Abstract
Transmissible mink encephalopathy (TME) is a rare disease of the North American mink, which has never been successfully transmitted to laboratory mice. We generated transgenic mice expressing the mink prion protein (PrP) and inoculated them with TME or the mouse-adapted scrapie strain 79A. TME infected mink PrP-transgenic mice on a murine PrP knockout background. The absolute species barrier between the infectious agent of TME and mice was therefore broken. Following TME and 79A infection of mice carrying both mink and murine PrPC, only proteinase-resistant PrP homologous to the incoming agent was detectable. The presence of the murine PrPC prolonged the incubation time of TME substantially.
Transmissible spongiform encephalopathies (TSEs) or prion diseases are a group of neurodegenerative diseases including Creutzfeldt-Jakob disease (CJD) in humans, bovine spongiform encephalopathy (BSE) in cattle and scrapie in sheep. These diseases are characterized by the accumulation of an abnormal isoform of the cellular prion protein (PrPC), termed PrPSc, in the brain of the affected individuals. PrPSc is thought to be the principal component of the infectious agent (23).
Experimental transmission to laboratory rodents has been achieved with TSE agents from many naturally affected species. In these studies, the concept of the species barrier was developed, which refers to the relative resistance to disease encountered following experimental inoculation with TSE agents derived from a different species (21). The adaptation of a TSE agent to its new host requires one or more passages. The species barrier can be quantified by the reduction in the incubation time between primary and secondary passage in the new host.
Transmissible mink encephalopathy (TME) of the North American mink (Mustela vison) was first recognized in 1947 and subsequently described as a TSE in 1965 by Hartsough and Burger (13, 19). Only very few outbreaks of this disease in farmed mink have been described and the cause for these outbreaks is unknown. As scrapie and BSE were experimentally transmitted to mink and, vice versa, TME was transmitted to sheep and cattle, a causal link between the ruminant diseases and TME seems plausible (11, 12, 24, 25). TME was experimentally transmitted to hamsters, but has never been successfully transmitted to mice (2, 18, 30). The species barrier between TME and mice could therefore be termed as an ‘absolute’ one.
The coding region of the mink PrP gene was sequenced and the deduced amino acid sequence showed a similarity to mouse PrPC of around 90% in the mature protein (17) (Fig. 1A). There is strong evidence that transmission of TSEs is tightly controlled by the PrP-encoding gene (PRNP for humans, Prnp for mice and PrP gene for all other species). Studies with transgenic animals have shown that the species barrier encountered during transmission from nonmurine TSE strains to mice is overcome by introducing the respective nonmurine PrP gene into mice (5, 8, 26, 28, 29, 34). Subsequent investigations, however, have revealed that the expression of a donor-derived PrP transgene may not always be sufficient to erase the species barrier to TSE transmissions and host and strain-specific factors may play a role as well (6, 14, 31, 32).
FIG. 1.
(A) Sequence comparison between murine and mink PrP. The sequence of the murine Prnpa allele (36) is shown and differences in the mink PrP sequence (17) are indicated underneath. The numbering is according to the murine PrP. The single letter code for amino acids is used and only the sequences of the mature PrP devoid of the N- and C-terminal signal peptides are shown. Amino acids proposed to be involved in protein X-binding (15) are underlined and amino acids proposed to be involved in the PrPSc/PrPC interface (28) are highlighted in bold and italics. (B) Schematic representation of cosMink. Exonic sequences of the hamster PrP gene are represented by white boxes and the coding region of the mink PrP gene is highlighted in gray.
In the report provided here, we have expanded these transgenic studies to TME and introduced the mink PrP gene into transgenic mice.
Generation of transgenic mice.
