Abstract
PsbP and PsbQ proteins are extrinsic subunits of photosystem II (PSII) and participate in the normal function of photosynthetic water oxidation. Both proteins exist in a broad range of the oxygenic photosynthetic organisms; however, their physiological roles in vivo have not been well defined in higher plants. In this study, we established and analyzed transgenic tobacco (Nicotiana tabacum) plants in which the levels of PsbP or PsbQ were severely down-regulated by the RNA interference technique. A plant that lacked PsbQ showed no specific phenotype compared to a wild-type plant. This suggests that PsbQ in higher plants is dispensable under the normal growth condition. On the other hand, a plant that lacked PsbP showed prominent phenotypes: drastic retardation of growth, pale-green-colored leaves, and a marked decrease in the quantum yield of PSII evaluated by chlorophyll fluorescence. In PsbP-deficient plant, most PSII core subunits were accumulated in thylakoids, whereas PsbQ, which requires PsbP to bind PSII in vitro, was dramatically decreased. PSII without PsbP was hypersensitive to light and rapidly inactivated when the repair process of the damaged PSII was inhibited by chloramphenicol. Furthermore, thermoluminescence studies showed that the catalytic manganese cluster in PsbP-deficient leaves was markedly unstable and readily disassembled in the dark. The present results demonstrated that PsbP, but not PsbQ, is indispensable for the normal PSII function in higher plants in vivo.
Plants, algae, and cyanobacteria are unique in their ability to catalyze the oxidation of water to molecular oxygen by using light energy (for review, see Barber, 2004). This process occurs in a PSII protein complex embedded in thylakoid membranes (Hillier and Babcock, 2001). In the luminal side of PSII, a cluster of three inorganic ions, manganese (Mn), calcium (Ca), and chloride (Cl), catalyzes water oxidation (Debus, 2000). Since ancestral cyanobacteria are the progenitors of chloroplasts in plants and algae, many PSII subunits are conserved between plants, algae, and cyanobacteria (Hankamer et al., 2001). However, the compositions of the PSII extrinsic proteins, which are associated with the luminal side of PSII and involved in oxygen evolution, are significantly different among these organisms (Seidler, 1996): Higher plants and green algae have a set of three extrinsic proteins (PsbO [33 kD], PsbP [23 kD], and PsbQ [17 kD]; Murata and Miyao, 1985), whereas cyanobacteria have a different set of proteins (PsbO, PsbU [12 kD], and PsbV [cytochrome c550]; Shen and Inoue, 1993; Enami et al., 2000). In addition, recent genomic and proteomic studies have demonstrated the existence of PsbP and PsbQ homologs in cyanobacteria (Kashino et al., 2002; De Las Rivas et al., 2004; Thornton et al., 2004). Therefore, it is reasonable to assume that five extrinsic proteins, PsbO, PsbP, PsbQ, PsbU, and PsbV, existed in ancestral cyanobacteria, but PsbU and PsbV were lost from the chloroplasts of higher plants during evolution.
The molecular functions of PSII extrinsic proteins have been studied in both higher plants and cyanobacteria. In higher plants, the functional characteristics of each protein in oxygen evolution in vitro have been extensively analyzed by release-reconstitution experiments using isolated oxygen-evolving PSII preparations. PsbO is responsible for the stable binding of the Mn cluster by facilitating Cl− retention in PSII (Miyao and Murata, 1984a). PsbP is involved in Ca2+ and Cl− retention in PSII (Ghanotakis et al., 1984a), and PsbQ mainly participates in Cl− retention (Akabori et al., 1984; Miyao and Murata, 1985). Finally, PsbP and PsbQ protect the Mn cluster from a bulky reductant (Ghanotakis et al., 1984b). The functions of cyanobacterial extrinsic proteins, elucidated by both in vitro and in vivo studies (Philbrick et al., 1991; Shen et al., 1998; Debus, 2000), are similar to those in higher plants. However, the protein nature of PsbP and PsbQ is greatly different between species, which suggests that they have changed considerably during evolution (Thornton et al., 2004).
Although in vitro studies have demonstrated the mechanistic roles of higher-plant PsbP and PsbQ under artificial conditions and their crystal structures have recently been determined to high resolution (Calderone et al., 2003; Ifuku et al., 2004), there is almost no information available on the physiological roles of these proteins in vivo. In previous studies, a Chlamydomonas reinhardtii mutant lacking PsbP showed decreased oxygen-evolving activity (Mayfield et al., 1987), and an increased concentration of Cl− was required to restore oxygen evolution in isolated thylakoid membranes (Rova et al., 1994). However, the in vivo photosynthetic properties of the mutant cells were not well characterized in these studies. Furthermore, algal PsbP and PsbQ are different from those in higher plants in many respects, including primary sequence, binding features, and functional contributions to oxygen evolution (Suzuki et al., 2003, 2004), and these differences presumably reflect differences in ionic conditions in the thylakoid lumen between land plants and aqueous algae. Thus, the physiological functions of PsbP and PsbQ need to be determined in higher plants.
To address the above issues, we produced two transgenic tobacco (Nicotiana tabacum) lines, ΔPsbP and ΔPsbQ, in which the level of accumulation of PsbP or PsbQ was severely decreased by the RNA interference technique (RNAi). ΔPsbQ plants did not show any distinguishable differences from wild-type plants under the conditions examined, whereas ΔPsbP showed distinct phenotypes, such as a drastic reduction in growth rate and pale-green-colored leaves. In ΔPsbP leaves, photochemical reaction was severely impaired and PSII was hypersensitive to light. Thermoluminescence (TL) studies showed that in intact leaves of ΔPsbP, the Mn cluster was released from PSII in the dark and reassembled in the light. This study demonstrated that higher-plant PsbP, but not PsbQ, is essential for the normal function of PSII and plays a crucial role in stabilizing the Mn cluster in vivo.
RESULTS
The psbP and psbQ Genes Were Effectively Silenced by RNAi
In tobacco, PsbP is encoded by four nuclear isogenes, and all four are expressed in plants (Hua et al., 1992). In the initial trials to obtain tobacco plants lacking PsbP protein (ΔPsbP), a cDNA fragment of psbP (550 bp) was used as an RNAi trigger. However, we could not obtain ΔPsbP lines. Then we created a new RNAi vector, pBE-psbP37 bpir, which was designed to express a hairpin RNA with a 37-bp self-complementary region that contained a 35-bp perfect match among all of the psbP isogenes (Fig. 1A). After transformation, two of 17 kanamycin-resistant lines showed a marked decrease in the amount of all of the psbP transcripts.
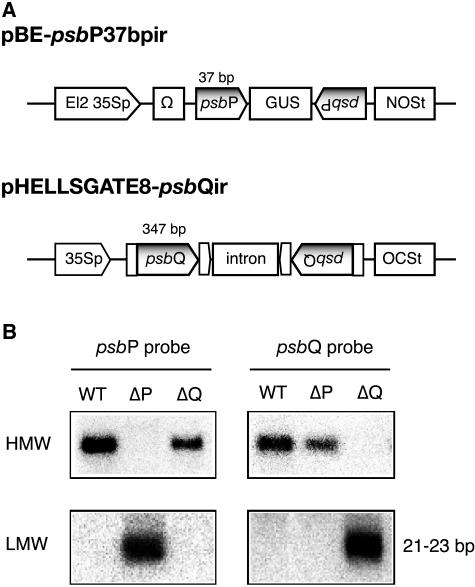
Figure 1.
