Abstract
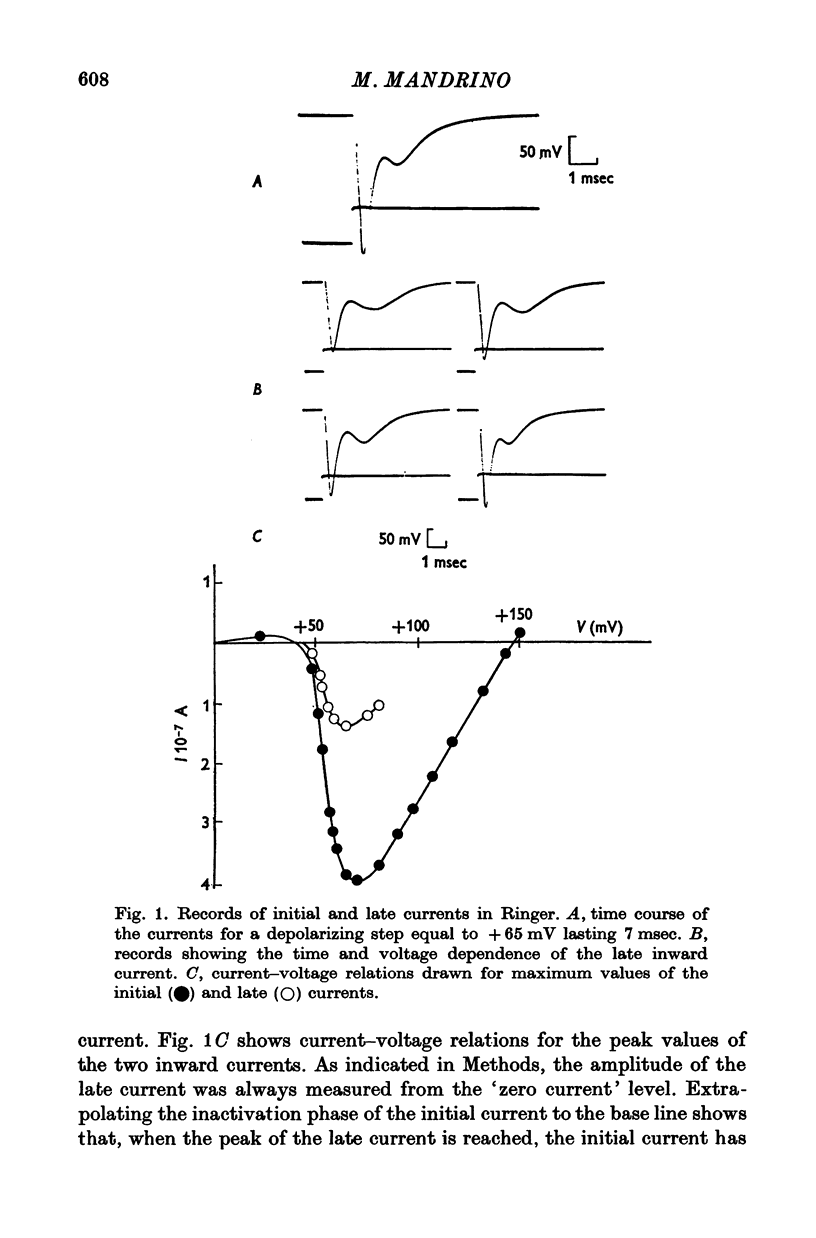
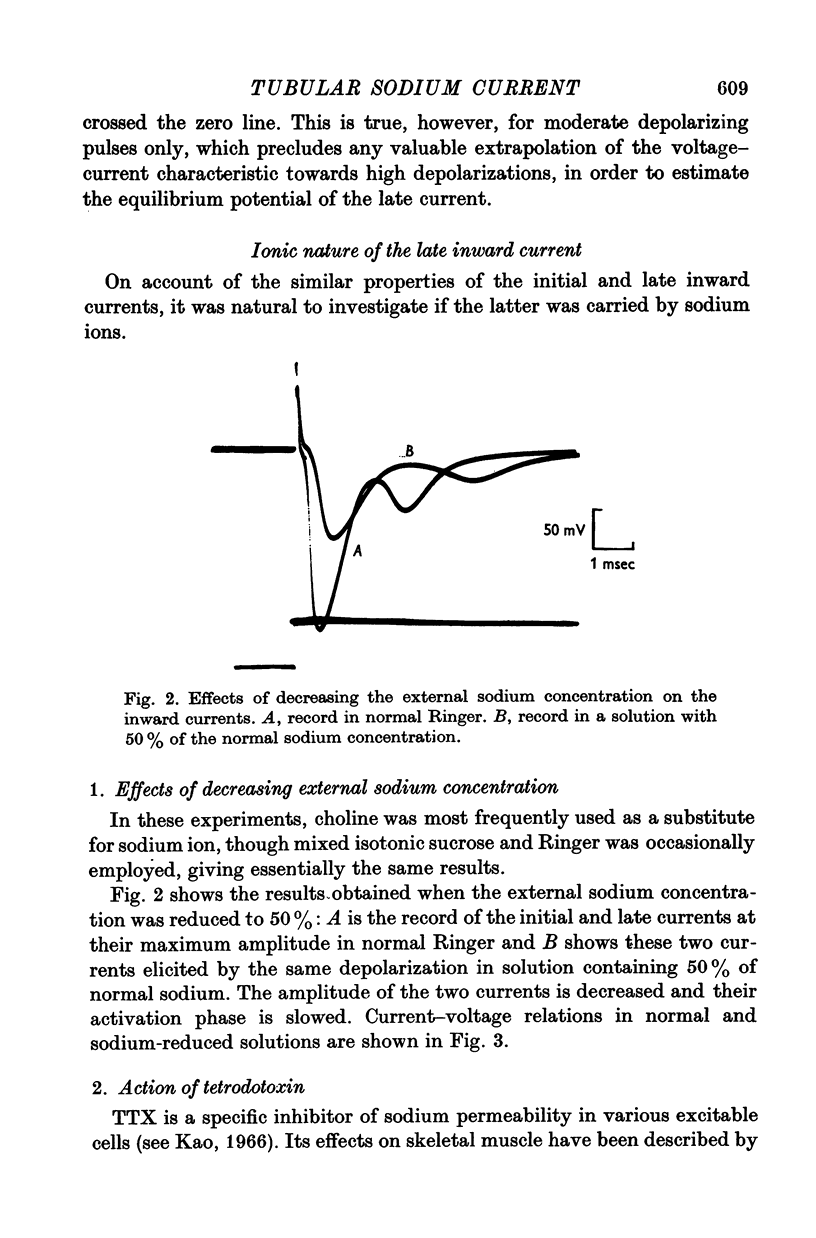
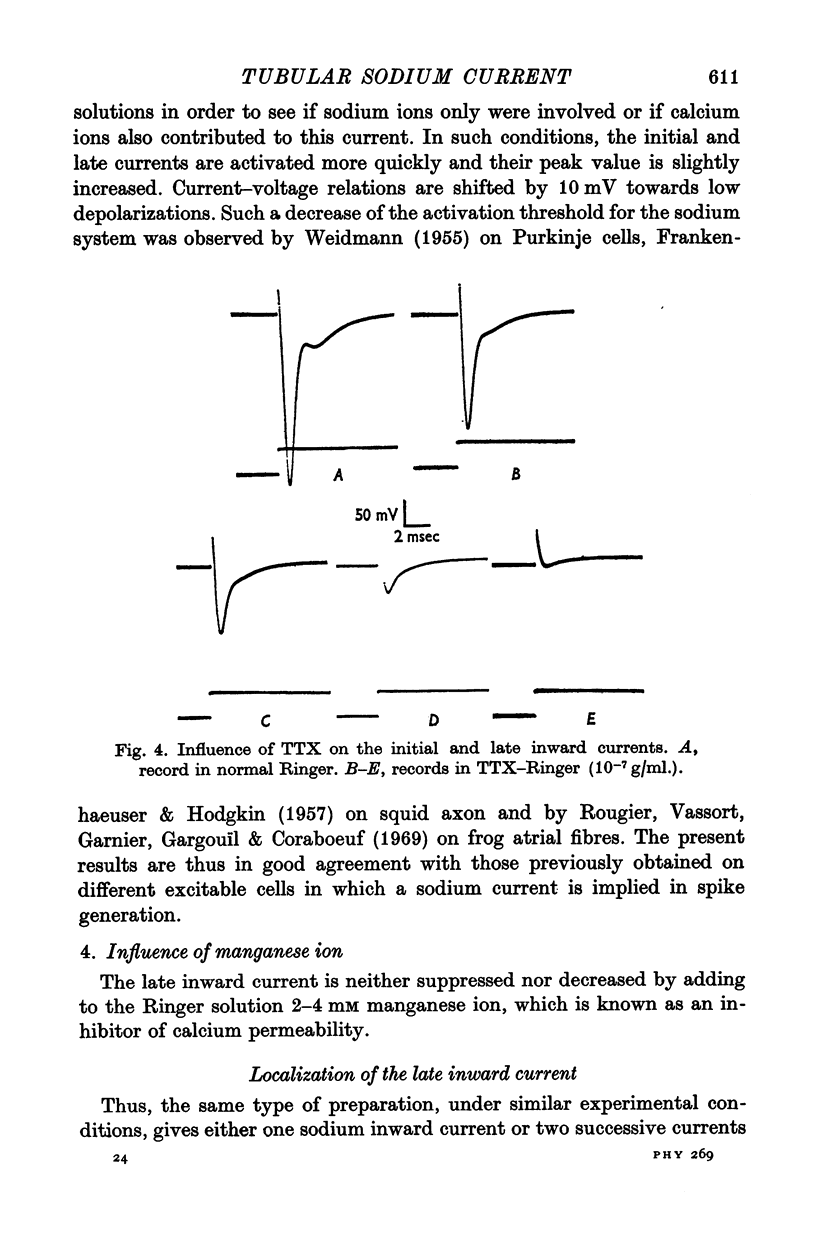
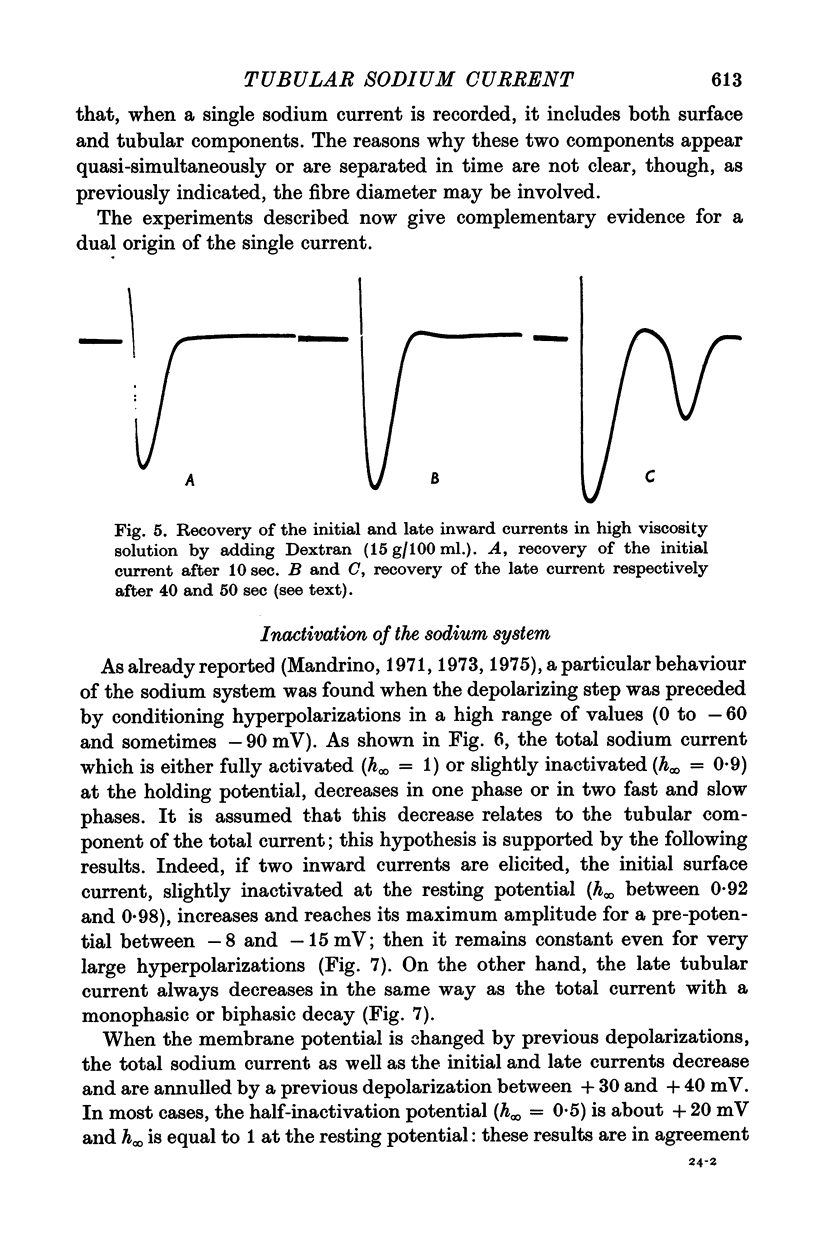
1. A late inward current following the initial sodium current is sometimes observed on frog single skeletal muscle fibres studied in voltage-clamp conditions by the double sucrose-gap method. 2. The late inward current is time and voltage dependent; it is sensitive to extracellular sodium concentration and inhibited by tetrodotoxin. Therefore, it is very probably carried by sodium ions. 3. It is assumed that the late current reflects a regenerative increase of sodium conductance in the tubular membrane. The main argument favouring this assumption is that the late current is never observed on detubulated fibres. 4. Inactivation studies show a marked difference between the evolution of the two inward currents when the membrane resting potential is increased by previous hyperpolarizations in a high range of values: the late current is decreased while the initial current remains constant. The decrease of the late current is suppressed by tetraethylammonium ions or in a potassium-free medium. On detubulated fibres, the sodium current is never decreased by conditioning hyperpolarizations. 5. It is postulated that a fast transient potassium current, located in the bubular membrane, should develop contemporaneously with the sodium current. 6. The decay of the sodium inactivation is anaylsed on semilogarithmic plot: on normal fibres, it runs in two phases while on detubulated fibres, one phase only is found.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H., FREYGANG W. H. Potassium conductance of frog muscle membrane under controlled voltage. J Physiol. 