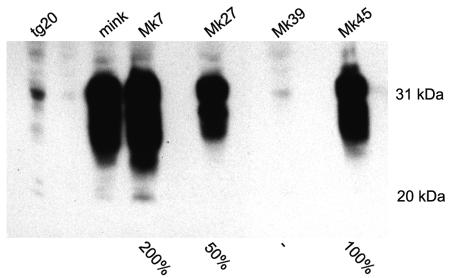
The construct cosMink was derived from the vector cosSHa.Tet (27) and contained the coding region of the mink PrP gene under the control of the hamster PrP gene promoter (Fig. 1B). The vector does not contain the gene coding for Doppel (20). The large NotI fragment of cosMink was injected into fertilized oocytes of either FVB or C57BL/6 mice. The offspring was screened for the presence of the transgene by using PCR and four founder animals were identified (MK7, MK27, MK39, and MK45). The copy number of the transgene was determined by a densitometric analysis of a Southern blot hybridization of EcoRI-digested genomic DNA (Table 1). Expression of the transgene in brain tissue was detected by Western blot analysis using the antibody L42 (35). The expression levels of the transgenic protein in MK7, MK27, and MK45 were compared with the expression of PrP in the brain of an American mink and were 200%, 50%, and 100% respectively, while no synthesis of the transgenic protein was visible in MK39 (Table 1; Fig. 2).
TABLE 1.
Transgenic mouse lines carrying the mink PrP gene
| Line no. | Background | Copy no./haploid genome | Expression |
|---|---|---|---|
| MK7 | FVB | 4-5 | 200% |
| MK27 | C57Bl/6 | 1-2 | 50% |
| MK39 | C57Bl/6 | 2-3 | |
| MK45 | FVB | 4-5 | 100% |
FIG. 2.
Western blot analysis of transgenic mouse lines carrying the mink PrP gene. Brain homogenates of F1 animals of the four different transgenic lines carrying the mink PrP gene, MK7, MK27, MK39, and MK45 were analyzed alongside of North American mink and tg20, a transgenic mouse line overexpressing the murine Prnp (9). Antibody L42 was used as primary antibody in the Western blot analysis following SDS-PAGE. Molecular size markers are indicated to the right of the blot and estimates of the amount of mink PrP in the different transgenic lines compared to the mink are indicated underneath.
Infection of lines MK7 and MK45 with TME on a murine PrP-deficient background.
Lines MK7 and MK45 were selected for transmission experiments and were crossed with Prnp0/0 mice (4) over two generations to generate mice hemizygous for the transgene and homozygous for Prnp0/0. A TME isolate was received from a mink experimentally infected with the Stetsonville TME agent (1). In parallel, a mouse-adapted scrapie strain, 79A, was used (3, 10). The transgenic mice together with normal inbred FVB and C57BL/6 mice were inoculated intracerebrally with brain homogenate from either of these isolates or with Hanks' balanced salt solution (HBSS) as control.
The TME agent did not infect C57BL/6 or FVB inbred mice after more than 800 days (Table 2). In contrast, transgenic mice expressing the mink PrP gene on a Prnp0/0 background were readily infected with the TME agent and succumbed to disease with a mean incubation time of 164 days (MK7/Prnp0/0) or 312 days (MK45/Prnp0/0). The absolute species barrier of mice towards the TME agent was therefore broken by the introduction of the mink PrP gene and the incubation time was inversely proportional to the level of mink PrP gene expression.
TABLE 2.
Susceptibility of transgenic mouse lines carrying the mink PrP gene to TME and 79A
| Line no. | Isolates for primary transmission
|
Isolaets for secondary transmission
|
|||
|---|---|---|---|---|---|
| TMEa | 79Aa | HBSSb | TME in MK7/Pmp0/0a | TME in MK45/Pmp0/0a | |
| MK7/FVB | 459 ± 100.8 (5/5)d | 149 ± 3.8 (5/5)c | NAe | ||
| MK7/Pmp0/0 | 164 ± 9.6 (6/6)c | >800 (1/6) | >800 (0/6) | 148 ± 7.4 (10/10)c | 147 ± 7.3 (10/10)c |
| MK45/FVB | >800 (0/6) | 167 ± 12.2 (6/6)c | >800 (0/6) | ||
| MK45/Pmp0/0 | 312 ± 12.1 (6/6)c | NA | >800 (0/6) | ||
| FVB | >800 (0/6) | NA | NA | ||
| C57BL/6 | >800 (0/6) | 173 ± 6.6 (6/6)c | NA | ||
Six (primary transmission) or ten (secondary transmission) animals were intracerebrally inoculated per group with 10% brain homogenate.
Six animals were intracerebrally inoculated per group with HBSS.
Values are means of days to terminal disease following inoculation ± standard deviation. Each value in parenthesis is the number of animals terminally ill/number of animals inoculated. Mice that died from intercurrent diseases were not taken into consideration.
Single values of days to terminal disease were 323, 387, 507, 512, and 568.
NA, not analyzed.
