Short abstract
This article is part of a series examining the cost effectiveness of strategies to achieve the millennium development goals for health
Abstract
Objective To determine the costs and effectiveness of selected child health interventions—namely, case management of pneumonia, oral rehydration therapy, supplementation or fortification of staple foods with vitamin A or zinc, provision of supplementary food with counselling on nutrition, and immunisation against measles.
Design Cost effectiveness analysis.
Data sources Efficacy data came from published systematic reviews and before and after evaluations of programmes. For resource inputs, quantities came from literature and expert opinion, and prices from the World Health Organization Choosing Interventions that are Cost Effective (WHO-CHOICE) database
Results Cost effectiveness ratios clustered in three groups, with fortification with zinc or vitamin A as the most cost effective intervention, and provision of supplementary food and counselling on nutrition as the least cost effective. Between these were oral rehydration therapy, case management of pneumonia, vitamin A or zinc supplementation, and measles immunisation.
Conclusions On the grounds of cost effectiveness, micronutrients and measles immunisation should be provided routinely to all children, in addition to oral rehydration therapy and case management of pneumonia for those who are sick. The challenge of malnutrition is not well addressed by existing interventions.
Introduction
After the 1990 United Nations children's summit, 167 countries pledged to further intensify their efforts in child health and nutrition to meet a child related set of human development goals for the year 2000 (www.un.org/geninfo/bp/child.html). But by 2000, 10.6 million children were still dying yearly, most due to pneumonia, diarrhoea, and neonatal causes and, in sub-Saharan Africa, malaria as well.1,2 Malnutrition has been identified as an underlying cause in over 50% of cases,1 with zinc and vitamin A deficiencies contributing.1,3
In September 2000, 189 countries endorsed the UN millennium declaration, which set goals for human development by 2015 (www.who.int/mdg/en/). Millennium development goal 4 was specific to child health, aiming to have reduced mortality in children aged less than 5 by two thirds between 1990 and 2015. Other millennium development goals aimed at reducing poverty and malnutrition and improving access to safe water, sanitation, and air quality would also contribute to improving child health.4
Five years on, there is some, although uneven, progress, and if practice continues as usual until 2015, then many countries, particularly in sub-Saharan Africa and south Asia, will miss this goal.5 It is now incumbent on countries and the international community to reconsider if the resources currently used to improve child health are being used as effectively as possible, and what strategies would ensure that any new resources achieve the maximum benefit.
Some evidence already exists on the cost effectiveness of selected interventions aimed at improving child health in the developing world,6-8 but results have generally been based on interventions undertaken in isolation, without accounting for costs that can be shared across interventions or the impact of changing coverage on unit costs (for example, costs per child treated). In these new cost effectiveness analyses, the WHO Choosing Interventions that are Cost Effective (CHOICE) Millennium Development Goals Team standardised framework, methods, and tools9-11 are used for selected interventions for major causes of childhood morbidity and mortality. They allow combinations of interventions to be analysed at the same time and the impact of increasing coverage to be incorporated explicitly. Full details of the methods are published in the paper by Evans et al of this series.9-11
Methods
We evaluated nine single interventions, each at three levels of coverage (50%, 80%, and 95%), and various combinations thereof. The single interventions evaluated are oral rehydration therapy; case management of pneumonia; supplementation and fortification with vitamin A or zinc; provision of supplementary food during weaning, with counselling on nutrition (with and without growth monitoring and targeting); and measles immunisation. See annex A on bmj.com for a detailed description of the individual interventions.
Effectiveness
We analysed the prevented cases and deaths due to pneumonia, diarrhoea, and measles in the under 5s age group. These are converted to the number of disability adjusted life years (DALYs) averted. We obtained data on epidemiological rates by region and health state valuations primarily from the year 2000 update on burden of disease, supplemented by other published literature.12,13 Children with nutritional comorbidities have a higher risk of diarrhoea and pneumonia and dying from these diseases than do other children. We obtained relative risks from systematic reviews14-16 and we applied these to the relevant epidemiological rates for the specific subpopulations (table 1).
Table 1.
