Abstract
In humans, the primary visual cortex (V1) is essential for conscious vision. However, even without V1 and in the absence of awareness, some preserved ability to accurately respond to visual inputs has been demonstrated, a phenomenon referred to as blindsight. We used transcranial magnetic stimulation (TMS) to deactivate V1, producing transient blindness for visual targets presented in a foveal, TMS-induced scotoma. Despite unawareness of these targets, performance on forced choice discrimination tasks for orientation (experiment 1) and color (experiment 2) were both significantly above chance. In addition to demonstrating that TMS can be successfully used to induce blindsight within a normal population, these results suggest a functioning geniculoextrastriate visual pathway that bypasses V1 and can process orientation and color in the absence of conscious awareness.
Keywords: consciousness, perception, vision, blindsight, transcranial magnetic stimulation
An abundance of evidence suggests that the human primary visual cortex (V1) is essential for conscious visual perception. Most notably, following a lesion to a portion of V1, patients report no phenomenological awareness of stimuli presented in the corresponding region of their visual field (1). Despite this lack of visual awareness, some patients with V1 damage can nonetheless discriminate and localize these “unseen” stimuli at above chance levels. For example, it has been shown that these blindsight patients have preserved saccadic eye movements (2) and manual responses (3, 4) to stimuli presented in their blind field for which they are not consciously aware. Furthermore, they have even shown the remarkable ability of distinguishing within their blind field line orientation differences as small as 10° (5), wavelengths differing between 20 and 30 nm (6, 7), direction of motion (8), and basic shapes (3, 9).
The proposed visual processing mechanisms subserving these residual visual abilities have been controversial. It has been suggested, for example, that the residual vision without conscious awareness may be mediated by preserved “islands” of cortex in V1 (10), the superior colliculus via the extrageniculate retinotectal pathway (2, 3, 11, 12), or a geniculo-extrastriate pathway (6, 7). Of these theories of blindsight, the extrageniculate vision account has received the strongest support. After lesions to V1, humans and monkeys frequently demonstrate preserved abilities to make accurate saccades to stimuli in their blind field (2), an effect that is abolished in monkeys by a subsequent lesion to the superior colliculus (13). Furthermore, directionally sensitive cells in area MT, which respond to stimuli in the monkey's blind field, fail to do so after a lesion to the superior colliculus (14).
Although the superior colliculus has been shown to be directly involved with the spatial coding of visual stimuli, sufficient to subserve a role in reflexive saccade generation and target selection (15-17), its role in the unconscious processing of other visual features may be minimal. The superior colliculus is not visually selective, as it responds to both large and small stimuli, moving and stationary objects, and has little to no orientation specificity and no color opponent processing (18). Thus, the neural mechanisms responsible for the extensive types of unconscious visual analyses in blindsight patients may be a consequence of other visual pathways that bypass V1 as well as the superior colliculus. Consistent with this geniculoextrastriate account of blindsight, direct projections from the lateral geniculate nucleus to areas MT (19), V2 (20), and V4 (21, 22) in the macaque have been identified. In each case the number of fibers was small in comparison to the primary geniculostriate pathway, but may be sufficient enough to be responsible for the preserved motion and wavelength discrimination abilities in the absence of V1 and conscious awareness in blindsight patients. Thus, it may be that in blindsight patients the colliculus is only involved with coding of the target location, whereas other forms of discriminatory visual processing may be occurring within the geniculoextrastriate pathway.
The goals of the current studies were to examine what types of stimuli can be processed without primary visual cortex, and through careful selection of visual stimuli, to narrow down which subcortical pathway(s) may be subserving the residual visual abilities seen in blindsight. We used transcranial magnetic stimulation (TMS) to disrupt neural processing in V1 while presenting stimuli in the corresponding region of the visual field. In experiment 1, we presented either a horizontal or vertical line in the TMS-induced scotoma to investigate orientation processing in the absence of V1 functioning and awareness. In experiment 2, subjects were presented with either a red or a green disk in the TMS-induced scotoma to investigate color processing without V1 and awareness. We hypothesized that, if a geniculoextrastiate pathway is involved with some aspects of blindsight, then orientation and color discrimination, which cannot be processed through the retinotectal pathway, should be at above chance levels despite a lack of conscious awareness.