The mouse-adapted scrapie strain 79A led to disease in wild-type mice (C57BL/6) with a mean incubation time of 173 days. In contrast, only one of six MK7/Prnp0/0 mice infected with the 79A agent showed clinical and neuropathological signs of prion disease after an incubation time of more than 800 days. This finding suggested that mink PrPC is a poor mediator of disease progression for mouse-adapted scrapie.
Brain material of terminally ill MK7/Prnp0/0 and MK45/Prnp0/0 mice infected with TME was inoculated into MK7/Prnp0/0 mice in a secondary transmission (Table 2). Both transmission experiments showed remarkable similar mean incubation times of 147 or 148 days, which is slightly shorter than 164 days of the primary transmission of TME into MK7/Prnp0/0 mice. This indicated that there may be a small species barrier to overcome in the primary transmission, but reliable estimate of the titers in the primary and secondary passage will require titration experiments.
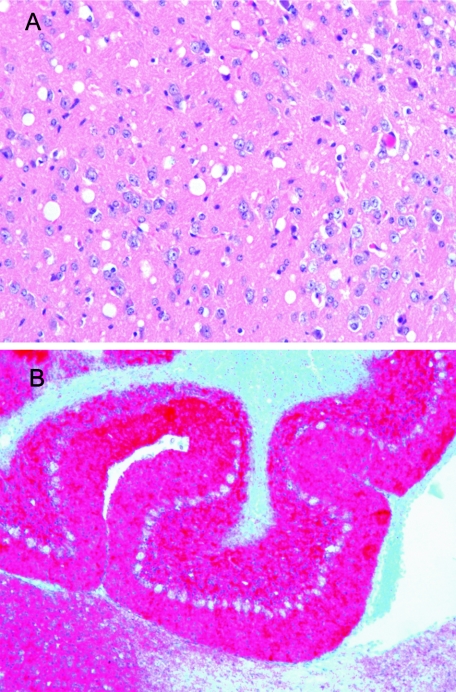
Histopathological examination revealed massive spongiosis, neuronal loss and gliosis in the cortex of MK7/Prnp0/0 mice (Fig. 3), while these changes were completely absent in mock-infected animals (data not shown). Immunohistochemical examination using antibody L42 revealed strong deposition of the mink PrPSc in the cortex and delicate granular staining in the cerebellum (Fig. 3). Quite distinctly, the Purkinje cells were devoid of staining. A mock-infected MK7/Prnp0/0 mouse did not show detectable PrPSc deposition (data not shown).
FIG. 3.
Histopathological analysis of infected MK7/Prnp0/0 mice. (A) Cerebral cortex showing numerous delicate vacuoles (spongiform change) stained with hematoxylin and eosin Original magnification, ×20. (B) Cerebellum showing strong immunostaining for PrPSc in red in the molecular and internal granule cell layer using L42 as primary antibody. Original magnification, ×10.
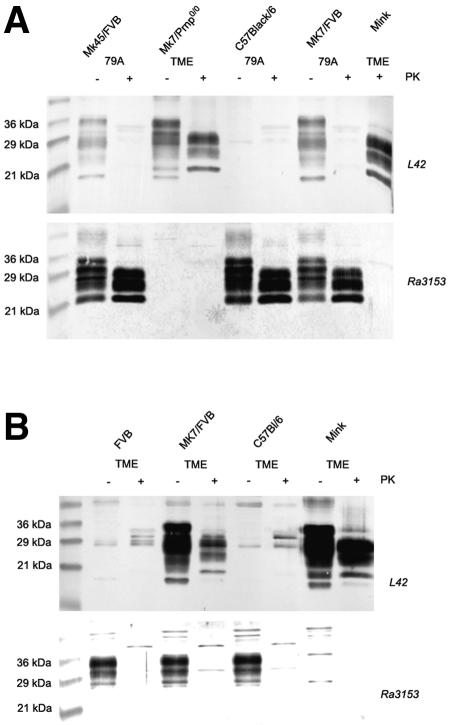
PrPSc deposited in the brain of terminally ill animals was further examined by Western blot analysis. These analyses benefited from two antibodies, L42, which detected mink PrP but not mouse PrP, and Ra3153 (37), which detected mouse PrP but not mink PrP (Fig. 4A). Following proteinase K (PK) digestion, the typical banding pattern of three PrPSc isoforms between about 20 and 30 kDa was visible in brain homogenates of all terminally ill animals, but not in normal mice or mice that proved to be resistant to TME infection (Fig. 4A and 4B). The distribution of the three PrPSc-specific bands, which most likely represent un-, mono-, and diglycosylated PrPSc, was clearly different in animals infected with TME from those infected with 79A. TME-infected animals presented with an overrepresentation of diglycosylated PrPSc, while in 79A-infected mice, the unglycosylated and monoglycosylated forms of PK-resistant PrPSc were prominent.