Relative risks applied to epidemiological rates for specific subpopulations
| Disease and nutritional comorbidities | Incidence relative risk (95% CI) | Case fatality rate relative risk (95% CI) |
|---|---|---|
| Diarrhoea | ||
| Vitamin A deficiency alone | 1 | 2.15 (1.83 to 2.56) |
| Zinc deficiency alone | 1.28 (1.1 to 1.5) | 1.28 (1.1 to 1.5) |
| Underweight: | ||
| Mild | 1 | 2.32 (1.93 to 2.79) |
| Moderate | 1.23 (1.12 to 1.35) | 5.39 (3.73 to 7.79) |
| Severe | 1.23 (1.12 to 1.35) | 12.50 (7.19 to 21.73) |
| Pneumonia | ||
| Vitamin A deficiency alone | 1 | 1 |
| Zinc deficiency alone | 1.69 (1.2 to 2.44) | 1.69 (1.2 to 2.44) |
| Underweight: | ||
| Mild | 1 | 2.01 (1.63 to 2.47) |
| Moderate | 1.86 (1.06 to 3.28) | 4.03 (2.67 to 6.08) |
| Severe | 1.86 (1.06 to 3.28) | 8.09 (4.36 to 15.01) |
For case management of pneumonia17 and supplementation with vitamin A or zinc,15-19 we obtained data on efficacy from systematic reviews with meta-analysis of numerous large community based trials in several developing countries. The estimate of effectiveness for oral rehydration therapy came from before and after evaluations of national diarrhoea control programmes in two countries. These reports were selected as being of high quality because quantitative and qualitative analyses were undertaken to isolate the impact of oral rehydration therapy from other interventions occurring at the same time.20,21 The estimate of effectiveness for provision of supplementary feeding and nutrition counselling came from a systematic review of several efficacy trials and evaluations of larger scale programmes.22 Although growth monitoring and promotion has not been shown to be effective by itself,23 we used it in this analysis for identifying children to be targeted for provision of supplementary food.
Data for vitamin A fortification came from a report on several programmes in Central America.24 In the absence of experience with national programmes on zinc fortification, we modelled its effectiveness relative to zinc supplementation at about the same level of vitamin A supplementation relative to fortification. For measles immunisation, we used an 85% seroconversion or vaccine efficacy rate.25,26 To model more realistically the attainable effectiveness outside research settings, we multiplied estimates of efficacy by an estimate of patient adherence to the therapy or, in the case of fortification, access to processed food (table 2).
Table 2.
Effectiveness of interventions to improve child health
| Interventions and parameters | Effectiveness* |
|---|---|
| Oral rehydration therapy: | |
| Adherence | 1† |
| Efficacy‡ | 0.36 (0.21 to 0.42) |
| Case management of pneumonia: | |
| Adherence | 0.85 |
| Efficacy‡ | 0.5 (0.25 to 0.67) |
| Vitamin A supplementation: | |
| Adherence | 0.75 |
| Efficacy‡§ | 1¶ |
| Vitamin A fortification: | |
| Access to processed food | 0.6 to 0.75 |
| Efficacyठ| 0.6 |
| Zinc supplementation: | |
| Adherence | 0.6 |
| Efficacy‡§ | 1¶ |
| Zinc fortification: | |
| Access to processed food | 0.6 to 0.75 |
| Efficacyठ| 0.5 |
| Provision of supplementary food and counselling on nutrition: | |
| Adherence | 0.8 |
| Efficacyठ| 0.16 (SD) |
| Measles immunisation: | |
| Adherence | 1 |
| Efficacy§ | 0.85 |
Relative change from baseline.
Adherence of 1 assumed because data on effectiveness came from evaluations of national programmes.
Case fatality rate.
Incidence rate.
Effectiveness in model is mediated through relative risks calculated from trials.
Costs
We adopted the standardised WHO ingredients approach, with separate specification of units of utilisation and costs.10 Utilisation rates and unit costs were derived from the literature, unpublished data, and expert opinion (see paper by Evans et al in this series for a description of the methods; table 3).7,27-29 All costs are summarised in international dollars ($Int), with 2000 as the base year and future costs discounted at 3%. The cost effectiveness ratios in this analysis are relatively higher than those in the literature because of the use of international dollars. International dollars are derived by dividing local currency units by an estimate of their purchasing power parity compared with a US dollar. Purchasing power parities are the rates of currency conversion that equalise the purchasing power of different currencies by eliminating the differences in price levels between countries.
Table 3.
