a long wave-length than a short wave-length background equated for its effect on rods.
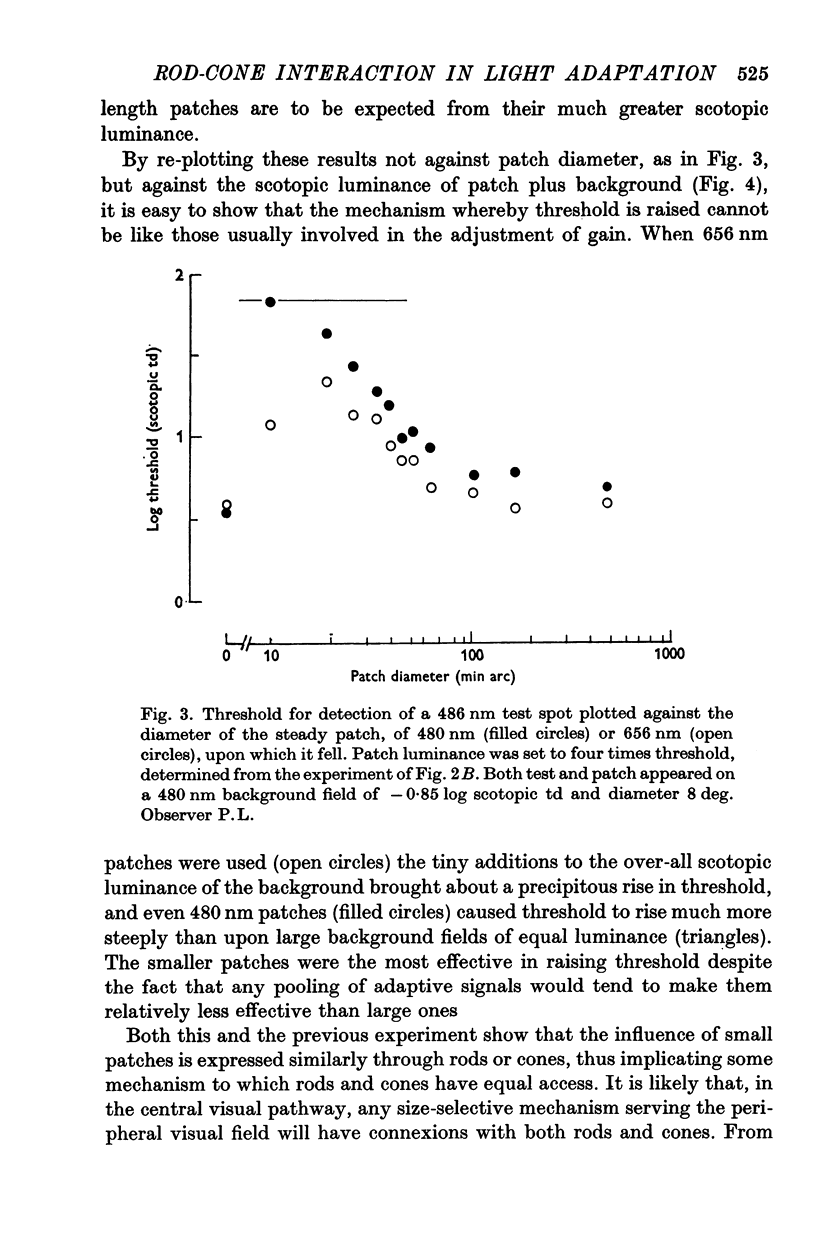
3. The influence of cones was explored in a further experiment. The scotopic increment-threshold was established for a short wave-length test spot on a large, short wave-length background. Then a steady red circular patch, conspicuous to cones, but below the increment-threshold for rod vision, was added to the background. When it was small, but not when it was large, this patch substantially raised the threshold for the test.