Methods
Experiment 1. Participants. Six neurologically normal subjects (two males) from Rice University participated in this experiment. One subject was excluded from the analyses because of an insufficient number of unaware trials. All subjects had normal or corrected to normal vision and participated only after informed consent.
Stimuli, apparatus, and procedure. Each session began with a V1 localization procedure and TMS threshold intensity determination. A Cadwell MES-10 polyphasic stimulator with a 9-cm diameter round coil was used. For localization and threshold determination, the TMS intensity was initially set at 50% of maximum output (2.2 T). The base of the coil was initially placed ≈2 cm above and 1 cm left of the inion, with the main axis of the coil oriented parallel to the sagittal plane and the handle positioned ventrally. A small green dot measuring 0.25° in diameter was presented for 14 ms, with the center 0.25° to the right of the center of fixation. A TMS pulse was time-locked to stimulus presentation and initially administered 100 ms after the onset of the green dot. During this localization and TMS threshold identification procedure, each subject was to report in full detail on each trial the percept of the briefly presented dot. The location of the coil was moved on the scalp according to the reported percept. For example, if only the top half of the dot was perceived, the coil was moved slightly lower on the head. Concurrently, the TMS output timing and intensity was adjusted over several trials until we obtained the suppression threshold, which was defined as the intensity at which at least three of five visual stimuli were undetected. A second localization procedure was used to ensure complete suppression of conscious visual processing. In this task, four digits were presented in the center of the screen followed by a TMS pulse at the optimal position and latency for each subject. We were interested in the reported percept of the last two digits, which corresponds to the location of the scotoma as localized by using the dot stimulus. If necessary, the location of the coil on the scalp and the TMS intensity were adjusted until the digits were no longer consciously perceived, and the first procedure (with the green dot) was used again to ensure complete suppression. After full visual suppression was achieved by using both types of stimuli, the location of the coil on the scalp was marked and this position was used for the remainder of the experiment. The TMS intensity for the main experiment was set at 10% above the visual suppression threshold to ensure optimal suppression of visual input while maintaining comfort for the participants (compare ref. 23). An even greater intensity was not used to avoid inducing visual phosphenes that may have interfered with the ability to report attributes of the stimulus and to minimize any discomfort. The mean intensity of the TMS used during testing was 73% of maximum output, with a range of 64-84%.
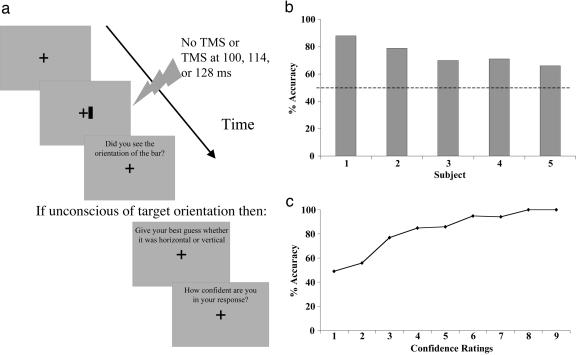
A fixation cross was displayed throughout the experiment in the center of the screen and was 0.2° of visual angle in height and width. Subjects were instructed to keep their eyes on the fixation cross at all times. On each trial, a vertical or horizontal bar, measuring 0.25° × 0.05° or 0.05° × 0.25°, respectively, was presented 0.25° to the right of the center of fixation (corresponding to the location of the TMS induced scotoma) for 14 ms (see Fig. 1a). On three-fourths of trials, this bar was followed by a TMS pulse at a stimulus onset asynchrony (SOA) of 100, 114, or 128 ms. These SOAs, each of which were used an equal number of times throughout the experiment, were chosen based on previous studies showing that TMS presented 80-140 ms after a stimulus onset produces optimal visual suppression (24-26). These TMS studies have shown that temporal intervals <80 ms or >140 ms do not cause visual suppression and that maximal suppression occurs with TMS ≈100-110 ms after visual stimulus presentation (23-25). On the remaining one-fourth of trials, no TMS pulse was administered. The different TMS SOA and no-TMS conditions were randomly interleaved throughout the experiment. Immediately after each stimulus presentation, the subject was asked to report whether there was any awareness of the orientation of the stimulus. If awareness of the orientation was reported, the next trial was then administered. However, if the orientation of the stimulus was not detected, the participants were asked to guess the orientation of the stimulus and to rate the confidence of their guess. Confidence ratings were made on a scale of 1-9, with 1 representing not at all confident and 9 representing extremely confident. All stimuli were dark gray presented on a light gray background and were viewed from a distance of 57 cm.