Costs of interventions to improve child health at 95% coverage in Afr-E and Sear-D
| Proportions/Nos for all regions
|
Cost per episode consulting ($Int)*
|
Cost per case using antibiotic ($Int)†
|
Cost per case admitted to hospital ($Int)‡
|
Cost per capita per year ($Int)
|
Cost per recipient per year ($Int)
|
|||
|---|---|---|---|---|---|---|---|---|
| Intervention and WHO regions | Outpatient visits | Inpatient days | Drugs or supplements for all regions | |||||
| Oral rehydration therapy: | ||||||||
| Afr-E
|
1 | 3.5 | Two packets of oral rehydration solution per patient ($Int0.07 per pack)
|
6.13
|
—
|
78.59
|
—
|
—
|
| Sear-D
|
|
|
4.28
|
—
|
72.83
|
—
|
—
|
|
| Case management of pneumonia: | ||||||||
| Afr-E
|
1.186 | 4.9 | Cotrimoxazole suspension (100 ml bottle $Int0.30) for outpatient and chloramphenicol injection (7 vials: $Int1.06/500 mg vial), and intravenous fluid/set ($Int2.40)
|
5.8
|
10.16
|
158.18
|
—
|
—
|
| Sear-D
|
|
|
3.52
|
6.34
|
147.76
|
—
|
—
|
|
| Provision of supplementary food and nutrition counselling: | ||||||||
| Afr-E
|
1.2 (one full initial visit plus two other visits at 10% each)
|
— | Food per capita per year $Int116.23 | —
|
—
|
—
|
—
|
317.30
|
| Sear
|
|
|
—
|
—
|
—
|
—
|
310.91
|
|
| Vitamin A supplementation: | ||||||||
| Afr-E
|
0.66 (two visits at 33% per visit)
|
— | Vitamin A supplement per capita per year $Int0.05 | —
|
—
|
—
|
0.33
|
5.42
|
| Sear-D
|
|
|
—
|
—
|
—
|
0.23
|
2.45
|
|
| Zinc supplementation: | ||||||||
| Afr-E
|
0.1 (i.e. 1 visit at 10%)
|
— | Zinc supplement per capita per year $Int1.00
|
—
|
—
|
—
|
0.17
|
0.91
|
| Sear-D
|
|
—
|
—
|
—
|
0.10
|
0.65
|
||
| Vitamin A fortification: | ||||||||
| Afr-E
|
— | — | Vitamin A fortificant per capita per year $Int0.03-0.05
|
—
|
—
|
—
|
|
|
| Sear-D
|
|
|
—
|
—
|
—
|
|
|
|
| Zinc fortification: | ||||||||
| Afr-E
|
— | — | Zinc fortificant per capita per year $Int0.01-0.03
|
—
|
—
|
—
|
|
|
| Sear-D
|
|
|
—
|
—
|
—
|
|
|
|
| Measles immunisation: | ||||||||
| Afr-E | 1 | Measles vaccine at $Int0.12 per dose and $Int0.02 for syringe | 3.89 | |||||
| Sear-D | 5.94 | |||||||
Afr-E=WHO defined region comprising countries in sub-Saharan Africa with high child mortality. Sear-D=WHO defined region comprising countries in South East Asia with high child mortality.
Proportion of cases consulting is 0.19 for oral rehydration therapy and 0.475 for case management of pneumonia.
Proportion of cases using antibiotics for case management of pneumonia is 0.095.
Proportion of cases admitted to hospital is 0.004 for oral rehydration therapy and 0.01 for case management of pneumonia.
Cost effectiveness analysis
For our analysis we assumed that interventions run for 10 years, after which time managers re-evaluate their strategies. This means that costs are incurred for only 10 years. However, any improvements in health that accrue because of the activities in those 10 years are included, regardless of when they occur.
To be able to assess the cost effectiveness of the current mix of interventions, we first compared interventions with a scenario of doing nothing to improve child health from today.11 If more resources are available, the decision whether to add a new intervention or to expand the first intervention is made on the basis of the incremental cost effectiveness ratio compared with the first intervention, and this sequential comparison is continued until there are no more additional health gains. This maps out the expansion path.
We carried out a sensitivity analysis to enable reporting of results with or without 3% discounting for DALYs and with or without age weighting.