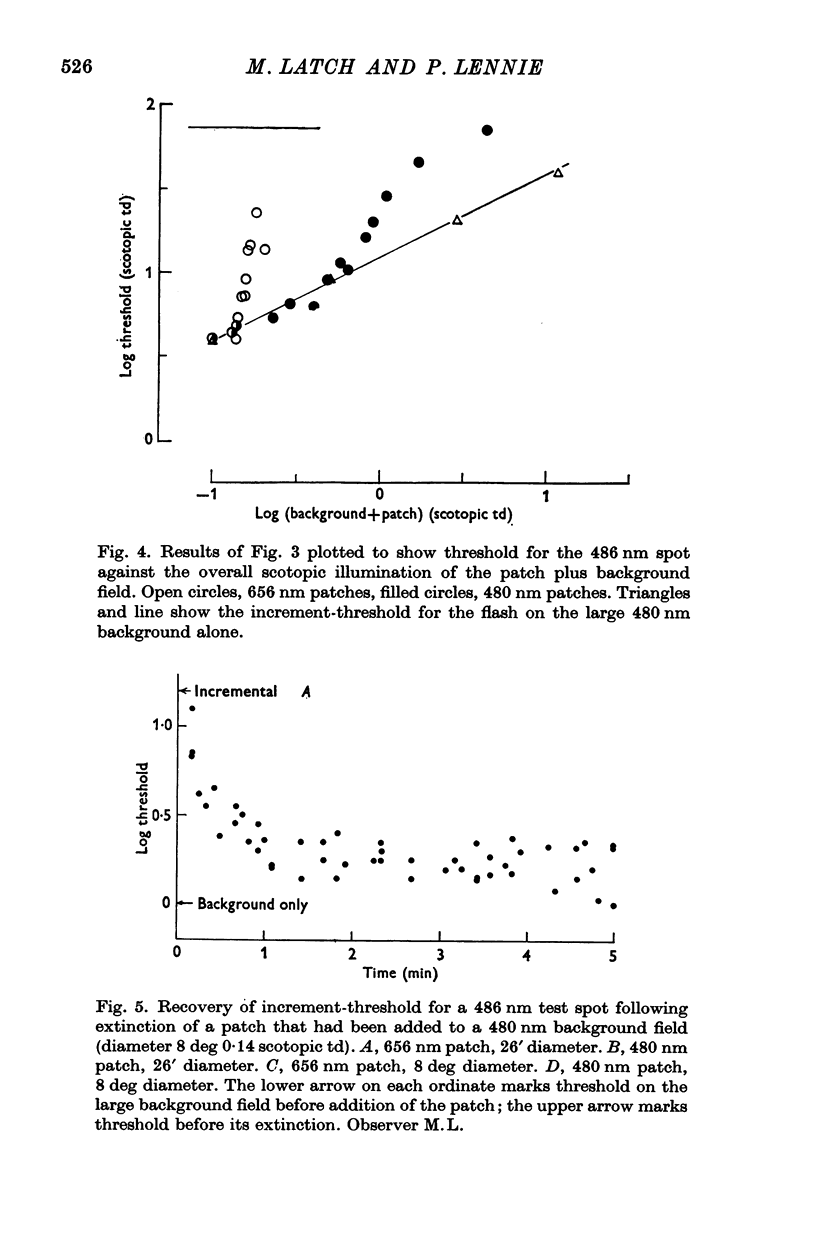
4. When a similar experiment was made using, instead of a red patch, a short wave-length one that was conspicuous in rod vision, threshold varied similarly with patch size. These results support the notion that the influence of small backgrounds arises in some size-selective mechanism that is indifferent to the receptor system in which visual signals originate. Two corollaries of this hypothesis were tested in further experiments.
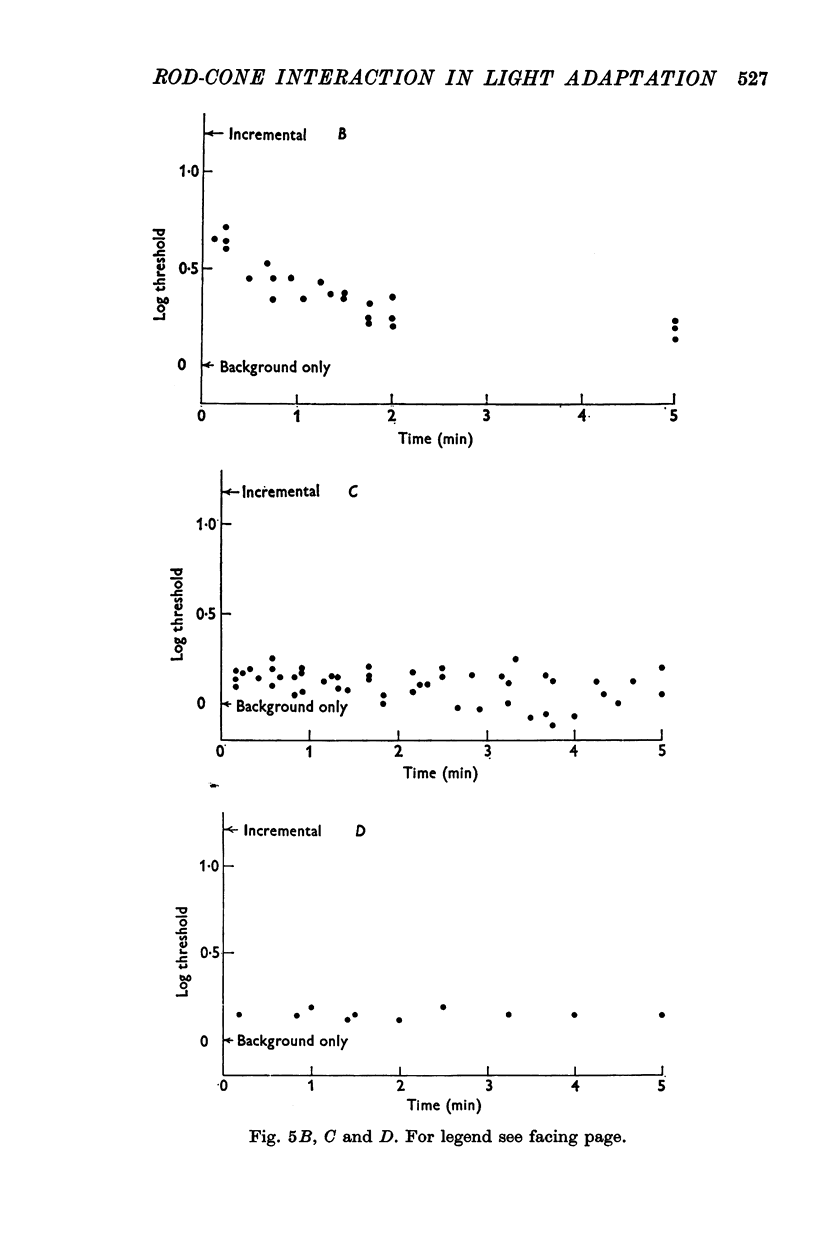
5. A small patch was chosen so as to lift scotopic threshold substantially above its level on a uniform field. This threshold elevation persisted for minutes after extinction of the patch, but only when the patch was small. A large patch made bright enough to elevate threshold by as much as the small one gave rise to no corresponding after-effect.
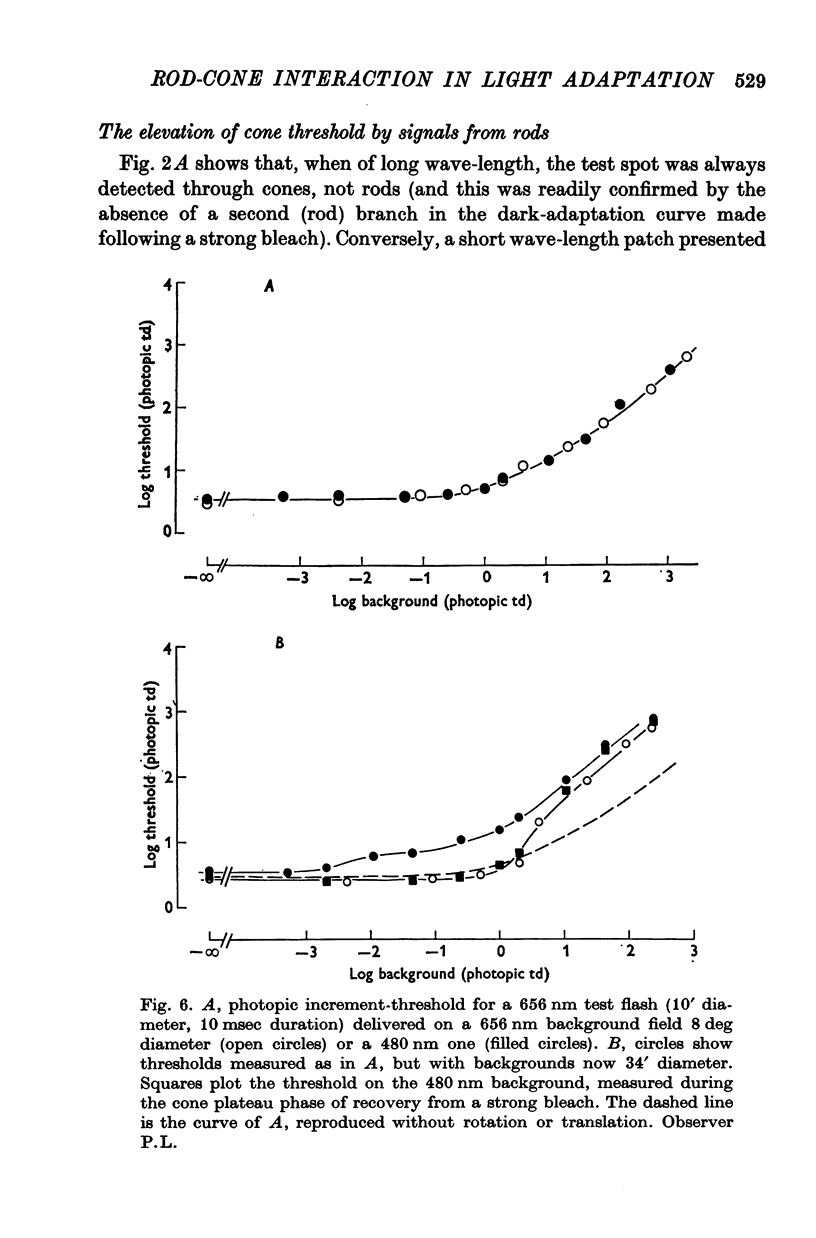
6. Increment-thresholds for a small red test spot, detected through cones, followed the same course whether a large uniform background was long- or short wave-length. When the background was small, threshold upon the short wave-length one began to rise for much lower levels of background illumination, suggesting the influence of rods. This was confirmed by repeating the experiment after a strong bleach when the cones, but not rods, had fully recovered their sensitivity. Increment-thresholds upon small backgrounds of long or short wave-lengths then followed the same course.