RNAi constructs and suppression of the psbP and psbQ genes. A, RNAi vectors used to down-regulate the psbP and psbQ genes. El2, Doubled enhancer-like elements; 35Sp, cauliflower mosaic virus 35S promoter; Ω, Ω sequence of Tobacco mosaic virus; NOSt, nopaline synthase terminator; OCSt, octopine synthase terminator. B, Northern analysis for high-molecular-weight (HMW) and low-molecular-weight (LMW) RNAs. RNAs (5 μg) extracted from leaves of wild type and transformants were separated on agarose/formaldehyde gel and hybridized with the indicated [32P]-labeled DNA probes.
To obtain a tobacco plant that lacked PsbQ protein (ΔPsbQ), we first cloned the psbQ cDNA in tobacco by reverse transcription-PCR with the degenerated primers. All five clones sequenced contained an identical 687-bp open reading frame encoding a product consisting of 228 amino acids (GenBank accession no. AB188569), indicating that the obtained psbQ gene was mainly expressed in tobacco. A 347-bp fragment of psbQ cDNA was then used as an RNAi trigger in an RNAi vector, pHELLSGATE8-psbQir (Fig. 1A). After transformation, 12 of 15 kanamycin-resistant lines showed a marked decrease in the amount of psbQ transcripts.
The ΔPsbP and ΔPsbQ lines, which showed strong and stable gene silencing, were then selected and used for the subsequent analyses. In these lines, accumulation of the silenced gene could scarcely be detected by northern analysis (Fig. 1B). Quantitative analyses using real-time PCR showed that the amount of total psbP transcripts in ΔPsbP was decreased to 3% ± 1% of that in the wild type, whereas in ΔPsbQ the amount of psbQ transcripts was below the level of detection (data not shown). The accumulation of short interfering RNA (siRNA), 21 to 23 bp of double-stranded RNA with a silenced-gene sequence, confirmed that the silenced phenotypes of the selected lines were caused by the normal RNAi pathway (Fig. 1B).
The plants were then transferred to soil, cultivated until they flowered, and self-fertilized. Since the segregation ratio for silenced:nonsilenced plants in T1 seedlings was approximately 3:1, our selected lines of ΔPsbP and ΔPsbQ seem to have a transgene(s) in a single locus. The T1 seedlings of ΔPsbP hardly grew in soil, so plants transferred to soil from in vitro culture (T0 generation) were used in subsequent analyses.
PSII Activity and Photoautotrophic Growth Were Impaired in ΔPsbP But Not in ΔPsbQ Tobacco
The ΔPsbQ plant grew normally under the conditions examined (25°C, 100 μE m−2 s−1), whereas ΔPsbP showed distinct phenotypes, such as a drastic reduction in growth rate and pale-green-colored leaves (Fig. 2). The chlorophyll (chl) content and the chl a/b ratio were lower in leaves of the ΔPsbP plant than in those of the wild-type and ΔPsbQ plants (Table I). The effects of RNAi on photosynthetic activity were evaluated by measuring chl fluorescence, and the results are summarized in Table I. The ΔPsbP plants showed much lower Fv/Fm values (an indicator of the potential PSII activity [quantum yield of PSII at the maximum level]) than wild-type and ΔPsbQ plants (approximately 0.82), although the values fluctuated within a range from 0 to 0.6, depending on leaf development and light intensity during growth. The calculated quantum yield of PSII (ΦPSII) under these growth conditions also was low (0–0.2) in ΔPsbP.
Figure 2.
Phenotypes of wild-type, ΔPsbP, and ΔPsbQ plants. Tobacco plants precultivated on 0.5× LS agar medium supplemented with 1.5% Suc were transplanted into soil and grown at 25°C under continuous light (100 μE m−2 s−1) for 3 weeks.
Table I.
Chl fluorescence and content of wild-type, ΔPsbP, and ΔPsbQ tobacco plants
Data presented are mean ± sd of three measurements.
| Wild Type | ΔPsbP | ΔPsbQ | |
|---|---|---|---|
| Fv/Fma | 0.82 ± 0.01 | 0.47 ± 0.03 | 0.81 ± 0.01 |
| ΦPSIIb | 0.73 ± 0.02 | 0.22 ± 0.09 | 0.73 ± 0.01 |
| Total chl (mg/g fresh weight) | 0.69 ± 0.14 | 0.42 ± 0.05 | 0.72 ± 0.12 |
| Chl a/b ratio | 3.25 ± 0.05 | 2.12 ± 0.11 | 3.24 ± 0.03 |
The Fv/Fm value was measured after incubation in the dark for 2 h.
The ΦPSII was measured under illumination in a growth chamber (100 μE m−2 s−1).
The PSII activity of isolated thylakoid membranes was then measured using an oxygen electrode with p-phenylbenzoquinone and K3Fe(CN)6 as an artificial electron acceptor. The thylakoid membranes isolated from the wild-type and ΔPsbQ leaves showed high O2 activities of around 250 μmol O2 (mg chl)−1 h−1, whereas those from ΔPsbP showed very low activity (approximately 30 μmol O2 [mg chl]−1 h−1; Table II). Further addition of Ca2+ and Cl− scarcely enhanced the activity of ΔPsbP membranes. The Mn content in thylakoid membrane was relatively lower in ΔPsbP than in wild type and ΔPsbQ (Table II).
Table II.
PSII activity and the Mn content of thylakoid membranes isolated from wild-type, ΔPsbP, and ΔPsbQ tobacco plants
Data presented are mean ± sd of three measurements.
| Wild Type | ΔPsbP | ΔPsbQ | |
|---|---|---|---|
| O2 evolutiona (μmol O2 [mg chl]−1 h−1) | 241 ± 10 | 26 ± 3 | 242 ± 12 |
| +5 mm CaCl2b | 245 ± 15 | 53 ± 3 | 240 ± 9 |
| Mn (ng [mg chl]−1)c | 0.66 ± 0.04 | 0.44 ± 0.01 | 0.64 ± 0.03 |
The buffer for measurement was 50 mm HEPES/NaOH, pH 7.6, containing 0.4 m Suc, 10 mm NaCl, 5 mm MgCl2, and 5 mm NH4Cl. As electron acceptors for PSII, 0.5 mm K3Fe(CN)6 and 0.5 mm p-phenylbenzoquinone were used.
Five millimolar CaCl2 was added to the buffer for measurements instead of 10 mm NaCl.
The Mn content was determined using an atomic absorption spectrophotometer.