FIG. 4.
Western blot analysis of PK-resistant PrP in brain homogenates of infected animals. (A) Brain homogenates of transgenic mice (MK45/FVB, MK7/FVB) infected with 79A were compared to normal mice (C57/Black6) infected with the same agent as well as to Mk7/Prnp0/0 mice and mink infected with TME. Homogenates were either treated with PK (+) or left untreated (−). The upper panel was immunostained using L42 as primary antibody, while the lower panel was stained using Ra3153. A molecular marker was loaded in the left lane, and the molecular sizes are indicated. (B) Brain homogenates of normal mice (FVB and C57/Black6), transgenic MK7/FVB and mink, all infected with TME, were compared using either L42 (upper panel) or Ra3153 (lower panel) as the primary antibody.
It is noteworthy that the use of a gene chimeric for the mink and mouse PrP genes similar to the human-mouse chimera used by Telling and colleagues was not necessary to generate mice susceptible to TME (15, 31, 32). The mink sequence in the C-terminal end of the protein seems fully sufficient to interact with the postulated host-specific protein X to support the conversion process between the incoming PrPSc and the residual PrPC. The amino acids 167, 171, 214, and 218 (numbered according to the murine PrP) postulated to form the discontinuous, protein X-binding epitope are all identical between the murine and the mink PrP (Fig. 1A), which is in line with this hypothesis.
Infection of lines MK7 and MK45 with TME on a murine PrP background.
In a separate line of breeding, the transgenic animals were crossed with nontransgenic inbred mice (FVB) instead of Prnp0/0 mice. These mice produced the mink PrP along with with the murine PrP and were inoculated with the TME agent and scrapie strain 79A. The TME agent caused disease in MK7/FVB mice after a mean incubation time of 459 days, which was dramatically prolonged compared with the incubation time of 164 days in MK7/Prnp0/0 mice (Table 2). TME did not cause disease in the lower-expressing MK45/FVB within 800 days. The presence of the endogenous murine PrP had therefore a strong inhibitory effect on the disease progression of TME in mice expressing mink PrP genes.
In sharp contrast to the TME agent, the mouse-adapted scrapie strain 79A infected both transgenic lines on normal background with a mean incubation time of 149 (MK7/FVB) and 167 days (MK45/FVB). These incubation times are comparable to or even slightly shorter than the 173 days following infection of normal mice with 79A. The presence of the mink transgene had therefore no recognizable inhibitory effect on the efficiency of the infection of 79A in mice.
The analysis of the brain homogenates without PK digestion showed that indeed PrP of both species was present in transgenic mice on a murine PrP-normal background (Fig. 4A for MK7/FVB mice and MK45/FVB mice infected with 79A and Fig. 4B for MK7/FVB mice infected with TME), while after PK digestion, only murine PrPSc was visible in 79A-infected mice (Fig. 4A) and only mink PrPSc could be detected in TME infected animals (Fig. 4B). Similar to an experiment expressing the hamster PrP gene in transgenic mice and infecting them with either hamster- or mouse-adapted scrapie (22), the infecting agent determined the type of PrP that accumulated in the brain of the diseased transgenic mouse. It is particularly noteworthy that also the banding pattern of the PK-resistant PrP corresponded the pattern of the inoculum. This banding pattern is considered as a strain signature and it has been shown in experimentally infected normal and transgenic mice to be faithfully transmitted (7, 16, 33).
