Abstract
Stomatal movement is important for plants to exchange gas with environment. The regulation of stomatal movement allows optimizing photosynthesis and transpiration. Changes in vacuolar volume in guard cells are known to participate in this regulation. However, little has been known about the mechanism underlying the regulation of rapid changes in guard cell vacuolar volume. Here, we report that dynamic changes in the complex vacuolar membrane system play a role in the rapid changes of vacuolar volume in Vicia faba guard cells. The guard cells contained a great number of small vacuoles and various vacuolar membrane structures when stomata closed. The small vacuoles and complex membrane systems fused with each other or with the bigger vacuoles to generate large vacuoles during stomatal opening. Conversely, the large vacuoles split into smaller vacuoles and generated many complex membrane structures in the closing stomata. Vacuole fusion inhibitor, (2s,3s)-trans-epoxy-succinyl-l-leucylamido-3-methylbutane ethyl ester, inhibited stomatal opening significantly. Furthermore, an Arabidopsis (Arabidopsis thaliana) mutation of the SGR3 gene, which has a defect in vacuolar fusion, also led to retardation of stomatal opening. All these results suggest that the dynamic changes of the tonoplast are essential for enhancing stomatal movement.
Guard cells can perceive environmental and intracellular signals, such as atmospheric CO2 concentration, light intensity, humidity, auxin, Ca2+, extracellular calmodulin, and abscisic acid (ABA) to regulate the stomatal aperture and gas exchange (Blatt, 2000; Allen et al., 2001; Schroeder et al., 2001; Chen et al., 2004). The regulation of guard cell movement plays an important role in the optimization of photosynthesis and transpiration (Wang and Chen, 2001). Studies have shown that the vacuoles in guard cells are also involved in stomatal movement. Vacuoles are the primary calcium pool in guard cells. Ca2+ channels and transporters in the tonoplast contribute to Ca2+ movement between vacuoles and the cytoplasm. Such Ca2+ movements between the reservoir and the cytoplasm result in the spatiotemporal changes in [Ca2+]cyt, generating [Ca2+]cyt oscillations and [Ca2+]cyt waves that modulate stomatal movement(Allen et al., 2001; Schroeder et al., 2001). Vacuoles also are the largest H+ storage sinks in guard cells. The accumulation of H+ in the vacuoles is dependent on the tonoplast proteins, V-ATPase and H+-PPase (Gaxiola et al., 2002). Studies revealed that cytosolic pH could act as a second messenger in ABA signaling in guard cells (Blatt, 2000). The H+ electrochemical gradient across the tonoplast mediates the movement of other ions, such as K+, Cl−, Suc, or malate between vacuoles and the cytoplasm, resulting in the changes of the water potential in guard cells (Schroeder et al., 2001), which drives water influx and efflux across the tonoplast. Such a process results in the changes of turgor pressure in the guard cells, driving stomatal movement.
During stomatal movement, the volume of the guard cells can change by more than 40% (Shope et al., 2003). The total vacuolar volume of the guard cell also changes rapidly during stomatal movement. The change of vacuole volume has been implicated to have a major contribution to the change of guard cell volume. In Commelina communis, for example, the total vacuolar volume in a guard cell can be changed from 2.5 to 6 picoliters during the period from the closed situation to an open width of 16 μm (Fricker and White, 1990). Changes of guard cell volume are accompanied by the changes of the plasma membrane and tonoplast surface area. In some plants, the plasma membrane and tonoplast surface area of a guard cell can be increased up to 1.5 times the original during the stomatal opening (Blatt, 2002). It is commonly believed that the membrane elasticity does not allow the change of more than 5% surface area (Wolfe and Steponkus, 1983). Therefore, the stretching and shrinkage of the plasma membrane and the tonoplast are insufficient to support the changes of guard cells and vacuole surface area during stomatal movement (Blatt, 2000; Shope et al., 2003). Other complementary mechanisms must also be involved. Recent studies on leaf epidermis and guard cell protoplasts have revealed that endocytotic vacuoles (or vesicles) were formed as the guard cell volume and cell surface area decreased. These small vacuoles (or vesicles) could fuse with the plasma membrane, increasing its surface area as guard cell volumes increase (Diekmann et al., 1993; Homann, 1998; Kubitscheck et al., 2000; Shope et al., 2003). Moreover, scanning electron microscopy also showed that the exocytotic extrusions on the plasma membrane appeared in shrunk protoplasts but disappeared in swelled protoplasts, indicating that these extrusions may be involved in the dynamic change of the surface area in the guard cells (Lambrechts et al., 1992). All these results indicate that another membrane system may serve as a reservoir for the plasma membrane to support the rapid dynamic volume change of guard cells during stomatal movement.
In spite of vacuolar importance to stomatal movement, little has been known about the regulatory mechanism underlying the change of the vacuolar surface area in response to rapid changes of vacuolar volume. There are a great number of small vacuoles in guard cells of the closed stomata, and only a few big ones in the guard cells of the fully opened stomata (Couot-Gastelier et al., 1984). The small vacuoles may fuse with each other to increase vacuolar volume during stomatal opening, implying that these small vacuoles also may be a reservoir of the tonoplast in guard cells. However, it is not an economical process for guard cells to change vacuolar volume rapidly and frequently by such fusion process, because the fusion of vacuoles consumes a huge amount of energy (Jahn et al., 2003). There must be other mechanisms adopted by the vacuoles of guard cells to meet the requirement for the rapid change of their volume. Here we report that the complex vacuolar membrane system may play an important role in the rapid increase of vacuolar volume in the guard cells during stomatal movement in Vicia faba. Small vacuoles fused rapidly with each other to form big ones in V. faba guard cells during stomatal opening. Dynamic changes in vacuolar shapes, which could be spherical, tubular, and vesicle like, were also observed. During stomatal closure, the reverse process, in which large vacuoles split into small vacuoles, could be observed. These results suggested that these complex intravacuolar membrane systems might be directly involved in rapid change of vacuolar volume. We propose that these membrane structures may serve as tonoplast reservoirs to support the rapid changes of vacuolar volume in the guard cells during stomatal movement in V. faba.
RESULTS
The Small Vacuoles Fused with Each Other to Form Bigger Vacuoles in Guard Cells during Stomatal Movement
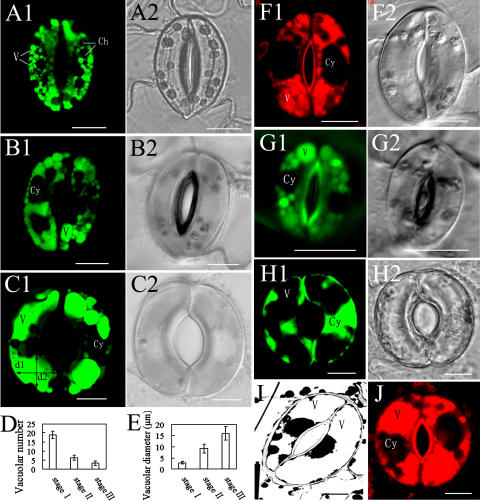
To visualize the vacuoles in guard cells, we stained V. faba guard cells with 10 μm acridine orange (AO) and imaged the vacuoles using confocal laser scanning microscopy (CLSM). As can be seen in Figure 1A, the guard cells contained many small vacuoles when the stomata were closed. They were largely spherical and had a diameter of 1 to 5 μm (Fig. 1A). As stomata opening proceeded, the number of vacuoles decreased in each guard cell, but the size of vacuoles increased accordingly (Fig. 1, A–C).