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARDEN G. B., WEALE R. A. Nervous mechanisms and dark-adaptation. J Physiol. 1954 Sep 28;125(3):417–426. doi: 10.1113/jphysiol.1954.sp005169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpern M., Rushton W. A., Torii S. The size of rod signals. J Physiol. 1970 Jan;206(1):193–208. doi: 10.1113/jphysiol.1970.sp009006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Campbell F. W. On the existence of neurones in the human visual system selectively sensitive to the orientation and size of retinal images. J Physiol. 1969 Jul;203(1):237–260. doi: 10.1113/jphysiol.1969.sp008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Enroth-cugell C. Quantitative aspects of sensitivity and summation in the cat retina. J Physiol. 1968 Sep;198(1):17–38. doi: 10.1113/jphysiol.1968.sp008591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Hertz B. G., Lennie P. Convergence of rod and cone signals in the cat's retina. J Physiol. 1977 Jul;269(2):297–318. doi: 10.1113/jphysiol.1977.sp011903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamant F., Stiles W. S. The directional and spectral sensitivities of the retinal rods to adapting fields of different wave-lengths. J Physiol. 1948 Mar 15;107(2):187–202. doi: 10.1113/jphysiol.1948.sp004262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennie P., MacLeod D. I. Background configuration and rod threshold. J Physiol. 