1962 Aug;163:104–114. doi: 10.1113/jphysiol.1962.sp006960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Hodgkin A. L. Voltage clamp experiments in striated muscle fibers. J Gen Physiol. 1968 May 1;51(5):188–192. [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Hodgkin A. L. Voltage clamp experiments in striated muscle fibres. J Physiol. 1970 Jul;208(3):607–644. doi: 10.1113/jphysiol.1970.sp009139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Costantin L. L., Peachey L. D. Radial spread of contraction in frog muscle fibres. J Physiol. 1969 Sep;204(1):231–257. doi: 10.1113/jphysiol.1969.sp008910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Freygang W. H. The potassium and chloride conductance of frog muscle membrane. J Physiol. 1962 Aug;163(1):61–103. doi: 10.1113/jphysiol.1962.sp006959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Peachey L. D. Reconstruction of the action potential of frog sartorius muscle. J Physiol. 1973 Nov;235(1):103–131. doi: 10.1113/jphysiol.1973.sp010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W. Potassium conductance changes in skeletal muscle and the potassium concentration in the transverse tubules. J Physiol. 1972 Aug;225(1):33–56. doi: 10.1113/jphysiol.1972.sp009928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W. The decline of potassium permeability during extreme hyperpolarization in frog skeletal muscle. J Physiol. 1972 Aug;225(1):57–83. doi: 10.1113/jphysiol.1972.sp009929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian J., Nakajima S. Action potential in the transverse tubules and its role in the activation of skeletal muscle. J Gen Physiol. 1974 Feb;63(2):257–278. doi: 10.1085/jgp.63.