One possible hypothesis that would explain these findings in mice producing both mink and mouse PrPC is that mouse PrPSc does not interact with mink PrPC and, therefore, the interaction of the incoming mouse PrPSc (79A) with the homologous mouse PrPC can progress with the same efficiency as in the absence of mink PrP, i.e., in the normal mouse. The incoming mink PrPSc (TME), however, can interact with mouse PrPC, but the conversion of this heterologous complex either cannot progress efficiently or converted mouse PrPSc cannot bind mouse or mink PrPC or, if bound, cannot convert mink or mouse PrPC. The homologous mink PrP conversion takes place, but is much less efficient in the presence of endogenous mouse PrPC than without mouse PrPC, i.e., in the MK7-mice on a Prnp0/0 background. It is important to note that the amino acid residues 183, 202, and 204 (numbering according to the murine PrP), which define part of the PrPC/PrPSc interface according to the publication by Scott and colleagues (28), all differ between the murine and the mink PrP. The nature of the interaction between the mink PrPC and the mouse PrPSc or the mouse PrPC and the mink PrPSc or lack thereof could be tested in in vitro conversion assays.
Acknowledgments
We thank Peter Gruss for his support in generating transgenic mice, Ulli Francke and Rainer Libal for expert technical help with the generation of the transgenic mice, and Christina Oberdieck for her excellent technical support. We are grateful to Christina Ziegler and Ilka Mayer for conscientious animal care. We are indebted to Michael Scott and Stanley B. Prusiner for supplying us with cosSHa.Tet, to Debbie McKenzie for the TME isolate, and to Armin Giese for the 79A isolate.
This work was supported by the German Ministry of Education and Research, the European Commission, and the State of Bavaria.
REFERENCES
- 1.Bartz, J. C., R. A. Bessen, D. McKenzie, R. F. Marsh, and J. M. Aiken. 2000. Adaptation and selection of prion protein strain conformations following interspecies transmission of transmissible mink encephalopathy. J. Virol. 74:5542-5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bessen, R. A., and R. F. Marsh. 1992. Identification of two biologically distinct strains of transmissible mink encephalopathy in hamsters. J. Gen. Virol. 73:329-334. [DOI] [PubMed] [Google Scholar]
- 3.Bruce, M. E., P. A. McBride, M. Jeffrey, and J. R. Scott. 1994. PrP in pathology and pathogenesis in scrapie-infected mice. Mol. Neurobiol. 8:105-112. [DOI] [PubMed] [Google Scholar]
- 4.Büeler, H., M. Fischer, Y. Lang, H. Bluethmann, H. P. Lipp, S. J. DeArmond, S. B. Prusiner, M. Aguet, and C. Weissmann. 1992. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature 356:577-582. [DOI] [PubMed] [Google Scholar]
- 5.Buschmann, A., E. Pfaff, K. Reifenberg, H. M. Muller, and M. H. Groschup. 2000. Detection of cattle-derived BSE prions using transgenic mice-overexpressing bovine PrP(c). Arch. Virol. Suppl. 16:75-86. [DOI] [PubMed] [Google Scholar]
- 6.Collinge, J., M. S. Palmer, K. C. Sidle, A. F. Hill, I. Gowland, J. Meads, E. Asante, R. Bradley, L. J. Doey, and P. L. Lantos. 1995. Unaltered susceptibility to BSE in transgenic mice expressing human prion protein. Nature 378:21-28. [DOI] [PubMed] [Google Scholar]
- 7.Collinge, J., K. C. Sidle, J. Meads, J. Ironside, and A. F. Hill. 1996. Molecular analysis of prion strain variation and the aetiology of ‘new variant’ CJD. Nature 383:685-690. [DOI] [PubMed] [Google Scholar]