Fig. 1.
Unconscious processing of orientation. (a) A schematic illustration of the sequence of trial events in experiment 1. To examine orientation processing without primary visual cortex and in the absence of awareness, a horizontally or vertically oriented line was presented while suppressing primary visual cortex processing using TMS. When subjects were unaware of the stimulus, they were asked to guess the orientation and to provide a confidence rating for their guess. (b) The mean accuracy in guessing the stimulus orientation on unaware trials. Despite the absence of awareness, guessing performance was significantly above chance. (c) The correlation between accuracy in guessing the stimulus orientation on unaware trials and the confidence ratings for those guesses.
Experiment 2. Participants. Six neurologically normal subjects (two males; five new and one who participated in experiment 1) from Rice University participated in this experiment. One subject was excluded from the analyses because, despite our repeated instructions, she always guessed “red” and gave a confidence rating of 1, and reported that this strategy would at least yield an accuracy rate of 50%. In fact, this was the only participant whose performance was slightly below chance. All subjects had normal or corrected to normal vision and participated only after informed consent.
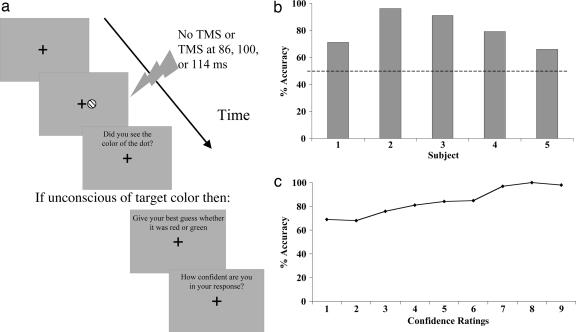
Stimuli, apparatus, and procedure. The stimuli and procedures were identical to that of experiment 1 with the exception that, instead of a horizontal or vertical line, either a red or a green dot measuring 0.25° in diameter was used as the visual stimuli, and the TMS SOAs were 86, 100, or 114 ms (see Fig. 2a). A heterochromatic flicker fusion procedure was performed for each subject at the beginning of the experiment to equate the red and green luminance values for each subject. The mean intensity of the TMS in this experiment was 76.4% of maximum output, with a range of 64-85%.
Fig. 2.
Unconscious processing of color. (a) A schematic illustration of the sequence of trial events in experiment 2. To examine color processing without primary visual cortex and in the absence of awareness, a red or green disk was presented while suppressing primary visual cortex processing using TMS. When subjects were unaware of the stimulus, they were asked to guess the color and to provide a confidence rating for their guess. (b) The mean accuracy in guessing the stimulus color on unaware trials. Despite the absence of awareness, guessing performance was significantly above chance. (c) The correlation between accuracy in guessing the stimulus color on unaware trials and the confidence ratings for those guesses.
Results
Experiment 1: Processing of Orientation Without V1. The percentage of correct guesses on the forced-choice orientation discrimination task was calculated for trials in which the subjects reported that they did not see the stimulus orientation (unaware trials; see Fig. 1b). An initial analysis showed that there was no difference in discrimination performance at the different TMS SOAs, F(2, 8) = 0.98, P = 0.42; all remaining analyses were therefore collapsed across this variable. Subjects were unaware of the orientation of the target on 61% of the TMS trials. Despite this unawareness of the orientation of the target, performance on the forced-choice orientation discrimination was nonetheless at significantly above chance levels (i.e., the discrimination accuracy was significantly different from 50%; mean = 75%, SD = 0.09): t(4) = 6.23, P = 0.003. This finding suggests that, even in the absence of normal V1 functioning and conscious visual perception, an alternative visual pathway may be involved with processing the orientation of a visual stimulus.