Figure 1.
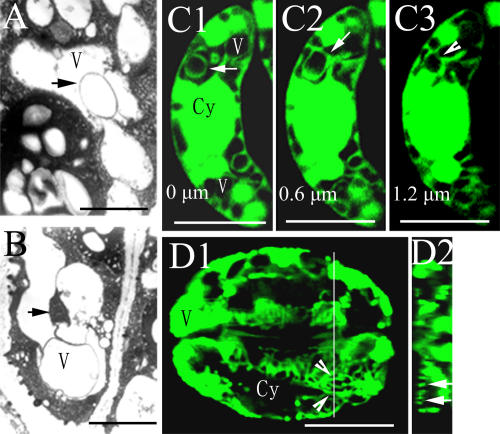
Vacuoles of guard cells at different stomatal apertures. A to C, The changes of vacuolar number and size with different stomatal apertures of V. faba, stained with AO and observed by CLSM. D and E, Quantitative analysis of the average number (D) and diameter (E) of vacuoles in guard cells of V. faba at different stages of stomatal movement (at stage I, stage II, and stage III, the stomatal apertures are <5 μm, 5–8 μm, and >8 μm, respectively). Only the central optical sections of guard cells were used to calculate the average number and diameter of vacuoles. The diameter of a vacuole is the average of d1 and d2, as shown in section C1. And d1 and d2 are the largest value of vacuolar diameter paralleling with the minor axis and major axis of stomatal apparatus, respectively. More than 100 guard cells were counted at each stage. Bars represent the standard errors of three independent experiments. F, Vacuoles of guard cells of V. faba stained with LysoTracker Red DND-99, observed by CLSM. G, The result of AO staining (G1) of Arabidopsis guard cell vacuoles, observed by CLSM. H to J, Only one or two vacuoles in a guard cell of opened stomata. H, Stained with FDA and observed by CLSM; I, observed by TEM; J, stained with LysoTracker Red DND-99 and observed by CLSM. Ch, Chloroplast; Cy, cytoplasma; V, vacuole. Bars = 10 μm in A to C and F to J.
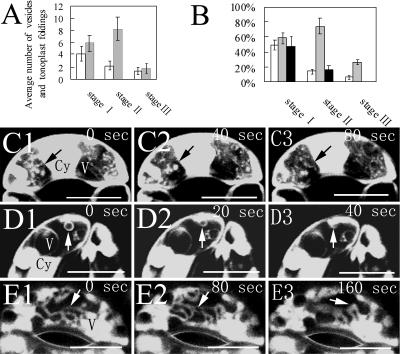
To facilitate the description of the dynamic changes in vacuole number and size during stomatal movement, we arbitrarily divided the movement of stomata into three particular stages according to the size of the stomatal aperture: stage I, <5 μm; stage II, 5 to 8 μm; and stage III, >8 μm. At stage I, on the average, more than 18 vacuoles could be found in each guard cell and the average diameter of the vacuoles was less than 3 μm. However, at stage III, they had less than four vacuoles with an average diameter of more than 15 μm (Fig. 1, D and E).
To confirm AO labeling of vacuoles, we also used LysoTracker Red DND-99 (an acidic organelle-selective cell-permeable probe; Molecular Probe) to stain the vacuoles of guard cells of V. faba, and similar results were obtained (Fig. 1F). In addition, AO staining pattern (Fig. 1, G1) of Arabidopsis (Arabidopsis thaliana) guard cell vacuoles was similar to that of V. faba, suggesting that AO can be used to label guard cell vacuoles in Arabidopsis. Fluorescein diacetate (FDA) can be deesterified by esterase in the cytoplasm, giving rise to the polar fluorescein that can't enter vacuoles (Fricker et al., 2001). Thus, FDA, like N-(3-triethylammoniumpropyl)-4-(6-(4-(diethylamino) phenyl) hexatrienyl) pyridinium dibromide, a tonoplast-specific probe, also can define the contour of vacuoles (Mathur et al., 2003). We also used FDA to label the vacuoles in guard cells in V. faba. In the opened stomata, each guard cell usually contained only a few big vacuoles, and the central-section image showed that the cytoplasm of the guard cell was pressed to the cell wall or nucleus (Fig. 1H). These results were consistent with those from AO labeling (Fig. 1C), transmission electron microscopy (TEM; Fig. 1I), and LysoTracker dyeing (Fig. 1J), verifying that FDA can be used to label the vacuoles of guard cells.
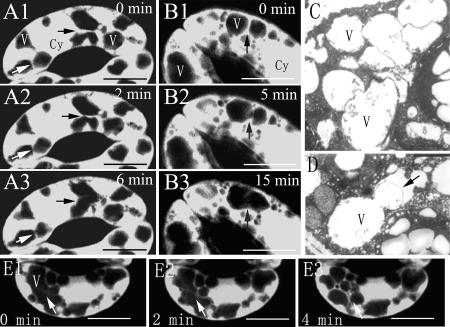
To understand the mechanism underlying the changes of vacuole numbers and sizes, we examined the motility of vacuoles and tonoplasts during stomatal movement by monitoring their structural changes using time-lapse imaging of FDA-stained cells. As shown in Figure 2, A and B, individual small vacuoles fused into bigger vacuoles during stage I of stomatal opening. Among the 52 observed V. faba guard cells at this stage, 88% of the cells showed the typical changes. In the opening stomata, the fusion of small vacuoles with each other (Fig. 2C) and the tonoplast remnants in the fusing vacuole lumen (Fig. 2D) also could be observed in TEM images. To test whether the dynamic changes in vacuoles are reversible, we investigated the change of vacuoles in the guard cells in closing stomata. Indeed, large vacuoles did split into many small vacuoles during stomatal closing induced by 10 μm ABA in V. faba (Fig. 2E). Thirty-six out of 44 guard cells showed the foregoing changes treated with ABA.
Figure 2.