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Caputo C., Gonzalez-Serratos H., Venosa R. A. Sodium dependence of the inward spread of activation in isolated twitch muscle fibres of the frog. J Physiol. 1972 Jun;223(2):507–523. doi: 10.1113/jphysiol.1972.sp009860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillé J., Ildefonse M., Rougier O. Proceedings: Relations between membrane potential, sodium currents and contraction in frog twitch muscle fibres. J Physiol. 1975 Jul;249(1):26P–28P. [PubMed] [Google Scholar]
- Caputo C., Dipolo R. Ionic diffusion delays in the transverse tubules of frog twitch muscle fibres. J Physiol. 1973 Mar;229(2):547–557. doi: 10.1113/jphysiol.1973.sp010153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantin L. L., Podolsky R. J. Depolarization of the internal membrane system in the activation of frog skeletal muscle. J Gen Physiol. 1967 May;50(5):1101–1124. doi: 10.1085/jgp.50.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantin L. L. The role of sodium current in the radial spread of contraction in frog muscle fibers. J Gen Physiol. 1970 Jun;55(6):703–715. doi: 10.1085/jgp.55.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. S., Howell J. N., Vaughan P. C. The maintenance of resting potentials in glycerol-treated muscle fibres. J Physiol. 1971 May;215(1):95–102. doi: 10.1113/jphysiol.1971.sp009459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M., Tanaka M., Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature. 1970 Oct 3;228(5266):34–36. doi: 10.1038/228034a0. [DOI] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Podolsky R. J. Regenerative calcium release within muscle cells. Science. 1970 Jan 2;167(3914):58–59. doi: 10.1126/science.167.3914.58. [DOI] [PubMed] [Google Scholar]