ΔPsbP Tobacco Was Hypersensitive to Light
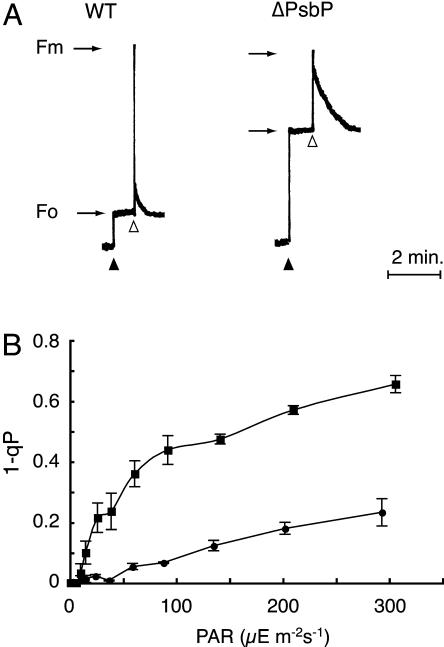
The low value of Fv/Fm indicates the susceptibility of the ΔPsbP plant to light-induced damage. In fact, exposure of ΔPsbP leaves to light at 100 μE m−2 s−1 resulted in a marked decrease in the Fv/Fm value by 90% within 150 min when chloramphenicol was added to prevent the recovery of PSII by inhibiting the de novo synthesis of the reaction center D1 protein (Fig. 3A). In contrast, under these conditions, the Fv/Fm value decreased less than 20% in wild-type and ΔPsbQ leaves. In the absence of inhibitor, the decrease was limited to approximately 20% in ΔPsbP leaves, indicating that light-induced damage is largely compensated by a repair process in ΔPsbP. Therefore, the hypersensitivity of ΔPsbP tobacco to light is caused by enhanced photodamage to PSII rather than by damage to a repair process, which has been proposed as a mechanism of photoinhibition in cyanobacterium (Nishiyama et al., 2001). ΔPsbP plants developed symptoms of photodamage, as manifested photobleached leaves after 1 to 2 weeks of growth and exposure to light (150 μE m−2 s−1), and the Fv/Fm values decreased to <0.2 (Fig. 3B).
Figure 3.
Hypersensitivity of ΔPsbP tobacco to light. A, Time course of photoinactivation in wild-type (circle), ΔPsbP (square), and ΔPsbQ (triangle) leaves exposed to 100 μE m−2 s−1 for 150 min with (black symbols) or without (white symbols) the vacuum infiltration of chloramphenicol (200 μg mL−1). The results are expressed as percentage of the initial Fv/Fm values (0.82 for wild type and ΔPsbQ, and 0.53 for ΔPsbP) prior to light incubation. B, Chronic photodamage observed in ΔPsbP. The ΔPsbP plants were grown under moderate light conditions (approximately 50 μE m−2 s−1) for 2 months. The plant on the right was then transferred to standard light conditions (150 μE m−2 s−1) and grown for another week.
While Most PSII Subunits Were Accumulated, PSI Was Markedly Decreased in ΔPsbP Tobacco
The accumulation of PSII subunits in wild type, ΔPsbP, and ΔPsbQ was analyzed by immunoblotting with protein-specific antibodies to clarify the biochemical consequences of these genetic modifications. All of the PSII subunits except for PsbQ were accumulated in ΔPsbQ as in wild-type plants (Fig. 4). On the other hand, PSII core proteins and PsbO were accumulated in ΔPsbP to an almost similar extent as in wild type. This result was quite different from the observation that PSII was not assembled in a PsbO-RNAi plant of Arabidopsis (Yi et al., 2005). Similar to a PsbO-RNAi plant of Arabidopsis, PsbQ was almost completely absent in ΔPsbP tobacco. Instead of PsbQ, a protein band with a faster migration rate in gel, a probable degradation fragment of PsbQ, was detected in ΔPsbP leaves.
Figure 4.
Immunoblot analyses of thylakoid membrane proteins. Proteins prepared from leaves and thylakoid membranes of tobacco plants grown under moderate light conditions (approximately 50 μE m−2 s−1) were subjected to SDS-PAGE or Tricine-SDS-PAGE, and then detected by immunoblotting using specific antibodies. To detect degraded fragments, total proteins from leaves were used to analyze the extrinsic proteins (PsbO, PsbP, and PsbQ). Each lane contained 5 μg of chl. WT, Wild-type tobacco; ΔP, ΔPsbP tobacco; ΔQ, ΔPsbQ tobacco.
The amounts of PSI subunit (PsaC) and PSI antenna (Lhca1) were markedly decreased in ΔPsbP. The amount of spectrophotometrically active PSI centers (P700) was significantly decreased in ΔPsbP leaves (approximately 30% of the wild type; data not shown). Although the mechanism that underlies this phenomenon is unknown, it might be the result of acclimation to a state of low PSII pressure (for review, see Walters, 2005). The amount of PSII antenna (light-harvesting complex II) and an ATPase subunit (AtpB) was not different between transgenic and wild type, whereas the amounts of PsbS, cytochrome f, and an NAD(P)H dehydrogenase subunit (Ndh-H) were significantly increased in ΔPsbP. The increased amount of PsbS, which plays a central role in nonphotochemical energy dissipation in PSII, indicates that the mechanism for dissipating light energy was activated in ΔPsbP. The up-regulation of NAD(P)H dehydrogenase in ΔPsbP may be caused by accumulated oxidative stress, as suggested by Martín et al. (1996).
Forward Electron Flow on Thylakoid Membranes Was Slowed in ΔPsbP Tobacco
PSII activity in vivo was further studied by analyzing induction curves of chl fluorescence. The Fo value, the minimum yield of chl fluorescence in dark-adapted leaves, was 3 times higher in ΔPsbP than in wild-type plants, resulting in a low Fv/Fm value in ΔPsbP (Fig. 5A). The high Fo level in ΔPsbP was not affected by additional far-red light illumination, which specifically excites PSI and leads to the oxidation of the plastoquinone pool (data not shown). This indicates that the high Fo level in ΔPsbP was not due to the reduction of the plastoquinone pool in the dark but rather to inactive PSII centers, which show a high chl fluorescence yield.
Figure 5.
Chl fluorescence induction and parameters obtained from wild-type and ΔPsbP plants. A, Fluorescence decay kinetics in wild type (WT) and ΔPsbP. The black and white arrowheads indicate the application of measuring light and a saturating light pulse, respectively. B, Values of 1 − qP under different light intensities in wild type (circle) and ΔPsbP (square). The value of 1 − qP represents the accumulation of the reduced quinone acceptor (QA−) within PSII and was determined 2 min after the application of actinic light at different intensities. The Fo′ level was determined with the application of far-red light after each saturating light pulse. The plants were adapted to the dark for 2 h before measurements.
Another marked difference between wild type and ΔPsbP was the slower decay of chl fluorescence after a saturating pulse (Fig. 5A). The slower decay indicates a slower oxidation of the reduced quinone acceptor QA− due to forward electron transfer to subsequent acceptors in ΔPsbP compared to wild-type thylakoid membranes. A very similar observation was reported in a tobacco mutant that lacked PsbJ protein, in which PsbP and PsbQ were both lost (Regel et al., 2001). The substantial accumulation of QA− in ΔPsbP (determined by the value 1 − qP) was also observed under continuous light even at low intensity (approximately 50 μE m−2 s−1; Fig. 5B). These results suggest that not only PSII activity but also subsequent electron transfer was inefficient in the thylakoid membrane of ΔPsbP, consistent with the low PSI level in ΔPsbP.