Fusion of small vacuoles during stomatal opening of V. faba. A and B, The fusion time-lapse images of two separated vacuoles (arrows) with stomatal opening under light, stained with FDA and observed by CLSM. Images were collected at the indicated time point. A1 to A3 were the central optical sections of stomata, and B1 to B3 were not. C, Fusing small vacuoles, observed by TEM. D, Tonoplast remnant in the lumen of two fusing vacuoles; observed by TEM. E, A big vacuole (E1, arrow) became some small vacuoles (E2 and E3, arrows) as the stomatal closing was treated with ABA, stained with FDA, and observed by CLSM. Images were collected at the indicated time point. Cy, Cytoplasma; V, vacuole. Bars = 10 μm in A, B, and E and 2 μm in C and D.
Fusion of Small Vacuoles Was Correlated to Stomatal Opening
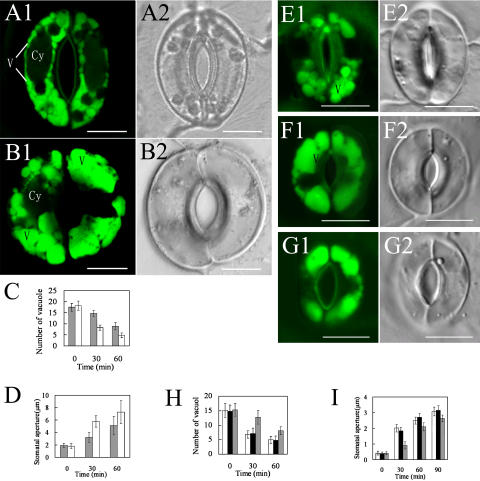
We also investigated whether the dynamic changes in vacuole morphology and size are required for stomatal movement using chemicals to inhibit vacuole fusion or mutation that is defective. To study the correlation between vacuole development and stomatal movement, first we used a membrane-permeable Cys protease inhibitor, (2s,3s)-trans-epoxy-succinyl-l-leucylamido-3-methylbutane ethyl ester (E-64d). Application of E-64d to the root cells of barley (Hordeum vulgare) caused the accumulation of starvation-induced autolysosomes (Moriyasu et al., 2003) or N-(3-triethylammoniumpropyl)-4-(6-(4-(diethylamino) phenyl) hexatrienyl) pyridinium dibromide-stainable small vesicles surrounding the nucleus (Yamada et al., 2003) in cultured tobacco (Nicotiana tabacum) Bright Yellow-2 (BY-2) suspension cells in a Suc-free condition. These results showed that E-64d could inhibit the fusion of endosomes with vacuoles (Yamada et al., 2003, 2005). In this study, we treated the epidermis peels of V. faba leaf for 2 h with 100 μm E-64d and then induced stomatal opening under light. The result clearly showed that the number of vacuoles in the treated guard cells was more than that in the untreated control epidermis peels (Fig. 3, A–C). Furthermore, the stomatal opening in the treated lower epidermis was slower than that in the untreated control (Fig. 3D). Among 158 V. faba guard cells, 73% of the cells showed the typical E-64d response.
Figure 3.
Fusion of small vacuoles is required for stomatal opening. A and B, Vacuoles in guard cells of V. faba present differences in number and size, with (A) or without (B) 100 μm E-64d pretreatment for 2 h, respectively, and induced 45 min under light, stained with AO, and observed by CLSM. C, The difference of stomatal opening of V. faba induced by light with 100 μm E-64d treatment for 2 h (black bar) and without 100 μm E-64d pretreatment (white bar). More than 150 stomata were counted at each stage. Error Bars represent the standard errors of three independent experiments. D, The difference of stomatal aperture of V. faba induced by cold light with 100 μm E-64d treatment (black bar) for 2 h and without 100 μm E-64d pretreatment (white bar). More than 150 stomata were counted at each stage. Error bars represent the standard errors of three independent experiments. E to G, Vacuoles in guard cells present differences in number and size of Arabidopsis mutant sgr3-1 (E), wild type (F), and complementary line sgr3-1/gSGR3 (G), after the stomata was induced 30 min under light, stained with AO, and observed by CLSM. H, The difference of vacuolar number in guard cells between Arabidopsis wild type (white bar), complementary line sgr3-1/gSGR3 (black bar), and mutant sgr3-1 (gray bar), after the stomata induced 30 min under light. More than 150 stomata were counted at each stage. Error bars represent the standard errors of three independent experiments. I, The difference of stomatal aperture between Arabidopsis wild type (white bar), complementary line sgr3-1/gSGR3 (black bar), and mutant sgr3-1 (gray bar), induced by light. More than 150 stomata were counted at each stage. Error bars represent the standard errors of three independent experiments. Cy, Cytoplasma; V, vacuole. Bars = 10 μm in A, B, and E to G.
To further confirm the above finding, we used an Arabidopsis mutant, sgr3-1, to study the correlation between vacuole fusion and stomatal movement. The Arabidopsis SGR3 gene encodes a syntaxin, SYP22 (syntaxin of plants 22), also called AtVAM3, which is localized in the prevacuole and the tonoplast (Uemura et al., 2002; Rojo et al., 2003; Carter et al., 2004). It is commonly believed that SGR3 protein plays an important role in transporting vesicles to vacuoles as well as the vacuolar fusion (Sato et al., 1997; Sanderfoot et al., 1999; Surpin and Raikhel, 2004). We found a significant reduction in the fusion of vacuoles in the guard cells of sgr3-1 (Fig. 3, E–H). The stomatal opening under light in sgr3-1 was slower compared to that in the wild type and the complementary line sgr3-1/gSGR3, especially obvious at the early stage of stomatal opening (Fig. 3I).This result further indicates that vacuolar fusion is involved in the stomatal movement.
Dynamic Tonoplast Structures in Guard Cells during Stomatal Movement of V. faba
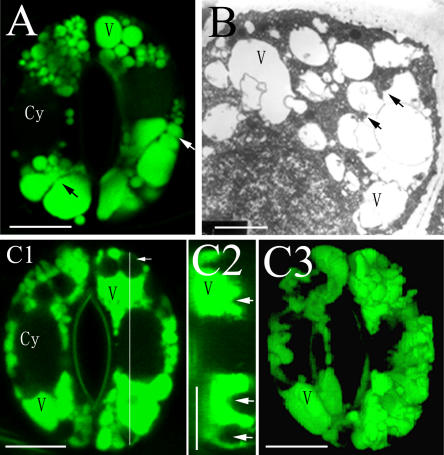
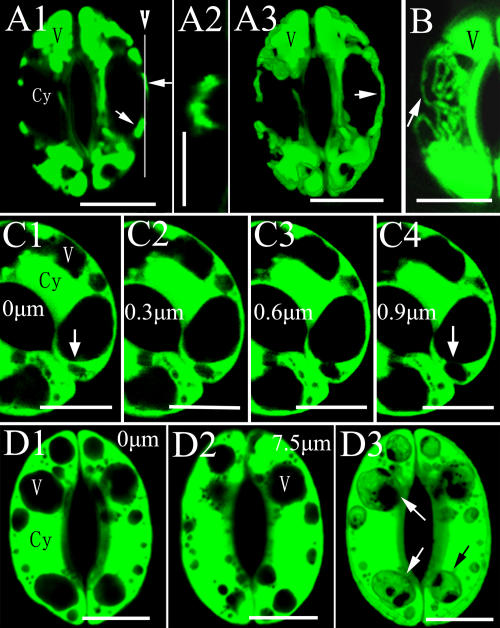
To further understand the structural basis for the dynamic changes in vacuolar morphology during stomatal movement, we next investigated the changes of vacuolar structure during stomatal opening. In the guard cells at stage I of stomatal opening, the small vacuoles with the diameters of 1 to 3 μm were spherical. In contrast, those with a diameter of more than 4 μm tended to be irregular (Fig. 4A). The guard cells also contained many complex membrane structures in their lumen, for example, foldings of the tonoplast (Fig. 4A). TEM images showed that the foldings of the tonoplast resulted from the invagination of the cytoplasm into the vacuolar lumen (Fig. 4B), generating ripple-like fringe in the vacuole (Fig. 4, C1 and C2). To visualize the spatial configuration of vacuoles in a readily accessible image, the three-dimensional (3D) projection was performed from a series of Z-axis optical section by CLSM. As shown in a 3D projection image of the guard cells in Figure 4, C1, the surface of globular vacuole was not smooth, but wavy (Fig. 4, C3).