When asked about their discrimination performance for unconscious stimuli presented in the blind field, some blindsight patients have reported that they were guessing randomly and were usually surprised by their above chance performance (3, 6). However, other patients have reported that they experience a “feeling” that something may have been presented, but have no conscious awareness of the stimuli (10, 27). By having subjects in the current experiment report whether they were aware of the orientation of the stimulus rather than whether or not they were conscious of any stimulus being presented, our study included trials with type I blindsight, when there was complete unawareness of the entire stimulus, in addition to trials with type II blindsight, when there was no awareness of the orientation of the stimulus, but awareness that something was presented (26). Although we did not assess on each trial whether type I or II blindsight occurred, several participants reported being aware that something was presented on some of the trials, but that they were unaware or uncertain of the orientation (i.e., type II blindsight). This latter type II blindsight may indicate that the conscious system may have some limited access to the processing of the unconscious system.
We examined the influences of the unconscious visual processing system on conscious judgments and phenomenal awareness by having participants provide confidence ratings for their guesses. To determine whether the unconscious processing of line orientation may have influenced feelings of awareness, confidence ratings were analyzed with respect to the accuracy of the orientation guesses (see Fig. 1c). A significant positive correlation was found between the confidence ratings and the accuracy in guessing the orientation of the stimulus, r = 0.93, P < 0.001. Accuracy was significantly above chance for confidence ratings of 4-9 (all P values < 0.05), but not for ratings between 1 and 3. This result demonstrates that a feeling of knowing may be associated with unconscious processes and that our study was measuring this type II form of blindsight on many of the trials.
Experiment 2: Processing of Color Without V1. The percentage of correct guesses on the forced-choice color discrimination task was calculated for trials in which the subject reported that they did not see the stimulus (see Fig. 2b). As in experiment 1, there was no significant difference between discrimination performance at the different TMS SOAs, F(2, 8) = 3.16, P = 0.10, so all subsequent analyses were collapsed across this variable. Subjects reported unawareness of the color of the target on 70% of TMS trials. When subjects reported being unaware of the target, they were nevertheless still able to accurately guess the color at significantly above-chance levels (M = 81%, SD = 0.13), t(4) = 5.43, P = 0.006. This result further demonstrates that, in the absence of normal V1 functioning and conscious visual perception, another structure(s) is still processing color information.
To determine whether the confidence ratings were related to the accuracy of their color guesses, a correlation analysis was performed (see Fig. 2c). As in experiment 1, a significant positive correlation was found between the confidence ratings and accuracy, r = 0.97, P < 0.001. Accuracy was significantly above chance for each of the confidence ratings, apart from the ratings of 1 and 3 (all P values < 0.05).
Discussion
When processing in primary visual cortex was suppressed by TMS, thereby suppressing visual awareness of a stimulus within the TMS-induced scotoma, subjects were nonetheless still able to accurately guess both the orientation and the color of the stimulus at significantly above chance levels. These findings suggest that a visual pathway that bypasses V1 must be involved with unconscious vision. The most likely visual pathway that supports our findings of unconscious visual processing of these features projects from the lateral geniculate nucleus directly to extrastriate cortex, and in particular V4. By excluding any contributions of the superior colliculus by using stimuli that it cannot discriminate (see ref. 18 for a review of collicular function), our results thus provide further functional evidence that there are direct projections from the lateral geniculate nucleus to extrastriate areas including area MT (19), V2 (20), and V4 (21, 22). V4 has long been known to be involved with color perception as it contains a high proportion of wavelength selective cells (28, 29). Furthermore, V4 also contains orientation and feature selective cells (30, 31). Therefore, a direct retinogeniculate-extrastiate pathway to V4 is most likely responsible for our findings of residual unconscious visual processing seen in the absence of normal primary visual cortex functioning.