Mn Cluster Was Unstable in the Dark in Leaves of ΔPsbP Tobacco
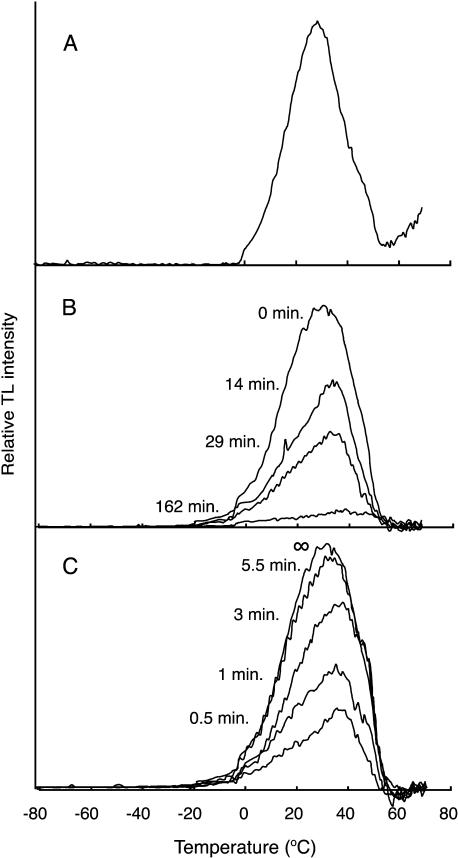
To investigate the redox properties of the donor and acceptor of PSII in vivo, we performed TL measurements in leaf segments of ΔPsbP plants. TL originates from a PSII reaction center that is re-excited by a charge recombination with an increase in the temperature of samples, where light-induced charge pairs in PSII are freeze-trapped (Vass and Inoue, 1992). The charge pairs involved can be identified by their emission temperatures. The B-band, which we investigated here, arises from a recombination of the S2 state of the Mn cluster with the semiquinone QB− (Rutherford et al., 1982). A single-flash excitation of the wild-type leaves after a short incubation (10 min) in the dark gave a prominent B-band (Fig. 6A). The ΔPsbP leaves also generated a B-band; however, the intensity of the flash-induced band gradually decreased during incubation in the dark, and only a very small residual band was induced after 162 min (Fig. 6B). No TL band was detected in ΔPsbP leaves that had been adapted to the dark for 5 h (data not shown). Incubation in the dark for 5 h did not affect either the intensity or peak temperature of the flash-induced B-band in wild-type leaves (data not shown). This indicates that dark incubation would cause the loss of the functional Mn cluster in PsbP leaves. Alternatively, dark incubation might cause the reduction of Tyr-D radical on D2 protein in PsbP leaves. The reduced Tyr-D was shown to reduce rapidly the S2 state of the Mn cluster to the S1 state after a single-flash excitation (Vass and Styring, 1991). In both cases, no TL signal would be observed.
Figure 6.
TL glow curves for the wild-type and ΔPsbP leaves. A, The TL band observed in the wild-type leaves. B, The ability of the TL band to decay in the dark in ΔPsbP leaves. The leaves were incubated in the dark at 20°C for the indicated period and then illuminated by a single flash at 0°C. B, The development of TL B-band capability during weak light illumination in dark-adapted ΔPsbP leaves. ΔPsbP leaves were dark adapted for 5 h and then incubated under dim light (0.5 μE m−2 s−1) at 20°C for the indicated period. The sample leaves were excited with a single flash at 0°C after 10 min of incubation in the dark. The TL B-band originates from recombination of the QB−/S2 charge pair in PSII.
Interestingly, the incubation of dark-adapted ΔPsbP leaves under dim light (0.5 μE m−2 s−1) resulted in a gradual restoration of the capacity for B-band formation within approximately 10 min (Fig. 6C). The observed kinetics of recovery of TL signal suggests that it would be due to the photoassembly of the functional Mn cluster (Tamura and Cheniae, 1987) but not due to the reoxidation of the reduced Tyr-D, which is known to occur in the nanosecond or microsecond time scale (Faller et al., 2001). A similar phenotype was reported in ΔPsbO cells of the cyanobacterium Synechocystis 6803, in which the Mn cluster was destabilized due to the absence of the Mn-stabilizing PsbO protein, to be dissembled in the dark and photoassembled in the light (Burnap et al., 1996). This phenotype has not been reported in any mutants in eukaryotes including higher plants. Therefore, the present results indicate that in ΔPsbP leaves, the functional Mn cluster was readily dissociated from PSII in the dark, although it could be reassembled under dim light. This view apparently accounts for the lower Mn content in thylakoids from ΔPsbP leaves, as shown in Table II. Presumably, some Mn was lost during the isolation of thylakoids, after which Mn clusters were continuously destroyed but the released Mn ions were preserved in the fraction. This situation may be responsible for the very low O2 evolution activity in ΔPsbP thylakoid preparations (Table II), and also partly for the low Fv/Fm (Table I) since chl fluorescence was measured after 2 h of incubation in the dark. The peak temperatures of the B-bands tended to decrease and increase during dark disassembly (Fig. 6B) and light assembly (Fig. 6C), respectively. This may be ascribed to the changes in the pH of the thylakoid lumen upon the inactivation and activation of water oxidation.
DISCUSSION
This work is the first to describe the properties of tobacco plants that are deficient in PsbP and PsbQ (ΔPsbP and ΔPsbQ, respectively). Down-regulation of PsbQ has not been reported in any eukaryotes. Interestingly, down-regulation of psbP in the obtained ΔPsbP plants was not perfect (approximately 5% of wild type) compared to that of psbQ in ΔPsbQ (<0.5% of wild type), suggesting that the complete down-regulation of psbP genes would cause fatal damage in higher plants. In our previous screening for high-Fo chl fluorescence mutants of Arabidopsis, we could obtain the psbo1 mutant (Murakami et al., 2002), whereas we could not obtain a mutant that has a defect in the psbP gene. Arabidopsis has two psbO genes (psbO1 and psbO2), and both of them are expressed. In the psbo1 mutant, the presence of psbO2 partially compensates the defect of psbO1 and allows plant survival (Murakami et al., 2005). However, Arabidopsis has a single psbP gene that is expressed (At1g06680), making it difficult to obtain knock-out or knock-down plants. Therefore, we used tobacco that has four psbP genes and successfully obtained ΔPsbP tobacco lines by RNAi. Although ΔPsbP tobacco could grow photoautotrophically, it showed very severe phenotype especially in the seedling stage, and such a knock-down plant will be very difficult to obtain Arabidopsis. Our ΔPsbP and ΔPsbQ tobacco clearly showed that the lack of PsbP severely impaired the photochemical reaction of PSII in the light and led to disassembly of the Mn cluster in the dark, whereas the lack of PsbQ did not alter the plant phenotype. Thus, we concluded that PsbP, but not PsbQ, is indispensable for plant survival and normal PSII functions in higher plants in vivo.