- González-Serratos H. Inward spread of activation in vertebrate muscle fibres. J Physiol. 1971 Feb;212(3):777–799. doi: 10.1113/jphysiol.1971.sp009356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F., TAYLOR R. E. Local activation of striated muscle fibres. J Physiol. 1958 Dec 30;144(3):426–441. doi: 10.1113/jphysiol.1958.sp006111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ildefonse M., Rougier O. Activation et inactivation du courant potassium de la fibre musculaire squelettique. C R Acad Sci Hebd Seances Acad Sci D. 1968 Dec 23;267(26):2344–2347. [PubMed] [Google Scholar]
- Ildefonse M., Rougier O. Voltage-clamp analysis of the early current in frog skeletal muscle fibre using the double sucrose-gap method. J Physiol. 1972 Apr;222(2):373–395. doi: 10.1113/jphysiol.1972.sp009803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ildefonse M., Roy G. Kinetic properties of the sodium current in striated muscle fibres on the basis of the Hodgkin-Huxley theory. J Physiol. 1972 Dec;227(2):419–431. doi: 10.1113/jphysiol.1972.sp010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaimovich E., Venosa R. A., Shrager P., Horowicz P. Density and distribution of tetrodotoxin receptors in normal and detubulated frog sartorius muscle. J Gen Physiol. 1976 Apr;67(4):399–416. doi: 10.1085/jgp.67.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C. Y., Stanfield P. R. Actions of some cations on the electrical properties and mechanical threshold of frog sartorius muscle fibers. J Gen Physiol. 1970 May;55(5):620–639. doi: 10.1085/jgp.55.5.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C. Y. Tetrodotoxin, saxitoxin and their significance in the study of excitation phenomena. Pharmacol Rev. 1966 Jun;18(2):997–1049. [PubMed] [Google Scholar]