Results
Results for the full set of interventions by region are available at www.who.int/choice. Consistent with all papers in this series, we present the results for two regions, both consisting of countries with high rates of child mortality.11 Sear-D is in South East Asia and Afr-E in sub-Saharan Africa.
Costs
The highest costs at the population level are those for the provision of supplementary food and nutrition counselling, but targeting food supplementation through growth monitoring and promotion cuts costs by 40% in Sear-D and about 52% in Afr-E. Fortification programmes are the least costly, and fortification with zinc or vitamin A costs between 5% and 30% of the costs of supplementation. The total population costs for case management of pneumonia are lower than that for oral rehydration therapy because there are fewer episodes of pneumonia than there are of diarrhoea.
The proportion of patient level costs for interventions aimed at the individual range from about 80% to 99% of the total costs because most of the interventions are directed to the individual and not to the population. The bulk comes from commodities (drugs, supplementary food), outpatient visits, and hospital days. These costs increase almost linearly with increasing coverage. Because of the relatively small proportion of programme to total costs for almost all interventions except for fortification, there is limited potential to spread them across a larger number of recipients, so the unit cost of interventions is not observed to fall with increases in coverage.
Effectiveness
In general, higher population health gains are obtained from case management of pneumonia, oral rehydration therapy, and measles immunisation, followed by the nutritional interventions. Supplementation averts more DALYs than does fortification, primarily because of the limited access in some areas to processed food. Combinations of interventions produce additive or near additive gains, with the highest health gains achieved with a bundle of interventions that includes oral rehydration therapy, case management of pneumonia and diarrhoea, measles immunisation, vitamin A and zinc supplementation, the provision of supplementary feeding, and nutrition counselling.
Cost effectiveness
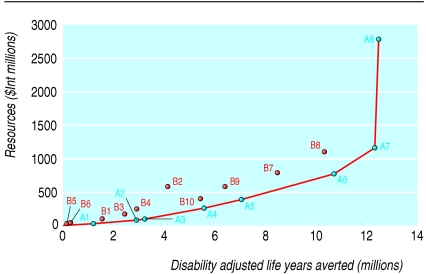
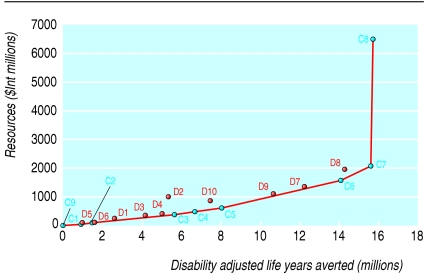
Tables 4 and 5 report the costs and effects of the most cost effective set of interventions in Afr-E and Sear-D, respectively. Figures 1 and 2 summarise these results. The expansion path—the bold line joining the points at the lower right of the figures—shows the interventions that would be chosen on purely cost effectiveness grounds for any level of resource availability.
Table 4.
Costs, effects, and cost effectiveness ratios for most cost effective interventions in Afr-E in 2000
| Intervention package | Description (coverage) of package | Additional interventions* | Yearly cost ($Int millions) | DALYs averted per year (millions) | ACER ($Int per DALY averted) | ICER ($Int per DALY averted) |
|---|---|---|---|---|---|---|
| A1 | Vitamin A and zinc fortification (95%) | Vitamin A and zinc fortification of food staple (95%) | 23 | 1 | 19 | 19 |
| A2 | A1 + measles immunisation (80%) | Measles immunisation | 72 | 3 | 25 | 29 |
| A3 | A2 + measles immunisation (95%) | Measles immunisation | 91 | 3 | 28 | 58 |
| A4 | A3 + case management of pneumonia (80%) | Case management of pneumonia | 261 | 6 | 47 | 73 |
| A5 | Vitamin A and zinc supplementation + case management of pneumonia (80%) + measles immunisation (95%) | Fortification replaced by supplementation | 386 | 7 | 55 | 85 |
| A6 | A5 + oral rehydration therapy (80%) | Oral rehydration therapy for diarrhoea | 772 | 11 | 72 | 106 |
| A7 | A6 (95%) | Coverage expanded to 95% | 1167 | 12 | 95 | 243 |
| A8 | A7 + provision of supplementary food and nutrition counselling, and growth monitoring and promotion (95%) | Provision of supplementary food and nutritional counselling, growth monitoring and promotion | 2 797 | 12 | 225 | 12 791 |
DALY=disability adjusted life year; ICER=incremental cost effectiveness ratio; ACER=average cost effectiveness ratio; Afr-E=WHO defined region comprising countries in sub-Saharan Africa with high child mortality.