Higher-Plant PsbP Is Required to Maintain the Active Mn-Ca-Cl Cluster in Vivo
The ΔPsbP plant accumulated most of the PSII subunits, whereas its PSII activity in vivo was considerably low, as indicated by chl fluorescence. PsbP has been shown to be involved in the high-affinity binding of Ca2+ and Cl− in PSII (Ghanotakis et al., 1984c; Miyao and Murata, 1984b, 1985). The cyanobacterial mutant that lacked PsbP was shown to require Ca2+ and Cl− in the growth media for optimal oxygen evolution (Thornton et al., 2004). Therefore, it is reasonable to assume that a shortage of these ions is responsible for the impairment of PSII function in ΔPsbP tobacco. Ca2+ has been proposed to be a metal constituent of the Mn-Ca-Cl cluster and to participate in water-oxidation chemistry (Debus, 1992). In vitro experiments indicate that Ca2+ bound to PSII is not equilibrated with bulk ions in solution in the presence of PsbP, but the absence of PsbP makes the Ca2+ site exchangeable with the bulk ions with an affinity on the order of several millimolar (Ghanotakis et al., 1984c; Miyao and Murata, 1984b; Homann, 1988). The concentration of free Ca2+ in the stroma is very low (2–6 μm; Kreimer et al., 1988; Johnson et al., 1995), and the Ca2+/H+ antiporter increases its concentration in the thylakoid lumen using light-induced ΔpH formed across the thylakoid membranes (Ettinger et al., 1999). However, acidification of the thylakoid lumen upon ΔpH generation facilitates the release of Ca2+ from PSII (Homann, 1988; Krieger and Weis, 1993), presumably via the partial dissociation of PsbP. Thus, the balance between the Ca2+ concentration and the pH inside the thylakoid lumen would regulate the PSII function in vivo. The absence of PsbP would push this balance toward a shortage of Ca2+ in PSII. On the other hand, higher plants usually contain the Cl− in abundance, and its concentration in stroma is reported to range from 30 to 60 mm (Demming and Gimmler, 1983). Considering the relatively high permeability of thylakoid membranes to Cl− (Schuldiner and Avron, 1971), Cl− deficiency in PSII is less likely to be the main contributor to the effects due to the absence of PsbP.
TL studies in intact leaves of ΔPsbP indicated that the Mn cluster was dissociated from PSII in the dark and reassembled in the light (Fig. 6, B and C). In vitro studies demonstrated that the removal of PsbP and PsbQ increased the susceptibility of the Mn cluster to exogenous reductants, such as NH2OH, hydroquinone, H2O2, and Fe2+, which cause the release of Mn from PSII by reducing Mn ions in higher oxidation states to Mn2+ (Ghanotakis et al., 1984b). Very recently, PsbP was reported to have the ability to bind Mn and facilitate the light-dependent ligation of the Mn cluster to PSII, a process called photoactivation (Bondarava et al., 2005). However, our results showed that photoactivation could rapidly occur without PsbP in vivo. Therefore, the function of PsbP would protect the Mn cluster from exogenous reductants or retain reduced Mn (Mn2+) in the vicinity of the PSII reaction center.
Although we could not identify the endogenous reductant that reduced Mn in ΔPsbP leaves, H2O2 may be a candidate of such a reductant. Reductants such as ascorbate or ferrocyanide do not release Mn from PSII depleted of PsbP and PsbQ (Ghanotakis et al., 1984b). Previous studies suggested that H2O2 is generated from the PSII acceptor side when the oxidation of QA− is perturbed (Schröder and Åkerlund, 1990), or from the PSII donor side when the function of the Mn cluster is impaired (Schröder and Åkerlund, 1986; Fine and Frasch, 1992; Hillier and Wydrzynski, 1993; Klimov et al., 1993; Arató et al., 2004). PSII depleted of the Mn cluster has also been reported to generate O2− efficiently (Chen et al., 1995). Since both the acceptor side and donor side reactions of PSII were impaired in ΔPsbP leaves, H2O2 may present around PSII and attack the Mn cluster. Further studies were obviously required to address this issue.
What Is the Physiological Role of PsbQ in Higher Plants?
The present data clearly demonstrate that PsbQ was not necessary for either PSII function or growth under normal conditions. We also grew ΔPsbQ tobacco in a greenhouse (28°C, 700–1,000 μE m−2 s−1 in the daytime), and no significant difference was observed between ΔPsbQ and wild type (data not shown). In in vitro studies, PsbQ has been shown to be required for optimum PSII activity under low Cl− conditions (Akabori et al., 1984; Miyao and Murata, 1985) or under conditions in which the function of PsbP is impaired by truncation of its N terminus (Ifuku and Sato, 2002). However, such conditions are unlikely to occur naturally in vivo.
Although the physiological function of PsbQ in higher plants is still unclear, our results and recent publications suggest that the function and relationship of PsbP and PsbQ in higher plants have evolved differently from those in cyanobacteria and green algae. (1) The function of PsbQ requires PsbP in both higher plants (Miyao and Murata, 1985) and green algae (Suzuki et al., 2003), but not in cyanobacteria (Thornton et al., 2004). (2) The binding of PsbQ to PSII requires PsbP in higher plants (Miyao and Murata, 1983, 1989), but not in cyanobacteria (Thornton et al., 2004) and green algae (Suzuki et al., 2003, 2004). In addition, PsbQ was degraded in ΔPsbP leaves, which suggests that unassembled PsbQ is degraded by a protease. Consistent with this observation, a protease that specifically degrades PsbQ has been reported in spinach (Spinacia oleracea) leaves (Kuwabara and Suzuki, 1994), and a degradation fragment of PsbQ was detected in ΔPsbP leaves by immunoblotting (Fig. 4). These observations were in contrast to those reported in cyanobacteria and green algae, where PsbQ can accumulate and bind to PSII in the absence of PsbP (Mayfield et al., 1987; de Vitry et al., 1989; Suzuki et al., 2003; Thornton et al., 2004). Therefore, we assume that PsbQ acts as an auxiliary, as if PsbQ is an accessory subunit to support the function of PsbP in higher plants. Alternatively, PsbQ could be required in vivo under some extreme conditions. In fact, cyanobacterial PsbQ has been shown to be essential for photoautotrophic growth under conditions of limited Ca2+, Cl−, and iron (Summerfield et al., 2005).
MATERIALS AND METHODS
Plant Material
Tobacco plants (Nicotiana tabacum cv Samsun NN) were grown on agar-solidified 0.5× Linsmaier-Skoog (LS) medium supplemented with 1.5% Suc under continuous light (10 μE m−2 s−1) at 25°C. For analytical purposes, tobacco plants (T0 generation) precultivated on LS agar medium were transplanted into soil and grown under continuous light (50–100 μE m−2 s−1) at 25°C for 2 to 3 weeks. Fully developed leaves (fourth and fifth leaves from the top) were used in all experiments.
RNAi Vector Construction and Transformation
To down-regulates the expression of the psbP gene, the RNAi vector pBE-psbP37 bpir, which has a fragment containing sense and antisense sequences of the psbP gene (37 bp, respectively) separated by a 20-bp fragment of the β-glucuronidase gene, was prepared. The binary vector pBE2113-GUS (Mitsuhara et al., 1996) was used as a backbone. To down-regulate the expression of the psbQ gene, the cDNA encoding tobacco PsbQ protein was cloned by reverse transcription-PCR to produce the RNAi vector pHELLSGATE8-psbQir, which has a fragment containing sense and antisense sequences of the psbQ gene (347 bp, respectively). The binary vector pHELLSGATE8 (Helliwell and Waterhouse, 2003) was used for construction. The experimental details of the vector construction were described elsewhere (Yamamoto et al., 2005). The above RNAi vectors were introduced into Agrobacterium tumefaciens LBA4404, and tobacco plants were transformed by the leaf-disc method.
Isolation and Analysis of RNA
Total RNA was extracted from leaves with TRI-reagent (Sigma). High-molecular-weight RNA (rRNA and mRNA) and low-molecular-weight RNA, including siRNA, were isolated and separated by the method described by Hamilton et al. (2002). High-molecular-weight RNA was analyzed on a formaldehyde-denaturing-agarose gel in 1× MOPS buffer (20 mm MOPS-KOH, pH 7.0, 5 mm sodium acetate, 1 mm EDTA) and blotted onto a charged nylon membrane (Hybond N+; Amersham). siRNA was analyzed as described by Hamilton and Baulcombe (1999). cDNA fragments of psbP or psbQ were labeled with 32P and used as probes.