- Mandrino M. Inactivation du courant sodium de la fibre musculaire striée de grenouille, normale et détubulée. Rôle du système tubulaire transverse. C R Seances Soc Biol Fil. 1973;167(6):908–913. [PubMed] [Google Scholar]
- Mandrino M. Relation entre l'inactivation du courant sodium de la fibre musculaire striée de Grenouille et une accumulation de potassium dans le système tubulaire transverse. C R Seances Soc Biol Fil. 1971;165(3):460–465. [PubMed] [Google Scholar]
- Mounier Y., Vassort G. Evidence for a transient potassium membrane current dependent on calcium influx in crab muscle fibre. J Physiol. 1975 Oct;251(3):609–625. doi: 10.1113/jphysiol.1975.sp011111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NARAHASHI T., DEGUCHI T., URAKAWA N., OHKUBO Y. Stabilization and rectification of muscle fiber membrane by tetrodotoxin. Am J Physiol. 1960 May;198:934–938. doi: 10.1152/ajplegacy.1960.198.5.934. [DOI] [PubMed] [Google Scholar]
- Neher E. Two fast transient current components during voltage clamp on snail neurons. J Gen Physiol. 1971 Jul;58(1):36–53. doi: 10.1085/jgp.58.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROUGIER O. MESURE DE LA R'ESISTANCE MEMBRANAIRE DE LA FIBRE MUSCULAIRE SQUELETTIQUE. ETUDE 'A L'AIDE DE LA M'ETHODE DITE DU "SUCROSE GAP". C R Seances Soc Biol Fil. 1964;158:343–347. [PubMed] [Google Scholar]
- Rougier O., Vassort G., Garnier D., Gargouil Y. M., Coraboeuf E. Existence and role of a slow inward current during the frog atrial action potential. Pflugers Arch. 1969;308(2):91–110. doi: 10.1007/BF00587018. [DOI] [PubMed] [Google Scholar]