This proposal may seem to be in direct conflict to patient studies providing evidence (2, 3, 11, 12) or showing activation (27) of the superior colliculus when processing unconscious stimuli, and for the hypothesized role of the colliculus in mediating saccades to unseen targets. Furthermore, these results may seem in contrast with studies suggesting processing in the intact hemisphere via small ipsilateral ganglion projections to V1 or across hemispheres via interhemispheric connections (32, 33). However, it is likely the case that, after damage or disruption to the primary visual pathway of the brain, many other pathways/structures are still functioning and may be recruited in an attempt to restore normal conscious visual functioning. Furthermore, these alternate visual routes may be selectively involved with other forms of visual processing, such that the superior colliculus may be coding for stimulus properties including onset or location for a saccade and the geniculoextrastriate or ipsilateral projection pathways coding for orientation, color, and form. These multiple alternate routes for unconscious vision may, in fact, be the basis for why blindsight has been demonstrated for so many different visual stimulus properties.
Our finding that TMS can be applied over the occipital cortex to more directly investigate blindsight builds on a prior study conducted in our laboratory examining the influence of unconscious distractors presented within a TMS-induced scotoma on saccade latency and manual button press responses (12). In that study, subjects reported on the presence or absence of an entire stimulus rather than on the perception of a given feature as in the current study. Thus, our previous work, despite using identical TMS intensities and procedures, measured only the effects of unconscious processing in type I blindsight. The previous Ro et al. study (26) found that saccadic eye movement latencies, but not manual button press responses, were significantly affected by unconscious distractors. In the current study, we not only show that some stimulus features can be discriminated without awareness, but also provide evidence that TMS can be used to examine both type I and type II blindsight, as assessed through reports of participants regarding their awareness of something being presented on some of the trials. Other indirect evidence suggesting that type II blindsight was measured on some of our trials is the high confidence ratings of their guesses that were given by the participants on some of the trials. Further studies directly examining whether discrimination is better with type II as compared to type I TMS-induced blindsight will not only provide insights into whether the mechanisms operating are similar to the naturally occurring blindsight phenomenon, but will also lead to a better understanding of conscious and unconscious vision.
It may be noted that the blindsight, as induced with the parameters of TMS used in this study, is in some ways different from the type I blindsight phenomena typically studied in patients with lesions in V1. Depending on stimulus parameters, patients in such blindsight studies never consciously perceive visual stimuli, but under other stimulus conditions may report an awareness of a visual event even if they do not “see” it (i.e., type II blindsight). In the scotoma induced by the TMS parameters used in the present study, the subject's unawareness is transient and awareness is occasionally reported. (It can be noted, however, that in some participants it is possible to produce unawareness on all or nearly all trials with very high, but typically uncomfortable, TMS intensities). This is one of the main reasons why a subjective, trial-by-trial assessment was made regarding the awareness of stimuli presented in the TMS-induced scotoma. By employing a subjective awareness procedure on each trial and analyzing the data according to these reports, discrimination performance on unaware trials is not influenced in any way by trials with awareness, which is not the case when most objective measures of awareness are used. Furthermore, our work demonstrating a dissociation of awareness with subjective reports and discrimination performance within the same trials relates well to typical dissociation observed in blindsight as well as the work by Azzopardi and Cowey (34), which suggests that, independent of response biases, forced-choice discrimination procedures may be more revealing of unconscious processing than simple detection tasks. Although no signal detection analyses were conducted in the current or previous (12) TMS studies from our laboratory, future studies examining sensitivity and response biases in TMS-induced blindsight would be highly informative, because these studies might, for example, determine that TMS over the visual cortex simply reduces visual stimuli to near-threshold levels and/or induces criterion shifts, making this phenomenon very different from that observed in naturally occurring blindsight (34).