1973 Aug;233(1):143–156. doi: 10.1113/jphysiol.1973.sp010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makous W., Boothe R. Cones block signals from rods. Vision Res. 1974 Apr;14(4):285–294. doi: 10.1016/0042-6989(74)90078-9. [DOI] [PubMed] [Google Scholar]
- RUSHTON W. A. VISUAL ADAPTATION. Proc R Soc Lond B Biol Sci. 1965 Mar 16;162:20–46. doi: 10.1098/rspb.1965.0024. [DOI] [PubMed] [Google Scholar]
- Sachs M. B., Nachmias J., Robson J. G. Spatial-frequency channels in human vision. J Opt Soc Am. 1971 Sep;61(9):1176–1186. doi: 10.1364/josa.61.001176. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Creutzfeldt O., Scheich H. An experimental comparison between the ganglion cell receptive field and the receptive field of the adaptation pool in the cat retina. Pflugers Arch. 1969;307(3):133–137. doi: 10.1007/BF00592079. [DOI] [PubMed] [Google Scholar]
- Sternheim C. E., Glass R. A. Evidence for cone and rod contributions to common "adaptation pools". Vision Res. 1975 Feb;15(2):277–281. doi: 10.1016/0042-6989(75)90219-9. [DOI] [PubMed] [Google Scholar]
- Westheimer G. Rod-cone independence for sensitizing interaction in the human retina. J Physiol. 1970 Jan;206(1):109–116. doi: 10.1113/jphysiol.1970.sp009000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westheimer G. Spatial interaction in the human retina during scotopic vision. J Physiol. 1965 Dec;181(4):881–894. doi: 10.1113/jphysiol.1965.sp007803. [DOI] [PMC free article] [PubMed] [Google Scholar]