SDS-PAGE and Immunoblot Analysis of Protein
To isolate thylakoid membranes, leaves were chopped in a blender with the ice-cold buffer (50 mm HEPES-NaOH, pH 7.6, containing 0.4 m Suc, 10 mm NaCl, 5 mm MgCl2, and 5 mm sodium ascorbate). The mixture was then filtrated and centrifuged, and the pellet was washed and resuspended in the same buffer without sodium ascorbate. The chl contents of thylakoid membranes were determined as described by Arnon (1949). Proteins corresponding to 5 μg chl were separated on 15% SDS-polyacrylamide gels. To analyze membrane-embedded protein, 6 m urea was included in the gels. PsaC was analyzed by a Tricine-SDS-PAGE system (Schagger and von Jagow, 1987). Separated proteins were transferred to a polyvinylidene difluoride membrane using a semidry blotting system (Bio-Rad). Immunoblot detection was performed using an enhanced chemiluminescence system (ECL; Amersham Biosciences).
Photosynthetic Measurements
Oxygen evolution from thylakoid membranes was measured at 25°C with a Clark-type oxygen electrode (Hanzatech) with 0.5 mm K3Fe(CN)6 and 0.5 mm p-phenylbenzoquinone as electron acceptors under saturating red light with an R-60 red long-pass filter (Kenko).
Chl fluorescence parameters were measured using a PAM-2000 chl fluorometer (Walz). The minimum chl fluorescence at an open PSII center (Fo and Fo′) was determined using light (655 nm) at an intensity of 0.05 to 0.1 μE m−2 s−1. A saturation pulse of white light (2,500 μE m−2 s−1 for 0.8 s) was applied to determine the maximum chl fluorescence at closed PSII centers in the dark (Fm) and during actinic light illumination (Fm′). The steady state of the chl fluorescence level (Fs) was recorded during actinic light illumination (3–300 μE m−2 s−1). The Fv/Fm and during steady-state photosynthesis (ΦPSII) were calculated as (Fm − Fo)/Fm and (Fm′ − Fs)/Fm′, respectively. Photochemical quenching qP was calculated as (Fm′ − Fs)/(Fm′ − Fo′).
The change in the absorbance of P700 at 820 nm was measured with a PAM-2000 chl fluorometer equipped with an emitter-detector unit (ED 800T; Walz; Schreiber et al., 1988). The change in absorbance induced by saturating far-red light represents the relative amount of photo-oxidizable P700.
TL was recorded with a home-built apparatus, as described elsewhere (Ono and Inoue, 1986). Leaf segments 10 mm in diameter were cooled to 0°C, illuminated with a saturating single turnover flash, and then quickly frozen in liquid N2. Light emission during sample warming was recorded against sample temperature.
Antisera
Rabbit antibodies against PsbP and D1 were produced by the authors. Rabbit antibodies against PsbO and PsbQ were provided by the late Dr. A. Watanabe, Tokyo University. Rabbit antibodies against CP47 and light-harvesting complex II were provided by Dr. A. Tanaka, Hokkaido University. Rabbit antibodies against D2 and CP43 were gifts from Dr. Y. Kashino, Hyogo Prefectural University. Rabbit antibodies against Ndh-H were produced by Mr. A. Takabayashi, Kyoto University. Rabbit antibodies against AtpB (TF1-B) were a gift from Dr. T. Hisabori, Tokyo Industrial University. Rabbit antibodies against PsaC and Lhc1a and hen antibodies against PsbS were purchased from AgriSera.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number AB188569.
Acknowledgments
We thank Dr. T. Endo, Kyoto University, for his technical assistance in P700 measurements. We thank Dr. T. Matoh and Dr. N. Ochiai, Kyoto University, for their help in measuring Mn using an atomic absorption spectrophotometer. We thank all of the investigators who kindly provided specific antibodies as listed in “Materials and Methods.” Finally, we thank Mr. Y. Watanabe, Kyoto University, for his excellent technical assistance throughout this research.
This work was supported in part by a Research for the Future Program Grant from the Japan Society for the Promotion of Science (JSPS-RFTF 00L01606 to F.S.), by a grant-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (grant no. 15770026 to K.I.), and a Research Grant from Nissan Science Foundation (to K.I.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Fumihiko Sato (fumihiko@kais.kyoto-u.ac.jp).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.068643.
References
- Akabori K, Imaoka A, Toyoshima Y (1984) The role of lipids and 17-kDa protein in enhancing the recovery of O2 evolution in cholate-treated thylakoid membranes. FEBS Lett 173: 36–40 [Google Scholar]
- Arató A, Bondarava N, Krieger-Liszkay A (2004) Production of reactive oxygen species in chloride- and calcium-depleted photosystem II and their involvement in photoinhibition. Biochim Biophys Acta 1608: 171–180 [DOI] [PubMed] [Google Scholar]
- Arnon DI (1949) Copper enzymes in isolated chloroplast. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber J (2004) Water, water everywhere, and its remarkable chemistry. Biochim Biophys Acta 1655: 123–132 [DOI] [PubMed] [Google Scholar]
- Bondarava N, Beyer P, Krieger-Liszkay A (2005) Function of the 23 kDa extrinsic protein of Photosystem II as a manganese binding protein and its role in photoactivation. Biochim Biophys Acta 1708: 63–70 [DOI] [PubMed] [Google Scholar]
- Burnap RL, Qian M, Pierce C (1996) The manganese stabilizing protein of photosystem II modifies the in vivo deactivation and photoactivation kinetics of the H2O oxidation complex in Synechocystis sp. PCC6803. Biochemistry 35: 874–882 [DOI] [PubMed] [Google Scholar]
- Calderone V, Trabucco M, Vujicic A, Battistutta R, Giacometti GM, Andreucci F, Barbato R, Zanotti G (2003) Crystal structure of the PsbQ protein of photosystem II from higher plants. EMBO Rep 4: 900–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GX, Blubaugh DJ, Homann PH, Golbeck JH, Cheniae GM (1995) Superoxide contributes to the rapid inactivation of specific secondary donors of the photosystem II reaction center during photodamage of manganese-depleted photosystem II membranes. Biochemistry 34: 2317–2332 [DOI] [PubMed] [Google Scholar]
- Debus RJ (1992) The manganese and calcium ions of photosynthetic oxygen evolution. Biochim Biophys Acta 1102: 269–352 [DOI] [PubMed] [Google Scholar]
- Debus RJ (2000) The polypeptides of photosystem II and their influence on manganotyrosyl-based oxygen evolution. Met Ions Biol Syst 37: 657–711 [PubMed] [Google Scholar]
- De Las Rivas J, Balsera M, Barber J (2004) Evolution of oxygenic photosynthesis: genome-wide analysis of the OEC extrinsic proteins. Trends Plant Sci 9: 18–25 [DOI] [PubMed] [Google Scholar]