Figure 4.
Foldings of tonoplasts in guard cells of V. faba. A and B, Foldings of tonoplasts (arrows). C, Wavy surface of vacuoles. C2, The cross section of a guard cell in section C1 through the line (arrow); C3, 3D projection image of the guard cells in section C1. A and C, Stained with AO and observed by CLSM. B, Observed by TEM. Cy, Cytoplasma; V, vacuole. Bars = 10 μm in A and C and 2 μm in B.
Many vesicle-like structures (named as bulb by Saito et al. [2002]) with diameters of 0.5 to 1.5 μm were found in spherical vacuole lumen (Fig. 5, A and B), bound with the single or double layer membrane. To study the full-view structure of the vesicle-like structures, we performed Z-axis optical sections at different foci on the guard cells stained with FDA. We found that a close, vesicle-like structure in vacuolar lumen (0 μm) was open at the 1.2-μm optical section (Fig. 5C). This result implied that these vesicle-like structures might come from the coalescence of tonoplast foldings. In addition, a guard cell also had tubular vacuolar membranes (TVMs) with the diameters of 0.5 to 1.5 μm in not fully opened stomata (Fig. 5D). Most of the TVMs were located at the central part of the guard cells. However, TVMs were not observed in the TEM images. The possible reason might be that the TVM structures had been destroyed by the conventional chemical fixation (Ashford and Allaway, 2002).
Figure 5.
Vesicle-like structures in vacuolar lumens and TVMs in guard cells of V. faba. A and B, Vesicles in vacuoles lumens (arrows), observed by TEM. C, A vesicle-like structure observed at different optical sections has a different morphology, stained with FDA and observed by CLSM. D, TVMs. D2 is the cross section of the guard cells in section D1 through the white line, showing the transection of TVMs (arrowheads) in section D1 is round (arrows), stained with AO, and observed by CLSM. Cy, Cytoplasma; V, vacuole. Bars = 2 μm in A and B and 10 μm in C and D.
The number of tonoplast foldings and vesicle-like structures in guard cells varied in different stages of stomatal aperture. As shown in Figure 6A, the average number of vesicles observed in the central optical section of a guard cell decreased from 4.1 to 1.3 when stomatal aperture proceeded from stage I to stage III. The largest average number of foldings was recorded at stage II, which then decreased as stomatal aperture proceeded to stage III. The statistical results showed a strong correlation between the emergence of tonoplast structures in guard cells and stomatal aperture (Fig. 6B). The proportion of guard cells containing vesicles in vacuolar lumen decreased from 49.3% to 6.7% when stomatal aperture developed from stage I to stage III. The guard cells with foldings were counted as more than 50% of the total guard cells at stage I and stage II. However, they occupied only 26.7% of the total guard cells at stage III. The percentage of guard cells with TVMs also decreased as the stomatal opening proceeded. When the stomata opened wider than 8 μm, the TVMs in guard cells disappeared.
Figure 6.
Dynamics of tonoplast structures during stomatal movement of V. faba. A, The number of tonoplast vesicle-like structures (white bars) and foldings (gray bars) in vacuolar lumen in guard cells changed at different stages of stomatal movement (at stage I, stage II, and stage III, the stomatal apertures are <5 μm, 5–8 μm, and >8μm, respectively). Only the central optical section of a guard cell is used to count the number of foldings and vesicles. More than 180 guard cells were counted at each stage. Scale bars represent the standard errors of three independent experiments. B, The proportion of guard cells with vesicles (white bars), foldings of tonoplasts (gray bars), and TVMs (black bars) at different stages of stomatal movement (at stage I, stage II, and stage III, the stomatal apertures are <5 μm, 5–8 μm, and >8μm, respectively). More than 180 guard cells were counted at each stage. Scale bars represent the standard errors of three independent experiments. C to E, The dynamics of foldings of tonoplasts (C, arrows), vesicle-like structures (D, arrows), and TVMs (E, arrows) during stomatal opening. Time-lapse images were collected at the indicated time points. The arrows indicate moving structures. C and D, Stained with FDA and observed by CLSM. E, Stained with AO and observed by CLSM. Cy, Cytoplasma; V, vacuole. Bars=10 μm in C to E.
To find the disappearance process of these tonoplast structures, we captured a series of time-lapse CLSM images during stomatal opening. The results showed that the foldings of the tonoplast disappeared, possibly due to the fact that they reverted to the tonoplast as stomatal opening proceeded (Fig. 6C). The vesicle-like structures probably fused with outer tonoplasts during stomatal opening and disappeared (Fig. 6D). The TVMs were converted to spherical vacuoles with the increase of stomatal aperture (Fig. 6E). These tonoplast structures were observed again in the vacuolar lumen of guard cells when stomata were closing.