- 8.Crozet, C., F. Flamant, A. Bencsik, D. Aubert, J. Samarut, and T. Baron. 2001. Efficient transmission of two different sheep scrapie isolates in transgenic mice expressing the ovine PrP gene. J. Virol. 75:5328-5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer, M., T. Rulicke, A. Raeber, A. Sailer, M. Moser, B. Oesch, S. Brandner, A. Aguzzi, and C. Weissmann. 1996. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J. 15:1255-1264. [PMC free article] [PubMed] [Google Scholar]
- 10.Giese, A., D. R. Brown, M. H. Groschup, C. Feldmann, I. Haist, and H. A. Kretzschmar. 1998. Role of microglia in neuronal cell death in prion disease. Brain Pathol. 8:449-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadlow, W. J., R. E. Race, and R. C. Kennedy. 1987. Experimental infection of sheep and goats with transmissible mink encephalopathy virus. Can. J. Vet. Res. 51:135-144. [PMC free article] [PubMed] [Google Scholar]
- 12.Hanson, R. P., R. J. Eckroade, R. F. Marsh, G. M. Zu Rhein, C. L. Kanitz, and D. P. Gustafson. 1971. Susceptibility of mink to sheep scrapie. Science 172:859-861. [DOI] [PubMed] [Google Scholar]
- 13.Hartsough G. R., and D. Burger. 1965. Encephalopathy of mink. I. Epizootiologic and clinical observations. J. Infect. Dis. 115:387-392. [DOI] [PubMed] [Google Scholar]
- 14.Hill, A. F., M. Desbruslais, S. Joiner, K. C. Sidle, I. Gowland, J. Collinge, L. J. Doey, and P. Lantos. 1997. The same prion strain causes vCJD and BSE. Nature 389:448-450. [DOI] [PubMed] [Google Scholar]
- 15.Kaneko, K., L. Zulianello, M. Scott, C. M. Cooper, A. C. Wallace, T. L. James, F. E. Cohen, and S. B. Prusiner. 1997. Evidence for protein X binding to a discontinuous epitope on the cellular prion protein during scrapie prion propagation. Proc. Natl. Acad. Sci. USA 94:10069-10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kascsak, R. J., R. Rubenstein, P. A. Merz, R. I. Carp, N. K. Robakis, H. M. Wisniewski, and H. Diringer. 1986. Immunological comparison of scrapie-associated fibrils isolated from animals infected with four different scrapie strains. J. Virol. 59:676-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kretzschmar, H. A., M. Neumann, G. Riethmuller, and S. B. Prusiner. 1992. Molecular cloning of a mink prion protein gene. J. Gen. Virol. 73:2757-2761. [DOI] [PubMed] [Google Scholar]
- 18.Marsh, R. F., D. Burger, R. Eckroade, G. M. Zu Rhein, and R. P. Hanson. 1969. A preliminary report on the experimental host range of the transmissible mink encephalopathy agent. J. Infect. Dis. 120:713-719. [DOI] [PubMed] [Google Scholar]
- 19.Marsh, R. F., and W. J. Hadlow. 1992. Transmissible mink encephalopathy. Rev. Sci. Tech. 11:539-550. [DOI] [PubMed] [Google Scholar]
- 20.Moore, R. C., I. Y. Lee, G. L. Silverman, P. M. Harrison, R. Strome, C. Heinrich, A. Karunaratne, S. H. Pasternak, M. A. Chishti, Y. Liang, P. Mastrangelo, K. Wang, A. F. Smit, S. Katamine, G. A. Carlson, F. E. Cohen, S. B. Prusiner, D. W. Melton, P. Tremblay, L. E. Hood, and D. Westaway. 1999. Ataxia in prion protein (PrP)-deficient mice is associated with upregulation of the novel PrP-like protein doppel. J. Mol. Biol. 292:797-817. [DOI] [PubMed] [Google Scholar]
- 21.Pattison, I. H. 1965. Experiments with scrapie with special refererence to the nature of the agent and the pathology of the disease, p. 249-257. In D. C. Gajdusek, C. J. Gibbs, Jr., and M. P. Alpers (ed.), Slow, latent and temperate virus infections. NINDB Monograph 2. U.S. Government Printing Office, Washington, D.C.