- Demming B, Gimmler H (1983) Properties of the isolated intact chloroplast at cytoplasmic K+ concentrations. 1. Light-induced cation uptake into intact chloroplasts is driven by an electrical potential difference. Plant Physiol 73: 169–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vitry C, Olive J, Drapier D, Recouvreur M, Wollman FA (1989) Post-translational events leading to the assembly of photosystem II protein complex: a study using photosynthesis mutants from Chlamydomonas reinhardtii. J Cell Biol 109: 991–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enami I, Yoshihara S, Tohri A, Okumura A, Ohta H, Shen JR (2000) Cross-reconstitution of various extrinsic proteins and photosystem II complexes from cyanobacteria, red algae and higher plants. Plant Cell Physiol 41: 1354–1364 [DOI] [PubMed] [Google Scholar]
- Ettinger WF, Clear AM, Fanning KJ, Peck M (1999) Identification of a Ca2+/H+ antiport in the plant chloroplast thylakoid membrane. Plant Physiol 119: 1379–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faller P, Debus RJ, Brettel K, Sugiura M, Rutherford AW, Boussac A (2001) Rapid formation of the stable tyrosyl radical in photosystem II. Proc Natl Acad Sci USA 98: 14368–14373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine PL, Frasch WD (1992) The oxygen-evolving complex requires chloride to prevent hydrogen peroxide formation. Biochemistry 31: 12204–12210 [DOI] [PubMed] [Google Scholar]
- Ghanotakis DF, Topper JN, Babcock GT, Yocum CF (1984. a) Water-soluble 17 and 23 kDa polypeptides restore oxygen evolution activity by creating a high-affinity binding site for Ca2+ on the oxidizing side of Photosystem II. FEBS Lett 170: 169–173 [Google Scholar]
- Ghanotakis DF, Topper JN, Yocum CF (1984. b) Structural organization of the oxidizing side of photosystem II: Exogenous reductants reduce and destroy the Mn-complex in photosystem II membranes depleted of the 17 and 23 kDa polypeptides. Biochim Biophys Acta 767: 524–531 [Google Scholar]
- Ghanotakis DF, Babcock GT, Yocum CF (1984. c) Calcium reconstitutes high rates of oxygen evolution in polypeptide-depleted Photosystem II preparations. FEBS Lett 167: 127–130 [Google Scholar]
- Hamilton AJ, Baulcombe DC (1999) A species of small antisense RNA in posttranscriptional gene silencing in plants. Science 286: 950–952 [DOI] [PubMed] [Google Scholar]
- Hamilton A, Voinnet O, Chappell L, Baulcombe D (2002) Two classes of short interfering RNA in RNA silencing. EMBO J 21: 4671–4679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankamer B, Morris E, Nield J, Carne A, Barber J (2001) Subunit positioning and transmembrane helix organization in the core dimer of photosystem II. FEBS Lett 504: 142–151 [DOI] [PubMed] [Google Scholar]
- Helliwell C, Waterhouse P (2003) Constructs and methods for high-throughput gene silencing in plants. Methods 30: 289–295 [DOI] [PubMed] [Google Scholar]
- Hillier W, Babcock GT (2001) Photosynthetic reaction centers. Plant Physiol 125: 33–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier W, Wydrzynski T (1993) Increases in peroxide formation by the Photosystem II oxygen evolving reactions upon removal of the extrinsic 16, 22, and 33 kDa proteins are reversed by CaCl2 addition. Photosynth Res 38: 417–423 [DOI] [PubMed] [Google Scholar]
- Homann PH (1988) The chloride and calcium requirement of photosynthetic water oxidation: effects of pH. Biochim Biophys Acta 934: 1–13 [Google Scholar]
- Hua SB, Dube SK, Barnett NM, Kung SD (1992) Photosystem II 23 kDa polypeptide of oxygen-evolving complex is encoded by a multigene family in tobacco. Plant Mol Biol 18: 997–999 [DOI] [PubMed] [Google Scholar]
- Ifuku K, Nakatsu T, Kato H, Sato F (2004) Crystal structure of the PsbP protein of photosystem II from Nicotiana tabacum. EMBO Rep 5: 362–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifuku K, Sato F (2002) A truncated mutant of the extrinsic 23-kDa protein that absolutely requires the extrinsic 17-kDa protein for Ca2+ retention in photosystem II. Plant Cell Physiol 43: 1244–1249 [DOI] [PubMed] [Google Scholar]
- Johnson CH, Knight MR, Kondo T, Masson P, Sedbrook J, Haley A, Trewavas A (1995) Circadian oscillations of cytosolic and chloroplastic free calcium in plants. Science 269: 1863–1865 [DOI] [PubMed] [Google Scholar]
- Kashino Y, Lauber WM, Carroll JA, Wang Q, Whitmarsh J, Satoh K, Pakrasi HB (2002) Proteomic analysis of a highly active photosystem II preparation from the cyanobacterium Synechocystis sp. PCC 6803 reveals the presence of novel polypeptides. Biochemistry 41: 8004–8012 [DOI] [PubMed] [Google Scholar]
- Klimov V, Ananyev G, Zastryzhnaya O, Wydrzynski T, Renger G (1993) Photoproduction of hydrogen peroxide in Photosystem II membrane fragments: a comparison of four signals. Photosynth Res 38: 409–416 [DOI] [PubMed] [Google Scholar]
- Kreimer G, Melkonian M, Latzko E (1988) Stromal free calcium concentration and light mediated activation of chloroplast fructose-1,6-bisphosphatase. Plant Physiol 86: 423–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger A, Weis E (1993) The role of calcium in the pH-dependent control of Photosystem II. Photosynth Res 37: 117–130 [DOI] [PubMed] [Google Scholar]
- Kuwabara T, Suzuki K (1994) A prolyl endoproteinase that acts specifically on the extrinsic 18-kDa protein of photosystem II: purification and further characterization. Plant Cell Physiol 35: 665–675 [DOI] [PubMed] [Google Scholar]
- Martín M, Casano LM, Sabater B (1996) Identification of the product of ndhA gene as a thylakoid protein synthesized in response to photooxidative treatment. Plant Cell Physiol 37: 293–298 [DOI] [PubMed] [Google Scholar]
- Mayfield SP, Rahire M, Frank G, Zuber H, Rochaix JD (1987) Expression of the nuclear encoding oxygen-evolving enhancer 2 is required for high levels of photosynthetic oxygen evolution in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 84: 749–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhara I, Ugaki M, Hirochika H, Ohshima M, Murakami T, Gotoh Y, Katayose Y, Nakamura S, Honkura R, Nishimiya S, et al (1996) Efficient promoter cassettes for enhanced expression of foreign genes in dicotyledonous and monocotyledonous plants. Plant Cell Physiol 37: 49–59 [DOI] [PubMed] [Google Scholar]
- Miyao M, Murata N (1983) Partial disintegration and reconstitution of the photosynthetic oxygen evolution system: binding of 24 kilodalton and 18 kilodalton polypeptides. Biochim Biophys Acta 725: 87–93 [Google Scholar]