In order of cost effectiveness.
Table 5.
Costs, effects, and cost effectiveness ratios for most cost effective interventions in Sear-D in 2000
| Intervention package | Description (coverage) of package | Additional interventions* | Yearly cost ($Int millions) | DALYs averted per year (millions) | ACER ($Int per DALY averted) | ICER ($Int per DALY averted) |
|---|---|---|---|---|---|---|
| C1 | Zinc fortification (95%) | Zinc fortification of food staple | 12 | 1 | 14 | 14 |
| C2 | C1 + vitamin A fortification (95%) | Vitamin A fortification of food staple | 49 | 1 | 35 | 70 |
| C3 | C2 + case management of pneumonia (80%) | Case management of pneumonia | 365 | 6 | 64 | 74 |
| C4 | C3 (95%) | Case management of pneumonia expanded to 95% | 470 | 7 | 70 | 99 |
| C5 | C4 + measles immunisation (95%) | Measles immunisation | 609 | 8 | 75 | 102 |
| C6 | Zinc supplementation + oral rehydration therapy + case management of pneumonia + measles immunisation (95%) | Zinc fortification replaced by supplementation, oral rehydration therapy for diarrhoea | 1560 | 14 | 111 | 158 |
| C7 | C6 + vitamin A supplementation (95%) | Vitamin A fortification replaced by supplementation | 2094 | 16 | 134 | 250 |
| C8 | C7 + provision of supplementary food and nutrition counselling, and growth monitoring and promotion (95%) | Provision of supplementary food and nutritional counselling, growth monitoring and promotion | 6546 | 16 | 416 | 44 384 |
DALY=disability adjusted life year; ICER=incremental cost effectiveness ratio; ACER=average cost effectiveness ratio; Sear-D=WHO defined region comprising countries in South East Asia with high adult and child mortality.
In order of cost effectiveness.
Fig 1.
Expansion path for most cost effective set of interventions to improve child health in Afr-E (WHO defined region comprising countries in sub-Saharan Africa with high child mortality)
Fig 2.
Expansion path for most cost effective set of interventions to improve child health in Sear-D (WHO defined region comprising countries in South East Asia with high child mortality)
For both Sear-D and Afr-E, the expansion path starts with some form of micronutrient fortification—that is, using vitamin A or zinc, followed by measles immunisation in Afr-E and then by case management of pneumonia and oral rehydration therapy. In Sear-D, oral rehydration therapy and measles immunisation follows closely after the case management of pneumonia. A shift occurs from fortification to supplementation at higher resource levels because, even if supplementation is more costly than fortification, a greater potential exists for health gain. The cost effectiveness of the interventions included in this set is either one or two orders of magnitude lower than those of the provision of supplementary food and nutrition counselling.
Removal of age weighting and discounting for DALYs increases the health gains and makes the interventions more cost effective. It does not change the ordering in either region.
Discussion
Cost effectiveness ratios vary across regions depending on the local epidemiology and existing cost structures. Despite this, there are enough similarities in the expansion paths in Sear-D and Afr-E (consisting of countries in South East Asia and sub-Saharan Africa with high rates of child mortality) to allow for some generalisations. At one extreme, fortification with vitamin A or zinc is very cost effective; provision of supplementary feeding with nutrition counselling is at the high end; and vitamin A or zinc supplementation, oral rehydration therapy, case management of pneumonia, and measles immunisation are in between.
Many countries have already made available oral rehydration therapy for diarrhoea, case management of pneumonia, and measles immunisation. These interventions achieve the largest health gains by an individual intervention, and the question is whether these should be scaled up further or if there are more cost effective choices. Purely on cost effectiveness grounds, countries should first consider fortification with micronutrients or supplementation with vitamin A or zinc.