Vacuoles Formed a Continuum in the Same Guard Cell of V. faba
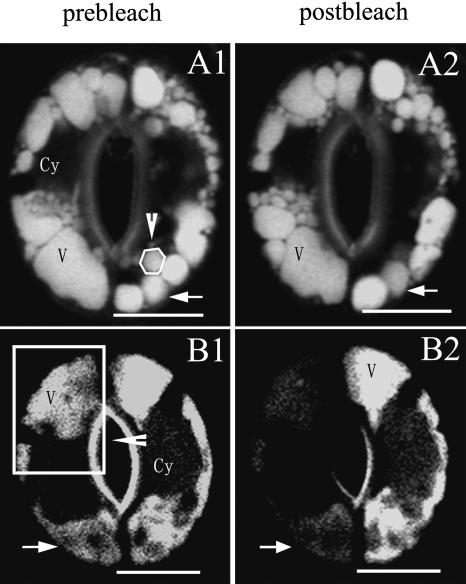
To examine the spatial relationship among vacuoles in a whole guard cell, a 3D projection for a series of Z-axis optical sections of stomata of V. faba at stages II and III was created. Lumens of two separated vacuoles in an optical section of Z-axis were interconnected in the vertical optical section (Fig. 7, A2). This finding suggested that the lumens of these two vacuoles might be connected with each other. As shown in Figure 7, A3, a projection image of the stoma in Figure 7, A1, a canal-like structure connected to the vacuoles at the two poles of a guard cell. In a single optical section, TVMs also were found linked to the spherical vacuoles (Fig. 7B). From a series of optical sections at different foci of a stomata stained with FDA, we found two separated small vacuoles at the 0-μm section (Fig. 7, C1) were connected at the 0.9-μm section (Fig. 7, C4). The projection image of another stoma stained with FDA (Fig. 7, D3) showed that the lumens of some vacuoles were interconnected, although they were found separated in the single optical section (Fig. 7D, 1 and 2). To gain further insight into the structural relationship between vacuolar lumens in a guard cell, photobleaching was performed with the strongest excitation light. As shown in Figure 8A, the fluorescence intensities of both a photobleached vacuole and a nearby vacuole were decreased after bleaching, indicating that the lumens of these two neighboring vacuoles were connected with each other. In another stoma, when the vacuoles at one end of the guard cell were bleached, the AO fading was observed in the vacuole lumen at the other end of the guard cell (Fig. 8B). These results indicated that TVMs might bridge the lumens of these vacuoles. We defined more than 20% of changes in fluorescence intensity of the nearby vacuole between pre- and after bleach as fluorescence intensity decreases. Among the 35 V. faba guard cells for this experiment, 69% of the cells showed the typical fluorescence intensity changes. Taken all together, we suggest that the lumens of different vacuoles in the same guard cell were interconnected, and their saps could flow freely.
Figure 7.
Vacuoles are interconnected in a guard cell of V. faba. A1, Two separated vacuoles (arrows). A2, A cross section image of section A1 through the white line (arrowhead), showing two separated vacuoles in section A1 are contacted. A3, The 3D projection image of the stoma in section A1, stained with AO and observed by CLSM. B, TVMs (arrow) link spherical vacuoles, stained with AO and observed by CLSM. C, Four optical sections of a stoma at different focus, showing two vacuoles are connected (arrows), stained with FDA and observed by CLSM. D, 3D projection showing interconnection of small vacuoles (arrows) in a guard cell. D1 and D2, Two optical sections of a stoma at different foci. D3, 3D projection image of the stoma in sections C1 and C2, stained with FDA and observed by CLSM. Cy, Cytoplasma; V, vacuole. Bars = 10 μm.
Figure 8.
Vacuolar lumens are interconnected in a guard cell of V. faba. The photobleaching was performed with the vacuoles stained with AO bleached 50 to 100 times with strong excitation light of 488 nm under the control of LSM 510 Meta (Zeiss). Bleaching was done in the circle (in A, arrowhead) or square (in B, arrowhead). The fluorescence intensities of neighboring vacuoles (arrows) decreased after bleaching. Cy, Cytoplasma; V, vacuole. Bars = 10 μm.
DISCUSSION
Fusion of Small Vacuoles Is Required for Stomatal Opening
In this study, we reported that the fusion of small vacuoles to form big vacuoles contributed to the increase of vacuolar volume in guard cells during stomatal opening. Inhibiting the fusion of small vacuoles by the inhibitor and the mutation of vesicle fusion gene inhibited not only the increase of vacuolar volume, but also the stomatal aperture in V. faba and Arabidopsis. This result indicates that the fusion of small vacuoles to form a bigger vacuole in the guard cell is required for the stomatal opening.
However, the mechanism for vacuolar fusion is still not clear. One possibility is that the uptaking of water results in the increase of vacuolar sizes that drives the two neighboring vacuoles to contact physically and fuse with each other passively. Another possibility is that there may be a regulatory mechanism that activates the fusion of the small vacuoles into the big vacuoles in the guard cells during the stomatal opening. The Arabidopsis SGR3 protein was involved in transporting vesicles to vacuoles as well as the vacuolar fusion (Sato et al., 1997; Sanderfoot et al., 1999; Carter et al., 2004). The sgr3-1 mutant produced abnormal vacuolar structure and had a reduced gravitropic response in the inflorescence stem (Yano et al., 2003), which implied that the mutation of SGR3 affected the morphology and function of vacuoles. The mutation of the SGR3 gene caused the fusion of vacuoles to be restrained in guard cells, resulting in the stomatal opening in sgr3-1 being much slower than that in the sgr3-1/gSGR3 and wild type (Fig. 3D). These results show that the fusion of vacuoles is partially, if not completely, regulated by signaling of guard cells. For example, the results of our group suggested that extracellular calmodulin and Ca2+ signals of guard cells mediated stomatal movement (Chen et al., 2004). Furthermore, in yeast (Saccharomyces cerevisiae), Ca2+/calmodulin signals were involved in vacuole fusion (Peters and Mayer, 1998).
As the number of vacuoles decreased, the volume per vacuole increased accordingly during stomatal opening. Only a few big vacuoles were found in each guard cell of the fully opened stomata. However, it is unclear why the change of cell volume required big vacuoles instead of multiple small vacuoles in V. faba. The possible explanation is that a big vacuole has a larger total volume than many small vacuoles when they have the same surface area. Therefore, the big vacuole is good for taking up more water than multiple small vacuoles under the condition of an unchangeable amount of tonoplasts. Alternatively, the formation of the big vacuoles via the fusion of small vacuoles may be also regulated by the physical and spatial signals. Nevertheless, our study clearly shows a strong correlation of the big vacuole formation to the stomatal opening. Taken all together, we conclude that the fusion of small vacuoles to form big vacuoles is required for the stomatal opening. The process may be regulated by the physical and cellular signals in guard cells.