One aspect of our data that requires some discussion, because it bears directly on the anatomy and physiology of unconscious vision, regards the late timing of the TMS pulse with respect to the visual stimulus to render it unconscious. Event-related potentials and single unit recordings have demonstrated an initial volley of activity in visual cortex occurring as early as 35 ms (for a review, see ref. 35). However, previous studies have shown that optimal visual suppression with TMS occurs much later, ranging from 80 to 140 after stimulus onset (23-25). This observation suggests that TMS is not disrupting the initial feedforward sweep into V1, but rather is disrupting processing for awareness at some later stage, either by affecting the magnitude of spiking activity within layer 4C of V1, affecting the interlaminar connections within V1, and/or the feedback projections onto V1 from “higher” cortical areas. Several studies suggest that feedback activity to primary visual cortex is necessary for visual awareness (26, 35-37). For example, one TMS study examining the awareness of visual motion has shown that feedback from MT to V1 is essential for visual awareness (37). Thus, in addition to disrupting any later afferent information from the lateral geniculate nucleus and interlaminar activity that may contribute to awareness, it is also highly likely that the unawareness of color and orientation from TMS of V1 is a consequence of disruption of the descending projections from V4 onto V1. This finding implies that some visual information can reach V1 through indirect geniculate projections, which further studies might be able to reveal.
In conclusion, this work provides direct evidence that V1 is necessary for visual awareness and validates TMS as a useful tool in examining the blindsight phenomenon in normal subjects. More importantly, these results provide indirect support not only for the existence of an alternate geniculoextrastriate visual pathway that bypasses V1, but demonstrate that this pathway may be involved with coding orientation and color unconsciously.
Author contributions: T.R. designed research; J.L.B., S.H., and T.R. performed research; J.L.B., S.H., and T.R. analyzed data; and J.L.B. and T.R. wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: TMS, transcranial magnetic stimulation; SOA, stimulus onset asynchrony.
References
- 1.Holmes, G. (1918) Br. J. Opthalmol. 2, 449-468 and 506-516. [Google Scholar]
- 2.Poppel, E., Held, R. & Frost, D. (1973) Nature 243, 295-296. [DOI] [PubMed] [Google Scholar]
- 3.Weiskrantz, L., Warrington, E. K., Sanders, M. D. & Marshall, J. (1974) Brain 97, 709-728. [DOI] [PubMed] [Google Scholar]
- 4.Blythe, I. M., Kennard, C. & Ruddock, K. H. (1987) Brain 110, 887-905. [DOI] [PubMed] [Google Scholar]
- 5.Weiskrantz, L. (1986) Blindsight: A Case Study and Implications (Oxford Univ. Press, Oxford).
- 6.Stoerig, P. & Cowey, A. (1989) Nature 342, 916-918. [DOI] [PubMed] [Google Scholar]
- 7.Stoerig, P. & Cowey, A. (1992) Brain 115, 425-444. [DOI] [PubMed] [Google Scholar]
- 8.Perenin, M. T. (1991) NeuroReport 2, 397-400. [DOI] [PubMed] [Google Scholar]
- 9.Sanders, M. D., Warrington, E. K., Marshall, J. & Weiskrantz, L. (1974) Lancet 20, 707-708. [DOI] [PubMed] [Google Scholar]
- 10.Fendrich, R., Wessinger, C. M. & Gazzaniga, M. S. (1992) Science 258, 1489-1491. [DOI] [PubMed] [Google Scholar]