Fortification seems to be a particularly cost effective option because it allows the possibility of fortifying a food staple with multiple micronutrients, not just those under discussion in this paper. In this analysis, the cost of fortification was based on adding the micronutrient to different food staples that would reach the entire population. In the analysis, however, we included only the benefits obtained in the under 5s age group. Furthermore, for both supplementation and fortification, we included only the impact on childhood pneumonia and diarrhoea even though provision of these micronutrients may also have a beneficial effect on malaria in children in sub-Saharan Africa and other causes of mortality in the under 5s age group.15,16 On the one hand this suggests that our estimates of the cost effectiveness of micronutrient interventions are conservative. On the other hand it might not be feasible to find suitable processed food that young children will eat or there may be constrained access to processed food by people living in remote areas or by the poor, which would bias the results in the opposite direction.
In terms of costing, all interventions except micronutrient supplementation were assumed to be delivered in health facilities. When some types of treatment for diarrhoea and pneumonia and the provision of micronutrient supplements could be provided in the community by trained non-health professionals, costs would be lower as would the cost effectiveness ratios, making our estimates conservative. Alternatively, measles immunisation can be delivered in national outreach activities or campaigns, but these delivery strategies are in general more costly than facility based interventions and will decrease the cost effectiveness of this intervention, unless other types of health interventions such as distribution of insecticide treated nets are piggy backed on immunisation campaigns.30
Because of different types of uncertainty, the results should be considered in bands of cost effectiveness. In both regions, fortification and supplementation with vitamin A or zinc and case management of pneumonia, oral rehydration therapy, and measles immunisation cost about $Int100 per DALY averted. Along similar lines, the cost effectiveness ratio for food supplements with nutrition counselling is several orders of magnitude more than these interventions. Accordingly, the outlines for policy recommendations at the beginning of this section still hold despite the uncertainty—on purely efficiency grounds, fortification or supplementation with vitamin A or zinc, treatment of pneumonia and diarrhoea, and measles immunisation should be pushed to their highest possible levels before supplementation of food along with nutrition counselling is provided.
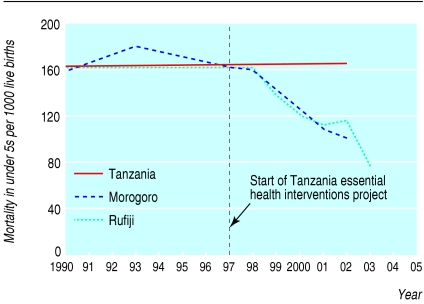
It is envisioned that analysts in countries will contextualise these results to their own settings and that policy makers can consider changing their investment decisions if so indicated.31 In figure 3, two districts in Tanzania have shown that, after considering the existing burden of disease, changing the allocation of health resources, and effectively implementing cost effective interventions (mostly aimed at the under 5s age group), they can achieve considerable health gains, including a 40% drop in child mortality in five years.32
Fig 3.
Decreases in child mortality in 10 years in two districts in Tanzania compared with national average, after analysis of burden of disease and reallocation of resources32
Our analysis presents cost effectiveness information on an initial set of interventions tackling pneumonia and diarrhoea, the two major causes of death in children aged under 5. Measles has also been included because it is specified as a target for millennium development goal 4. The absence of other interventions does not imply that these are not cost effective. Some interventions, such as treatment of malaria, use of insecticide treated nets, and promotion of breast feeding have been analysed in other papers in this series. Further reports will be completed in the future—such as for pneumococcal, haemophilus influenzae, and rotavirus immunisations—when more data on efficacy and region specific information on causes are available.
Progress in the other millennium development goals, such as the reduction of poverty and improvements in safe water, sanitation, and indoor air quality, will also impact on child health. People interested in public health could also engage in intersectoral action to encourage these developments. This is particularly true in the area of malnutrition, where it is striking that the health sector intervention we analysed is relatively cost ineffective, primarily because of the limited effectiveness of behaviour change interventions and the cost of providing ready to eat food supplements. Interventions to reduce poverty may, however, provide a more sustainable solution to malnutrition, although progress in reducing poverty might prove to be a more intractable goal than reducing child mortality.33 With malnutrition being an underlying cause of almost half of the deaths in children aged under 5, there is an urgent health research agenda to find more effective and less costly ways of ensuring availability of food to young infants and children. This is potentially the weakest point in the child survival strategy.