Complex Vacuolar Membrane Structures in Guard Cells May Serve as a Reservoir of Tonoplasts
It is usually believed that the plant vacuole is a large compartment with a smooth surface. However, several recent studies have revealed that it might not be true. Using the technologies of cryofixed and freeze-substituted fluorescence dye labeling, transgenic plants expressing green fluorescent protein combined with CLSM and 3D reconstruction, several research groups have found that the tonoplasts of plant cells actually have many complex and intricate structures, such as foldings of tonoplasts (Cutler et al., 2000; Yamamoto et al., 2003), bulb-like or barrel-like structures in vacuolar lumen (Saito et al., 2002; Uemura et al., 2002), wavy surface of vacuoles (Verbelen and Tao, 1998), and so on. We also found the wavy-vacuolar surface, tonoplast foldings, and vesicle-like structures in the vacuolar lumen in the guard cell of V. faba; and they were all dynamic structures during stomatal movement. Uemura et al. (2002) found that the vacuoles of root epidermal cells and Arabidopsis callus cells had the complex intravacuolar membrane structures in the hyperosmotic solution, but these structures were rarely seen in the hypo-osmotic condition using the expressed green fluorescent protein-AtVAM3P fusion protein as the reporter. They conjectured that the intravacuolar membranes might serve as a tonoplast reservoir and function in controlling the vacuolar surface area against various osmotic pressures. It has been reported that the bulb, another intravacuolar membrane structure found in adaxial epidermal cells of Arabidopsis young cotyledons, might serve as a reservoir of tonoplast reserved for the quick expansion of vacuoles, as they were very prominent in rapidly expanding epidermal cells and decreased in the late stage of cotyledon development (Saito et al., 2002). In V. faba, we found that the amount of foldings, vesicle-like structures, and TVMs in the guard cell changed with the changes of vacuolar volume during stomatal movement. Moreover, the transformation between the tonoplast and intravacuolar membrane structures was also observed during stomatal movement. Therefore, our study suggests that the intravacuolar membrane structures in guard cells may also serve as tonoplast reservoirs, which are reserved for the changes of vacuolar volume during stomatal movement in V. faba.
TVMs, another type of vacuolar structure found in many plant tissues, also appeared in the guard cells in V. faba. They formed in the guard cells of the opening stomata and disappeared in the fully opened stomata. As TVMs have been found to transform to spherical vacuoles during stomatal opening, we conjectured that TVMs in guard cells may also serve as a reservoir of tonoplasts for the increase of vacuolar volume. Many studies support this hypothesis. TVMs also were found in many dividing or growing cells, such as the cultured evacuolated oat (Avena sativa) mesophyll protoplasts (Newell et al., 1998), tobacco mitotic BY-2 cells (Kutsuna and Hasezawa, 2002; Kutsuna et al., 2003), germinating Arabidopsis pollen (Hicks et al., 2004), and dividing Allium mother guard cells (Palevitz and Okane, 1981). As the plant cell enlargement after cytokinesis was mainly from the expansion of vacuolar volume, TVMs in these cells might also take a role in such processes by serving as a reservoir of tonoplasts for the enlargement of vacuolar volume.
Vacuolar Interconnection in Guard Cells Also Is Important for Stomatal Movement
Our results showed that vacuoles in guard cells were interconnected and the spherical vacuoles were linked together with TVMs in V. faba. The photobleaching further confirmed that the vacuoles in the guard cells formed a continuum in V. faba. Several studies have shown that membrane linkage among the cells or organelles is important for cell signaling and cell function. Plasmodesmata, plasma-membrane-lined tubule-like channels, mediated the direct cell-to-cell communication in plants by facilitating the direct intercellular transport (Lucas and Lee, 2004). Stromules, tubule-like interconnections between chloroplasts, were involved in the exchanging of molecules (Kwok and Hanson, 2004) and cooperation between different plastids (Waters et al., 2004). In secretory trichomes of chickpea (Cicer arietinum; Lazzaro and Thomson, 1996) and dividing tobacco BY-2 cells (Kutsuna et al., 2003), TVMs are important for the solutes to diffuse freely among different parts. The TVMs in guard cells of V. faba appeared as the vacuolar-tubular continuum in the tobacco BY-2 cells (Kutsuna et al., 2003). Vacuolar continuum in guard cells of V. faba might function in stomatal movement via facilitating the diffusion of solutes and water among the different parts of guard cells to maintain the balance of osmotic potential and turgor pressure in the whole guard cell.
The changes of vacuolar morphology also were observed in the moving motor cell of Mimosa pudica pulvini, which contained two kinds of vacuoles, tannin vacuoles and aqueous vacuoles. When pulvini motor cells shrunk, the tannin vacuoles formed many connected tubules and the aqueous vacuoles formed many invaginations (Fleurat-Lessard et al., 1997). The fusion of many unattached vacuoles consumes much more energy (Jahn et al., 2003). Therefore, foldings of tonoplasts, vesicles, and TVMs, which serve as a tonoplast reservoir, not only avoid the fusion of small vacuoles during the increase of vacuolar volume, but also keep the integrity of the tonoplast during cell movement.
In conclusion, the fusion of small vacuoles to form big vacuoles in guard cells is important for the stomatal opening. However, it is not the only mechanism that is involved in the rapid increase of vacuolar volume during the stomatal opening. The complex tonoplast structures may also serve as a reservoir of tonoplast, which plays a role in the rapid change of vacuolar volume in the guard cells during the stomatal opening.
MATERIALS AND METHODS
Plant Materials
The plants of Vicia faba were grown in a growth chamber with 12 h of light and 12 h of darkness, with a photon flux density of 300 μmol m−2 s−1, and day and night temperature cycles of 25°C ± 2°C and 20°C ± 2°C, respectively. The fully extended leaves at the apex of V. faba plants were collected from 3- to 4-week-old plants for the analyses. The plants of Arabidopsis (Arabidopsis thaliana) wild type (Colombia ecotype), AtVAM3 mutant, sgr3-1, and it's complementary line sgr3-1/gSGR3 (which was sgr3-1 transformed with a 4-kb genomic fragment of SGR3) were grown in a growth chamber with 12 h of light and 12 h of darkness, with a photon flux density of 300 μmol m−2 s−1, at 22°C ± 2°C. Fully expanded rosette leaves of 4- to 6-week-old Arabidopsis plants were used for epidermal strip bioassay.
Epidermal Strip Bioassay
The epidermic strips from V. faba or Arabidopsis were peeled from the lower epidermis of leaves and cleaned up by gently brushing away the mesophyll cells. The cleaned strips were submerged in 10 mm MES (pH 6.1) and treated with the following procedures: For studying the vacuolar dynamic during stomatal movement, the closed stomata were induced to open under a halogen cold-light source (Colo-Parmer), and the opened stomata were induced to close in 10 μm ABA. For studying the effects of E-64d on stomatal opening of V. faba, the strips with closed stomata were transferred to 10 mm MES (pH 6.1) buffer with or without 100 μm E-64d (Sigma) diluted from 100 mm stock in methanol for 1 h, then transferred into 10 mm MES (pH 6.1) with 50 mm KCl to induce stomatal opening under a halogen cold-light source (Colo-Parmer). For studying the stomatal opening difference between sgr3-1, sgr3-1/gSGR3, and wild type of Arabidopsis, the strips with closed stomata were transferred to 10 mm MES (pH 6.1) buffer with 50 mm KCl to induce stomatal opening under a halogen cold-light source. Stomatal apertures were measured under a microscope at indicated times with more than 50 randomly selected stomata. Each assay was repeated three times.