- Rougier O., Vassort G., Ildefonse M. Analyse qualitative en voltage imposé du courant de membrane de la fibre musculaire squelettique. C R Acad Sci Hebd Seances Acad Sci D. 1968 Apr 22;266(17):1754–1757. [PubMed] [Google Scholar]
- STAEMPFLI R. DIE DOPPELTE SACCHAROSETRENNWANDMETHODE ZUR MESSUNG VON ELEKTRISCHEN MEMBRANEIGENSCHAFTEN MIT EXTRACELLULAEREN ELEKTRODEN. Helv Physiol Pharmacol Acta. 1963;21:189–204. [PubMed] [Google Scholar]
- STAMPFLI R. A new method for measuring membrane potentials with external electrodes. Experientia. 1954 Dec 15;10(12):508–509. doi: 10.1007/BF02166189. [DOI] [PubMed] [Google Scholar]
- Stanfield P. R. The differential effects of tetraethylammonium and zinc ions on the resting conductance of frog skeletal muscle. J Physiol. 1970 Jul;209(1):231–256. doi: 10.1113/jphysiol.1970.sp009164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield P. R. The effect of the tetraethylammonium ion on the delayed currents of frog skeletal muscle. J Physiol. 1970 Jul;209(1):209–229. doi: 10.1113/jphysiol.1970.sp009163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield P. R. The effect of the tetraethylammonium ion on the inwardly rectifying potassium channel of frog sartorius muscle. J Physiol. 1969 Jan;200(1):2P–3P. [PubMed] [Google Scholar]
- Vassort G. Voltage-clamp analysis of transmembrane ionic currents in guinea-pig myometrium: evidence for an initial potassium activation triggered by calcium influx. J Physiol. 1975 Nov;252(3):713–734. doi: 10.1113/jphysiol.1975.sp011167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDMANN S. Effects of calcium ions and local anesthetics on electrical properties of Purkinje fibres. J Physiol. 1955 Sep 28;129(3):568–582. doi: 10.1113/jphysiol.1955.sp005379. [DOI] [PMC free article] [PubMed] [Google Scholar]