The availability and scaling up of technological solutions such as antibiotics, oral rehydration therapy, vaccines, and, in particular, micronutrient supplements have a great potential in reducing child mortality. Major challenges in the achievement of the millennium development goal 4 will be to find a sustainable intersectoral solution to reducing malnutrition in children and tackling the root causes of poverty, lack of education, and sex inequality.
What is already known on this topic
Many countries are not on track to meet the millennium development goal for child health of reducing child mortality by two thirds
Malnutrition remains an underlying cause in half of the deaths in children
Interventions of proved effectiveness exist
What this study adds
Child health interventions to reduce mortality are not only effective but cost effective
More resources could be allocated to interventions tackling underlying causes; specifically to scale up micronutrient provision and to find intersectoral solutions for malnutrition
Failure to tackle malnutrition in a sustainable way is a potential weakness in the child survival strategy
Supplementary Material
 Additional tables and descriptions of the interventions are on bmj.com
Additional tables and descriptions of the interventions are on bmj.com
Contributors: TT-TE wrote the paper and carried out the effectiveness analysis. MA did the costing for all interventions except for measles immunisation. LW and RH calculated the incidence rates and costs of measles immunisation, respectively, at different levels of coverage. DBE and TT-TE helped to develop the methods and apply them to this problem. DBE and RB provided references, critiqued the work, and helped revise the manuscript. Ben Johns, Taghreed Adam, and Rob Baltussen helped produce the cost estimates for WHO-CHOICE. Jeremy Lauer, Dan Chisholm, and Yunpeng Huang assisted with the PopMod runs. Chika Hayashi provided the first template on effectiveness. Taghreed Adam, Dan Chisholm, and Stephen Lim assisted in finalising figures and tables and in checking versions of the manuscript. Margaret Squadrani and Marilyn Vogel assisted in referencing. Data and feedback were given by the Child Health and Development Unit in WHO. Chris Murray provided general guidance and critiqued earlier versions of the paper. TT-TE is guarantor. The opinions in this paper are those of the authors and not necessarily those of the institutions they represent.
Funding: None.
Competing interests: None declared.
Ethical approval: Not required.
References
- 1.Bryce J, Boschi-Pinto C, Shibuya K, Black RE. WHO estimates of the causes of death in children. Lancet 2005;365: 1147-52. [DOI] [PubMed] [Google Scholar]
- 2.Black RE, Morris SS, Bryce J. Where and why are 10 million children dying every year? Lancet 2003;361: 2226-34. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. The world health report 2002: reducing risks, promoting healthy life. Geneva: WHO, 2002. [DOI] [PubMed]
- 4.Jones G, Steketee R, Black RE, Bhutta ZA, Morris SS, and the Bellagio Child Survival Study Group. How many child deaths can we prevent this year? Lancet 2003;362: 65-71. [DOI] [PubMed] [Google Scholar]
- 5.Evans DB, Adam T, Tan-Torres Edejer T, Lim SS, Cassels A, Evans TG, et al. Achieving the millennium development goals for health: Time to reassess strategies for improving health in developing countries? BMJ 2005;331: 1133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Bank. World development report 1993: investing in health. New York: Oxford University Press, 1993.
- 7.Jamison DT, Mosley WH, Measham AR, Bobadilla JL. Disease control priorities in developing countries. New York: Oxford University Press, 1993.
- 8.Gelband H, Stansfield S. The evidence base for interventions to reduce under five mortality in low and middle-income countries. www.cmhealth.org/docs/wg5_paper9.pdf. WHO Commission on Macroeconomics and Health, Working Group 5 paper 9, 2001 (accessed 12 Dec 2001).
- 9.Murray CJ, Evans DB, Acharya A, Baltussen RM. Development of WHO guidelines on generalized cost-effectiveness analysis. Health Econ 2000;9: 235-51. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Making choices in health: WHO guide to cost-effectiveness analysis. Geneva: WHO, 2003.
- 11.Evans DB, Tan-Torres Edejer T, Adam T, Lim SS, for the WHO-CHOICE MDG Team. Achieving the millennium development goals for health: Methods to assess the costs and health effects of interventions for improving health in developing countries. BMJ 2005;331: 1137-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray CJL, Lopez AD. Global health statistics: a compendium of incidence, prevalence and mortality estimates for over 200 conditions. Cambridge, MA: Harvard University Press, 1996.