- 22.Prusiner, S. B., M. Scott, D. Foster, P. K. Pan, D. Groth, C. Mirenda, M. Torchia, S. Yang, D. Serban, G. A. Carlson, P. C. Hoppe, D. Westaway, and S. J. DeArmond. 1990. Transgenetic studies implicate interactions between homologous PrP isoforms in scrapie prion replication. Cell 63:673-686. [DOI] [PubMed] [Google Scholar]
- 23.Prusiner, S. B. 1998. Prions. Proc. Natl. Acad. Sci. USA 95:13363-13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson, M. M., W. J. Hadlow, T. P. Huff, G. A. Wells, M. Dawson, R. F. Marsh, and J. R. Gorham. 1994. Experimental infection of mink with BSE. J. Gen. Virol. 75:2151-2155. [DOI] [PubMed] [Google Scholar]
- 25.Robinson, M. M., W. J. Hadlow, D. P. Knowles, T. P. Huff, P. A. Lacy, R. F. Marsh, and J. R. Gorham. 1995. Experimental infection of cattle with the agents of transmissible mink encephalopathy and scrapie. J. Comp. Pathol. 113:241-251. [DOI] [PubMed] [Google Scholar]
- 26.Scott, M., D. Foster, C. Mirenda, D. Serban, F. Coufal, M. Walchli, M. Torchia, D. Groth, G. Carlson, S. J. DeArmond, D. Westaway, and S. B. Prusiner. 1989. Transgenic mice expressing hamster prion protein produce species-specific scrapie infectivity and amyloid plaques. Cell 59:847-857. [DOI] [PubMed] [Google Scholar]
- 27.Scott, M. R., R. Kohler, D. Foster, and S. B. Prusiner. 1992. Chimeric prion protein expression in cultured cells and transgenic mice. Protein Sci. 1:986-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott, M. R., J. Safar, G. Telling, O. Nguyen, D. Groth, M. Torchia, R. Koehler, P. Tremblay, D. Walther, F. E. Cohen, S. J. DeArmond, and S. B. Prusiner. 1997. Identification of a prion protein epitope modulating transmission of BSE prions to transgenic mice. Proc. Natl. Acad. Sci. USA 94:14279-14284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott, M. R., R. Will, J. Ironside, H. O. Nguyen, P. Tremblay, S. J. DeArmond, and S. B. Prusiner. 1999. Compelling transgenetic evidence for transmission of BSE prions to humans. Proc. Natl. Acad. Sci. USA 96:15137-15142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor, D. M., A. G. Dickinson, H. Fraser, and R. F. Marsh. 1986. Evidence that transmissible mink encephalopathy agent is biologically inactive in mice. Neuropathol. Appl. Neurobiol. 12:207-215. [DOI] [PubMed] [Google Scholar]
- 31.Telling, G. C., M. Scott, K. K. Hsiao, D. Foster, S. L. Yang, M. Torchia, K. C. Sidle, J. Collinge, S. J. DeArmond, and S. B. Prusiner. 1994. Transmission of CJD from humans to transgenic mice expressing chimeric human-mouse prion protein. Proc. Natl. Acad. Sci. USA 91:9936-9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Telling, G. C., M. Scott, J. Mastrianni, R. Gabizon, M. Torchia, F. E. Cohen, S. J. DeArmond, and S. B. Prusiner. 1995. Prion propagation in mice expressing human and chimeric PrP transgenes implicates the interaction of cellular PrP with another protein. Cell 83:79-90. [DOI] [PubMed] [Google Scholar]
- 33.Telling, G. C., P. Parchi, S. J. DeArmond, P. Cortelli, P. Montagna, R. Gabizon, J. Mastrianni, E. Lugaresi, P. Gambetti, and S. B. Prusiner. 1996. Evidence for the conformation of the pathologic isoform of the prion protein enciphering and propagating prion diversity. Science 274:2079-2082. [DOI] [PubMed] [Google Scholar]
- 34.Vilotte, J. L., S. Soulier, R. Essalmani, M. G. Stinnakre, D. Vaiman, L. Lepourry, J. C. Da Silva, N. Besnard, M. Dawson, A. Buschmann, M. Groschup, S. Petit, M. F. Madelaine, S. Rakatobe, A. Le Dur, D. Vilette, and H. Laude. 2001. Markedly increased susceptibility to natural sheep scrapie of transgenic mice expressing ovine PrP. J. Virol. 75:5977-5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vorberg, I., A. Buschmann, S. Harmeyer, A. Saalmuller, E. Pfaff, and M. H. Groschup. 1999. A novel epitope for the specific detection of exogenous prion proteins in transgenic mice and transfected murine cell lines. Virology 255:26-31. [DOI] [PubMed] [Google Scholar]
- 36.Westaway, D., P. A. Goodman, C. A. Mirenda, M. P. McKinley, G. A. Carlson, and S. B. Prusiner. 1987. Distinct prion proteins in short and long scrapie incubation period mice. Cell 51:651-662. [DOI] [PubMed] [Google Scholar]
- 37.Xiang, W., O. Windl, G. Wünsch, M. Dugas, A. Kohlmann, N. Dierkes, I. M. Westner, and H. A. Kretzschmar. 2004. Identification of differentially expressed genes in scrapie-infected mouse brains by using global gene expression. J. Virol. 78:11051-11058. [DOI] [PMC free article] [PubMed] [Google Scholar]