TEM
Leaves of V. faba with different stomatal apertures were cut into small pieces about 1 mm2, then prefixed in the fixation solution containing 4% (v/v) glutaraldehyde and 1% (w/v) tannic (Komis et al., 2002) for 3 to 4 h at room temperature. The prefixed leaves were washed with 0.1 m phosphate-buffered saline buffer (pH 7.2), then further fixed in a postfixation solution containing 1% osmic acid (Sigma) at room temperature for 4 h, and finally dehydrated with a gradient of ethanol solutions. The specimens were osmosed and embedded in Spurr. Ultrathin sections were performed using an ultramicrotome and were stained with acetate uranium and lead citrate. The specimens were observed in TEM.
Fluorescent Dye Loading
For vacuole fluorescent staining, the lower epidermal strips of V. faba and Arabidopsis leaves were dipped into 10 μm AO (Fluka; in MES buffer, pH 6.1) for 10 to 15 min, 1 μm FDA (Sigma; in MES buffer, pH 6.1) 5 to 10 min as described by Mathur et al. (2003), or 100 nm LysoTracker Red DND-99 (Molecular Probes) for 40 to 60 min at room temperature in dark, then washed three times with MES (pH 6.1) buffer.
CLSM and Images Process
The epidermal strips stained with AO or FDA were observed under MRC 1024 CLSM (Bio-Rad) or LSM 510 CLSM (Zeiss) equipped with an argon ion laser as the excitation source. With an excitation laser of 488 nm, a series of images were captured with a 505 to 550 nm band-pass filter. The epidermal strips stained with LysoTracker were observed under LSM 510 CLSM (Zeiss) equipped with a Helium/Neon laser as the excitation source. With an excitation laser of 543 nm, images were captured with a 560- to 615-nm band-pass filter. To register the dynamic of vacuoles in guard cells during stomatal movement, the epidermal strips preloaded with AO or FDA were treated for 10 to 15 min with 1 mg L−1 fusicoccin (Sigma) for stomatal opening or 10 μm ABA (Sigma) for stomatal closing, then observed under CLSM. Fusicoccin or ABA also was added directly to the buffer in which the strips were placed. The photobleaching was performed with the vacuoles stained with AO bleached 50 to 100 times with strong excitation light of 488 nm under the control of LSM 510 Meta (Zeiss). The images of prebleach and postbleach were collected. 3D projections were obtained from a series of 0.2 to 0.5 μm interval continuous optical sections with LSM 510 Meta (Zeiss). All images were processed using Confocal Assistant version 4.02 (Bio-Rad) or LSM 510 image browser version 3.0 (Zeiss) and were exported as TIFF files. The optical sections of X- or Y-axis were obtained from a series of Z-axis optical sections at different foci, using the ORTHO function of LSM 5 image browser version 3.0. Adobe Photoshop 6.0 was used for further processing of all the images.
Acknowledgments
We thank Dr. De Ye (China Agricultural University) and Dr. Zhenbiao Yang (University of California, Riverside) for their helpful discussion and critical reading of the manuscript. We also thank Dr. Masao Tasaka and Dr. Miyo T. Morita (Nara Institute of Science and Technology, Japan) for the generous gift of Arabidopsis seeds of sgr3-1 and sgr3-1/gSGR3.
This work was supported by the National Basic Research Program of China (grant nos. 2006CB100100 and 2003CB114300) and the National Science Foundation of China (grant nos. 30370129 and 30421002).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Xue-Chen Wang (xcwang@cau.edu.cn).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.067520.
References
- Allen GJ, Chu SP, Harrington CL, Schumacher K, Hoffmann T, Tang YY, Grill E, Schroeder JI (2001) A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature 411: 1053–1057 [DOI] [PubMed] [Google Scholar]
- Ashford AE, Allaway WG (2002) The role of the motile tubular vacuole system in mycorrhizal fungi. Plant Soil 244: 177–187 [Google Scholar]
- Blatt MR (2000) Cellular signaling and volume control in stomatal movements in plants. Annu Rev Cell Dev Biol 16: 221–241 [DOI] [PubMed] [Google Scholar]
- Blatt MR (2002) Toward understanding vesicle traffic and the guard cell model. New Phytol 153: 405–413 [DOI] [PubMed] [Google Scholar]
- Carter C, Pan S, Zouhar J, Avila E, Girke T, Raikhel N (2004) The vegetative vacuole proteome of Arabidopsis thaliana reveals predicted and unexpected proteins. Plant Cell 16: 3285–3303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YL, Huang R, Xiao YM, Lü P, Chen J, Wang XC (2004) Extracellular calmodulin-induced stomatal closure is mediated by heterotrimeric G protein and H2O2. Plant Physiol 136: 4096–4103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couot-Gastelier J, Laffray D, Louguet P (1984) Etude comparee de 1′utrastructure des stomates ouverts et fermes chez le Tradescantia viragimiana. Can J Bot 62: 1505–1512 [Google Scholar]
- Cutler SR, Ehrhardt DW, Griffitts JS, Somerville CR (2000) Random GFP∷cDNA fusions enable visualization of subcellular structures in cells of Arabidopsis at a high frequency. Proc Natl Acad Sci USA 97: 3718–3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekmann W, Hedrich R, Raschke K, Robinson DG (1993) Osmocytosis and vacuolar fragmentation in guard cell protoplasts: their relevance to osmotically-induced volume changes in guard cells. J Exp Bot 44: 1569–1577 [Google Scholar]
- Fleurat-Lessard P, Frangne N, Maeshima M, Ratajczak R, Bonnemain JL, Martinoia E (1997) Increased expression of vacuolar aquaporin and H+-ATPase related to motor cell function in Mimosa pudica L. Plant Physiol 114: 827–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker M, Parsons A, Tlalka M, Blancaflor E, Gilroy S, Meyer A, Plieth C (2001) Fluorescent probes for living plant cells. In C Hawes and B Satiat-Jeunemaitre, eds, Plant Cell Biology. Oxford University Press, New York, pp 35–84
- Fricker MD, White N (1990) Volume measurements of guard cell vacuoles during stomatal movements using confocal microscopy. Trans Roy Microsc Soc 1: 345–348 [Google Scholar]
- Gaxiola RA, Fink GR, Hirschi KD (2002) Genetic manipulation of vacuolar proton pumps and transporters. Plant Physiol 129: 967–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks GR, Rojo E, Hong S, Carter DG, Raikhel NV (2004) Germinating pollen has tubular vacuoles, displays highly dynamic vacuole biogenesis, and requires VACUOLESS1 for proper function. Plant Physiol 134: 1227–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homann U (1998) Fusion and fission of plasma-membrane material accommodates for osmotically induced changes in the surface area of guard-cell protoplasts. Planta 206: 329–333 [Google Scholar]