- 13.Perry R, Halsey N. The clinical significance of measles: a review. J Infect Dis 2004;189: S4-16. [DOI] [PubMed] [Google Scholar]
- 14.Fishman S, Caulfield L, de Onis M, Blossner M, Hyder A, Mullany L, et al. Chapter 2. childhood and maternal underweight undernutrition. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, eds. Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors (vol 1). Geneva: WHO, 2004.
- 15.Rice A, West K, Fishman S, Black R. Chapter 4. Vitamin A deficiency. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, eds. Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors (vol 1). Geneva: WHO, 2004.
- 16.Caulfield L, Black R. Chapter 5. Zinc deficiency. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, eds. Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. Geneva: WHO, 2004.
- 17.Sazawal S, Black RE. Meta-analysis of intervention trials on case-management of pneumonia in community settings. Lancet 1992;340: 528-33. [DOI] [PubMed] [Google Scholar]
- 18.Beaton GH, Martorell R, Aronson KJ, Edmonston B, McCabe G, Ross AC, et al. Effectiveness of vitamin A supplementation in the control of young child morbidity and mortality in developing countries. New York: United Nations, 1993. ACC/SCN State-of-the-Art Series. Nutrition policy discussion paper No 13.
- 19.Bhutta ZA, Bird SM, Black RE, Brown KH, Gardner JM, Hidayat A, et al. Therapeutic effects of oral zinc in acute and persistent diarrhea in children in developing countries: pooled analysis of randomized controlled trials. Am J Clin Nutr 2000;72: 1516-22. [DOI] [PubMed] [Google Scholar]
- 20.Victora CG, Olinto MT, Barros FC, Nobre LC. Falling diarrhoea mortality in northeastern Brazil: did ORT play a role? Health Policy Plan 1996;11: 132-41. [DOI] [PubMed] [Google Scholar]
- 21.Miller P, Hirschhorn N. The effect of a national control of diarrheal diseases program on mortality: the case of Egypt. Soc Sci Med 1995;40: S1-30. [DOI] [PubMed] [Google Scholar]
- 22.Caulfield L, Huffman S, Piwoz E. Interventions to improve intake of complementary foods by infants 6 to 12 months of age in developing countries: impact on growth and on the prevalence of malnutrition and potential contribution to child survival. Food Nutr Bull 1999;20: 183-99. [Google Scholar]
- 23.Pampanich R, Garner P. Growth monitoring in children. Cochrane Database Syst Rev 2000;(2): CD001443. [DOI] [PubMed]
- 24.Mora JO, Dary O, Chinchilla D, Arroyave G. Vitamin A sugar fortification in Central America. Experience and lessons learned. Arlington, VA: MOST, US Agency for International Development Micronutrient Program, 2000.
- 25.Dilraj A, Cutts FT, de Castro JF, Wheeler JG, Brown D, Roth C, et al. Response to different measles vaccine strains given by aerosol and subcutaneous routes to schoolchildren: a randomised trial. Lancet 2000;355: 798-803. [DOI] [PubMed] [Google Scholar]
- 26.Singh J, Datta KK. Measles vaccine efficacy in India: a review. J Commun Dis 1997;29: 47-56. [PubMed] [Google Scholar]
- 27.Adam T, Evans DB, Murray CJL. Econometric estimation of country-specific hospital costs. Cost Eff Resour Alloc 2003;1: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pieche S. Care seeking behaviour of caretakers of sick children (draft report). Geneva: WHO, 1998.
- 29.Horton S. Unit costs, cost-effectiveness, and financing of nutrition interventions. Washington, DC: Population and Human Resources Department, 1992. World Bank. WPS-952.
- 30.Pegurri E, Fox-Rushby JA, Damian W. The effects and costs of expanding the coverage of immunisation services in developing countries: a systematic literature review. Vaccine 2005;23: 1624-35. [DOI] [PubMed] [Google Scholar]
- 31.Hutubessy R, Chisholm D, Edejer TT, WHO-CHOICE. Generalized cost-effectiveness analysis for national-level priority-setting in the health sector. Cost Eff Resour Alloc 2003;1: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Savigny D, Kasale H, Mbuya C, Reid G. Fixing health systems. Ottawa, Canada: International Development Research Centre, 2004.
- 33.United Nations. The millennium development goals report 2005. New York: United Nations, 2005: 1-43.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.