- 11.Rafal, R., Smith, J., Krantz, J., Cohen, A. & Brennan, C. (1990) Science 250, 118-121. [DOI] [PubMed] [Google Scholar]
- 12.Ro, T., Shelton, D. J. M., Lee, O. L. & Chang, E. (2004) Proc. Natl. Acad. Sci. USA 101, 9933-9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohler, C. W. & Wurtz, R. H. (1977) J. Neurophysiol. 40, 74-94. [DOI] [PubMed] [Google Scholar]
- 14.Rodman, H. R., Gross, C. G. & Albright, T. D. (1990) J. Neurosci. 10, 1154-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McPeek, R. M. & Keller, E. L. (2004) Nat. Neurosci. 7, 757-763. [DOI] [PubMed] [Google Scholar]
- 16.Munoz, D. P. & Wurtz, R. H. (1995) J. Neurophysiol. 73, 2334-2348. [DOI] [PubMed] [Google Scholar]
- 17.Munoz, D. P. & Wurtz, R. H. (1995) J. Neurophysiol. 73, 2313-2333. [DOI] [PubMed] [Google Scholar]
- 18.Robinson, D. L. & McClurkin, J. W. (1989) Rev. Oculomot. Res. 3, 337-360. [PubMed] [Google Scholar]
- 19.Sincich, L., Park, K., Wohlgemuth, M. & Horton, J. (2004) Nat. Neurosci. 7, 1123-1128. [DOI] [PubMed] [Google Scholar]
- 20.Bullier, J. & Kennedy, H. (1983) Exp. Brain Res. 53, 168-172. [DOI] [PubMed] [Google Scholar]
- 21.Fries, W. (1981) Proc. R. Soc. London Ser. B 213, 73-86. [DOI] [PubMed] [Google Scholar]
- 22.Yukie, M. & Iwai, E. (1981) J. Comp. Neurol. 201, 81-97. [DOI] [PubMed] [Google Scholar]
- 23.Rossini, P. M., Barker, A. T., Berardelli, A., Caramia, M. D., Caruso, G., Cracco, R. Q., Dimitrijevic, M. R., Hallett, M., Katayama, Y., Lucking, C. H., et al. (1994) Electroencephalogr. Clin. Neurophysiol. 91, 79-92. [DOI] [PubMed] [Google Scholar]
- 24.Amassian, V. E., Cracco, R. Q., Maccabee, P. J., Cracco, J. B., Rudell, A. & Eberle, L. (1989) Electroencephalogr. Clin. Neurophysiol. 74, 458-462. [DOI] [PubMed] [Google Scholar]
- 25.Corthout, E., Uttl, B., Walsh, V., Hallett, M. & Cowey, A. (1999) NeuroReport 10, 2631-2634. [DOI] [PubMed] [Google Scholar]
- 26.Ro, T., Breitmeyer, B., Burton, P., Singhal, N. & Lane, D. (2003) Curr. Biol. 11, 1038-1041. [DOI] [PubMed] [Google Scholar]
- 27.Sahraie, A., Weiskrantz, L., Burbur, J. L., Simmons, A., Williams, S. C. R. & Brammer, M. J. (1997) Proc. Natl. Acad. Sci. USA 94, 9406-9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeki, S. (1980) Nature 284, 412-418. [DOI] [PubMed] [Google Scholar]
- 29.Zeki, S. (1990) Brain 113, 1721-1777. [DOI] [PubMed] [Google Scholar]
- 30.Desimone, R., Schein, S. J., Moran, J. & Ungerleider, L. G. (1985) Vision Res. 25, 441-452. [DOI] [PubMed] [Google Scholar]
- 31.Gallant, J. L., Braun, J. & Van Essen, D. C. (1993) Science 259, 100-103. [DOI] [PubMed] [Google Scholar]
- 32.Reinhard, J. & Trauzettel-Klosinski, S. (2003) Invest. Ophthalmol. Vis. Sci. 44, 1568-1572. [DOI] [PubMed] [Google Scholar]
- 33.Tomaiuolo, F., Ptito, M., Marzi, C. A., Paus, T. & Ptito, A. (1997) Brain 120, 795-803. [DOI] [PubMed] [Google Scholar]
- 34.Azzopardi, P. & Cowey, A. (1997) Proc. Natl. Acad. Sci. USA 94, 14190-14194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamme, V. A. & Roelfsema, P. R. (2000) Trends Neurosci. 23, 571-579. [DOI] [PubMed] [Google Scholar]
- 36.Super, H., Spekreijse, H. & Lamme, V. A. (2001) Nat. Neurosci. 4, 304-310. [DOI] [PubMed] [Google Scholar]
- 37.Pascual-Leone, A. & Walsh, V. (2001) Science 292, 510-512. [DOI] [PubMed] [Google Scholar]