- Miyao M, Murata N (1984. a) Role of the 33-kDa polypeptide in preserving Mn in the photosynthetic oxygen-evolving system and its replacement by chloride ions. FEBS Lett 170: 350–354 [Google Scholar]
- Miyao M, Murata N (1984. b) Calcium ions can be substituted for the 24-kDa polypeptide in photosynthetic oxygen evolution. FEBS Lett 168: 118–120 [Google Scholar]
- Miyao M, Murata N (1985) The Cl− effect on photosynthetic oxygen evolution: interaction of Cl− with 18-kDa, 24-kDa and 33-kDa proteins. FEBS Lett 180: 303–308 [Google Scholar]
- Miyao M, Murata N (1989) The mode of binding of three extrinsic proteins of 33 kDa, 23 kDa, and 18 kDa in Photosystem II complex of spinach. Biochim Biophys Acta 977: 315–321 [DOI] [PubMed] [Google Scholar]
- Murakami R, Ifuku K, Takabayashi A, Shikanai T, Endo T, Sato F (2002) Characterization of an Arabidopsis thaliana mutant with impaired psbO, one of two genes encoding extrinsic 33-kDa proteins in photosystem II. FEBS Lett 523: 138–142 [DOI] [PubMed] [Google Scholar]
- Murakami R, Ifuku K, Takabayashi A, Shikanai T, Endo T, Sato F (2005) Functional dissection of two Arabidopsis PsbO proteins PsbO1 and PsbO2. FEBS J 272: 2165–2175 [DOI] [PubMed] [Google Scholar]
- Murata N, Miyao M (1985) Extrinsic membrane proteins in the photosynthetic oxygen-evolving complex. Trends Biochem Sci 10: 122–124 [Google Scholar]
- Nishiyama Y, Yamamoto H, Allakhverdiev SI, Inaba M, Yokota A, Murata N (2001) Oxidative stress inhibits the repair of photodamage to the photosynthetic machinery. EMBO J 20: 5587–5594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono TA, Inoue Y (1986) Effects of removal and reconstitution of the extrinsic 33, 24 and 16 kDa proteins on flash oxygen yield in Photosystem II particles. Biochim Biophys Acta 850: 380–388 [Google Scholar]
- Philbrick JB, Diner BA, Zilinskas BA (1991) Construction and characterization of cyanobacterial mutants lacking the manganese-stabilizing polypeptide of photosystem II. J Biol Chem 266: 13370–13376 [PubMed] [Google Scholar]
- Regel RE, Ivleva NB, Zer H, Meurer J, Shestakov SV, Herrmann RG, Pakrasi HB, Ohad I (2001) Deregulation of electron flow within photosystem II in the absence of the PsbJ protein. J Biol Chem 276: 41473–41478 [DOI] [PubMed] [Google Scholar]
- Rova M, Franzén LG, Fredriksson PO, Styring S (1994) Photosystem II in a mutant of Chlamydomonas reinhardtii lacking the 23 kDa psbP protein shows increased sensitivity to photoinhibition in the absence of chloride. Photosynth Res 39: 75–83 [DOI] [PubMed] [Google Scholar]
- Rutherford AW, Crofts AR, Inoue Y (1982) Thermoluminescence as a probe of photosystem II photochemistry. The origin of the flash-induced glow peaks. Biochim Biophys Acta 682: 457–465 [Google Scholar]
- Schagger H, von Jagow G (1987) Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 166: 368–379 [DOI] [PubMed] [Google Scholar]
- Schreiber U, Klughammer C, Neubauer C (1988) Measuring P700 absorbance changes around 830 nm with a new type of pulse modulation system. Z Naturforsch 43C: 686–698 [Google Scholar]
- Schröder WP, Åkerlund HE (1986) H2O2 accessibility to the Photosystem II donor side in protein-depleted inside-out thylakoids measured as flash-induced oxygen production. Biochim Biophys Acta 848: 359–363 [Google Scholar]
- Schröder WP, Åkerlund HE (1990) Hydrogen peroxide production in photosystem II preparations. In C Baltscheffsky, ed, Current Research in Photosynthesis, Vol 1. Kluwer, Dordrecht, The Netherlands, pp 901–904
- Schuldiner S, Avron M (1971) Anion permeability of chloroplast. Eur J Biochem 19: 227–231 [DOI] [PubMed] [Google Scholar]
- Seidler A (1996) The extrinsic polypeptides of photosystem II. Biochim Biophys Acta 1277: 35–60 [DOI] [PubMed] [Google Scholar]
- Shen JR, Inoue Y (1993) Binding and functional properties of two new extrinsic components, cytochrome c-550 and a 12-kDa protein, in cyanobacterial photosystem II. Biochemistry 32: 1825–1832 [DOI] [PubMed] [Google Scholar]
- Shen JR, Qian M, Inoue Y, Burnap RL (1998) Functional characterization of Synechocystis sp. PCC 6803 delta psbU and delta psbV mutants reveals important roles of cytochrome c-550 in cyanobacterial oxygen evolution. Biochemistry 37: 1551–1558 [DOI] [PubMed] [Google Scholar]
- Summerfield TC, Shand JA, Bentley FK, Eaton-Rye JJ (2005) PsbQ (Sll1638) in Synechocystis sp. PCC 6803 is required for photosystem II activity in specific mutants and in nutrient-limiting conditions. Biochemistry 44: 805–815 [DOI] [PubMed] [Google Scholar]
- Suzuki T, Minagawa J, Tomo T, Sonoike K, Ohta H, Enami I (2003) Binding and functional properties of the extrinsic proteins in oxygen-evolving photosystem II particle from a green alga, Chlamydomonas reinhardtii having his-tagged CP47. Plant Cell Physiol 44: 76–84 [DOI] [PubMed] [Google Scholar]
- Suzuki T, Tada O, Makimura M, Tohri A, Ohta H, Yamamoto Y, Enami I (2004) Isolation and characterization of oxygen-evolving photosystem II complexes retaining the PsbO, P and Q proteins from Euglena gracilis. Plant Cell Physiol 45: 1168–1175 [DOI] [PubMed] [Google Scholar]
- Tamura N, Cheniae G (1987) Photoactivation of the water-oxidizing complex in Photosystem II membranes depleted of Mn and extrinsic proteins. I. Biochemical and kinetic characterization. Biochim Biophys Acta 890: 179–194 [Google Scholar]
- Thornton LE, Ohkawa H, Roose JL, Kashino Y, Keren N, Pakrasi HB (2004) Homologs of plant PsbP and PsbQ proteins are necessary for regulation of photosystem II activity in cyanobacterium Synechocystis 6803. Plant Cell 16: 2164–2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vass I, Inoue Y (1992) Thermoluminescence in the study of photosystem II. In J Barber, ed, The Photosystems: Structure, Function, and Molecular Biology. Elsevier, Amsterdam, pp 259–294
- Vass I, Styring S (1991) pH-dependent charge equilibria between tyrosine-D and the S states in photosystem II. Estimation of relative midpoint redox potentials. Biochemistry 30: 830–839 [DOI] [PubMed] [Google Scholar]
- Walters RG (2005) Towards an understanding of photosynthetic acclimation. J Exp Bot 56: 435–447 [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Ifuku K, Sato F (2005) Suppression of psbP and psbQ genes in Nicotiana tabacum by RNA interference technique. In A Est, D Brues, eds, Photosynthesis: Fundamental Aspects and Global Perspectives, Vol 2. Allen Press, Lawrence, KS, pp 798–799
- Yi X, McChargue M, Laborde S, Frankel LK, Bricker TM (2005) The manganese-stabilizing protein is required for photosystem II assembly/stability and photoautotrophy in higher plants. J Biol Chem 280: 16170–16174 [DOI] [PubMed] [Google Scholar]