- Jahn R, Lang T, Sudhof TC (2003) Membrane fusion. Cell 112: 519–533 [DOI] [PubMed] [Google Scholar]
- Komis G, Apostolakos P, Galatis B (2002) Hyperosmotic stress induces formation of tubulin macrotubules in root-tip cells of Triticum turgidum: their probable involvement in protoplast volume control. Plant Cell Physiol 43: 911–922 [DOI] [PubMed] [Google Scholar]
- Kubitscheck U, Homann U, Thiel G (2000) Osmotically evoked shrinking of guard-cell protoplasts causes vesicular retrieval of plasma membrane into the cytoplasm. Planta 210: 423–431 [DOI] [PubMed] [Google Scholar]
- Kutsuna N, Hasezawa S (2002) Dynamic organization of vacuole and microtubule structures during cell cycle progression in synchronized tobacco BY-2 cells. Plant Cell Physiol 43: 965–973 [DOI] [PubMed] [Google Scholar]
- Kutsuna N, Kumagai F, Sato MH, Hasezawa S (2003) Three-dimensional reconstruction of tubular structure of vacuolar membrane throughout mitosis in living tobacco cells. Plant Cell Physiol 44: 1045–1054 [DOI] [PubMed] [Google Scholar]
- Kwok EY, Hanson MR (2004) GFP-labelled rubisco and aspartate aminotransferase are present in plastid stromules and traffic between plastids. J Exp Bot 55: 595–604 [DOI] [PubMed] [Google Scholar]
- Lambrechts D, Schroeder JI, Verbelen JP (1992) The influence of osmolarity on the surface properties of the plasma membranes of isolated guard cell protoplasts of Vicia faba L. Plant Physiol 11: 25–32 [Google Scholar]
- Lazzaro MD, Thomson WW (1996) The vacuolar-tubular continuum in living trichomes of chickpea (Cicer arietinum) provides a rapid means of solute delivery from base to tip. Protoplasma 193: 181–190 [Google Scholar]
- Lucas WJ, Lee JY (2004) Plasmodesmata as a supracellular control network in plants. Nat Rev Mol Cell Biol 5: 712–726 [DOI] [PubMed] [Google Scholar]
- Mathur J, Mathur N, Kernebeck B, Hülskamp M (2003) Mutations in actin-related proteins 2 and 3 affect cell shape development in Arabidopsis. Plant Cell 15: 1632–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyasu Y, Hattori M, Jauh G, Rogers J (2003) Alpha tonoplast intrinsic protein is specifically associated with vacuole membrane involved in an autophagic process. Plant Cell Physiol 44: 795–802 [DOI] [PubMed] [Google Scholar]
- Newell JM, Leigh RA, Hall JL (1998) Vacuole development in cultured evacuolated oat mesophyll protoplasts. J Exp Bot 49: 817–827 [Google Scholar]
- Palevitz BA, Okane DJ (1981) Epifluorescence and video analysis of vacuole motility and development in stomatal cells of Allium. Science 214: 443–445 [DOI] [PubMed] [Google Scholar]
- Peters C, Mayer A (1998) Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature 396: 575–580 [DOI] [PubMed] [Google Scholar]
- Rojo E, Zouhar J, Kovaleva V, Hong S, Raikhel N (2003) The AtC-VPS protein complex is localized to the tonoplast and the prevacuolar compartment in Arabidopsis. Mol Biol Cell 14: 361–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito C, Ueda T, Abe H, Wadw Y, Kuroiwa T, Hisada A, Furuya M, Nakano A (2002) A complex and mobile structure forms a distinct subregion within the continuous vacuolar membrane in young cotyledons of Arabidopsis. Plant J 29: 245–255 [DOI] [PubMed] [Google Scholar]
- Sanderfoot AA, Kovaleva V, Zheng H, Raikhel NV (1999) The t-SNARE AtVAM3p resides on the prevacuolar compartment in Arabidopsis root cells. Plant Physiol 121: 929–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Nakamura N, Ohsumi Y, Kouchi H, Kondo M, Hara-Nishimura I, Nishimura M, Wada Y (1997) The AtVAM3 encodes a syntaxin-related molecule implicated in the vacuolar assembly in Arabidopsis thaliana. J Biol Chem 272: 24530–24535 [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D (2001) Guard cell signal transduction. Annu Rev Plant Physiol Plant Mol Biol 52: 627–658 [DOI] [PubMed] [Google Scholar]
- Shope JC, DeWald DB, Mott KA (2003) Changes in surface area of intact guard cells are correlated with membrane internalization. Plant Physiol 133: 1314–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surpin M, Raikhel N (2004) Traffic jams affect plant development and signal transduction. Nat Cell Biol 5: 100–109 [DOI] [PubMed] [Google Scholar]
- Uemura T, Yoshimura SH, Takeyasu K, Sato MH (2002) Vacuolar membrane dynamics revealed by GFP-AtVam3 fusion protein. Genes Cells 7: 743–753 [DOI] [PubMed] [Google Scholar]
- Verbelen JP, Tao W (1998) Mobile arrays of vacuole ripples are common in plant cells. Plant Cell Rep 17: 917–920 [DOI] [PubMed] [Google Scholar]
- Wang XC, Chen J (2001) Mechanism of stomatal movement, In CH Lou, XC Wang, eds, The Plant Physiological Principles for Crop Yield. China Agricultural Press, Beijing, pp 118–135
- Waters MT, Fray RG, Pyke KA (2004) Stromule formation is dependent upon plastid size, plastid differentiation status and the density of plastids within the cell. Plant J 39: 655–667 [DOI] [PubMed] [Google Scholar]
- Wolfe J, Steponkus PL (1983) Mechanical properties of the plasma membrane of isolated protoplasts. Plant Physiol 71: 276–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Fuji K, Shimada T, Nishimura M, Hara-Nishimura I (2005) Endosomal proteases facilitate the fusion of endosomes with vacuoles at the final step of the endocytotic pathway. Plant J 41: 888–898 [DOI] [PubMed] [Google Scholar]
- Yamada K, Nishimura M, Hara-Nishimura I (2003) E64d, an inhibitor of papain family proteases, inhibits endocytosis and degradation of plasma membrane proteins in BY-2 cells and Arabidopsis root cells (poster abstract no. 1189). In Plant Biology 2003, July 25–30, 2003, Honolulu. American Society of Plant Biologists, Rockville, MD, http://abstracts.aspb.org/pb2003/public/P69/0900.html
- Yamamoto Y, Nishimura M, Hara-Nishimura I, Noguchi T (2003) Behavior of vacuoles during microspore and pollen development in Arabidopsis thaliana. Plant Cell Physiol 44: 1192–1201 [DOI] [PubMed] [Google Scholar]
- Yano D, Sato M, Saito C, Sato MH, Morita MT, Tasaka M (2003) A SNARE complex containing SGR3/AtVAM3 and ZIG/VTI11 in gravity-sensing cells is important for Arabidopsis shoot gravitropism. Proc Natl Acad Sci USA 100: 8589–8594 [DOI] [PMC free article] [PubMed] [Google Scholar]