Abstract
The anthrax incidents in the United States in the fall of 2001 led to the use of electron beam (EB) processing to sanitize the mail for the U.S. Postal Service. This method of sanitization has prompted the need to further investigate the effect of EB irradiation on the destruction of Bacillus endospores. In this study, endospores of an anthrax surrogate, B. atrophaeus, were destroyed to demonstrate the efficacy of EB treatment of such biohazard spores. EB exposures were performed to determine (i) the inactivation of varying B. atrophaeus spore concentrations, (ii) a D10 value (dose required to reduce a population by 1 log10) for the B. atrophaeus spores, (iii) the effects of spore survival at the bottom of a standardized paper envelope stack, and (iv) the maximum temperature received by spores. A maximum temperature of 49.2°C was reached at a lethal dose of ∼40 kGy, which is a significantly lower temperature than that needed to kill spores by thermal effects alone. A D10 value of 1.53 kGy was determined for the species. A surface EB dose between 25 and 32 kGy produced the appropriate killing dose of EB between 11 and 16 kGy required to inactivate 8 log10 spores, when spore samples were placed at the bottom of a 5.5-cm stack of envelopes.
The genus Bacillus consists of aerobic and facultatively anaerobic gram-positive rods capable of forming endospores (8). As internal dormant structures, endospores can withstand heat (120°C, 15 min), chemical disinfection (0.05%, sodium hypochlorite at 30 min; 500 mg liter−1 ethylene oxide at 30 min; or 0.88 mol liter−1 hydrogen peroxide), and low-dose gamma (10 kGy) irradiation (24). There are over 40 species within the Bacillus genus, including B. anthracis which causes the disease anthrax. While B. anthracis is most closely related phylogenetically (by 16S rRNA sequencing) to B. cereus, B. atrophaeus (formerly known as B. globigii or B. subtilis var. niger) has historically been used as a B. anthracis surrogate due to its lack of pathogenicity and unique colonial characteristics, even though it is more closely related to B. subtilis (1, 5, 12, 17, 27). In fact, B. atrophaeus has been used as a genus-level representative for a number of inactivation techniques, such as chemical and gamma radiation sterilizations (4-6, 24). While B. atrophaeus is an accepted surrogate for such studies, inter-species variations preclude complete correlation between B. anthracis and any surrogate when evaluating biological activities. We present data evaluating electron beam (EB) inactivation of B. atrophaeus as an anthrax surrogate.
Anthrax has been known as a potential bioterror agent since World War I, due to its relative ease of preparation and dispersion (7, 23). Today, anthrax is considered by the Centers for Disease Control and Prevention as a category A agent because of the ease of dispersal, potential public health impact, high mortality rates (if untreated), potential for public panic, and requirement for public health preparedness (21). The deliberate dissemination of B. anthracis endospores in the fall of 2001 through the U.S. Postal Service (USPS) claimed the lives of 5 people, infected 17 others in seven states along the East Coast, and marked the first modern bioterror event on U.S. soil (15). The release of B. anthracis spores through the U.S. mail called for creative methods of decontamination in addition to a reevaluation of the natural history of the disease. As a result, a series of chemical techniques had to be used to decontaminate buildings, offices, equipment, and supplies. However, the use of chemical techniques was inappropriate for decontaminating the predominately paper-based mail while still maintaining the integrity of the information contained in it (20). Therefore, ionizing radiation (accelerated electrons, X rays, and gamma rays) was thought to be an effective alternate method for decontamination of mail (18, 20, 22).
Sterilization by ionizing radiation (successfully used in the medical device community for more than 50 years) seemed a rational choice based on its effectiveness in sterilizing products and the relative timely delivery of decontaminated mail (10, 11). The mechanism of ionizing radiation lethality is due to the interaction of charged particles (or indirect action of high-energy electromagnetic radiation) with matter to produce ions. The charged particles and ions destroy the cellular integrity, resulting in spore inactivation, i.e., a loss of viability. Other damage occurs when ionized water molecules form free radicals that also disrupt biological systems (13). The decision to use EB instead of gamma radiation was made because of the high-throughput capabilities of EB facilities (especially with conveyor systems) and to avoid the targeting of postal facilities that would house radioactive sources (2).
Unfortunately, there was a paucity of data in the literature regarding the use of EB radiation to inactivate anthrax spores (19, 24). Nonetheless, in 2001, anthrax-contaminated U.S. mail (packaged in double polyethylene bags, 54 by 25 by 10.5 cm) was irradiated with an EB dose of 56 kGy (14; http://www.epa.gov/radiation/sources/mail_irrad.htm). Justification for the 56-kGy dose was never provided. No data have been published on how that dose was determined or how many Bacillus spores that dose destroyed. Two subsequent studies evaluating the effects of EB irradiation on spore-contaminated mail suggest a total irradiation dose of greater than 20 kGy was required for spore (6 log10) lethality (3, 19). The data of those studies reflect results when other materials (dried milk or kaolin) were included in the spore preparation. The current study was designed to evaluate the effectiveness of EB irradiation on greater numbers (up to 8 log10) of Bacillus spores in paper envelopes, without additives, in order to determine the D10 (the dose to reduce a population by 90%), depth-dose kinetics, and the relationship between temperature with killing.
MATERIALS AND METHODS
Growth and preparation of Bacillus endospore culture.
Bacillus atrophaeus (ATCC 9372, formerly B. subtilis var. niger) was obtained from the American Type Culture Collection (Manassas, VA), rehydrated, and passaged on Trypticase soy agar (TSA) supplemented with 5% sheep blood (Becton Dickinson Co., Sparks, MD). The bacteria were harvested aseptically, grown on nutrient sporulation medium (9) at 36°C for 48 h, and then grown at room temperature. Sporulation was evaluated daily using microscopy. The spores were harvested with 5 ml cold (4°C) sterile distilled, deionized (18 MΩ) water (DDW) once they had >90% spore content. This was usually after 1 week. The spore suspension (70 ml) was then centrifuged at 2,504 × g, 10 min, 25°C (Centra MP4R; IEC, Needham Heights, MA), and the pellet was resuspended in 70 ml phosphate-buffered saline (PBS, pH 7.2) supplemented with 0.05% Tween 20 (Mallinckrodt Baker, Inc., Paris, KY), which was mixed for 5 min and centrifuged as described above. The spore pellet was then washed four times with 70 ml sterile DDW each time to remove the detergent. Finally, the spores were suspended in 40 ml sterile DDW with serial 10-fold dilutions prepared in 1 ml sterile water. Dilutions of 100 μl were incubated in nutrient agar (NA) pour plates (n = 3) to determine the concentration of spores in the stock suspension, which was found to be 9.6 ± 0.1 log10 CFU ml−1. A 10-ml spore suspension was adjusted to 9.0 log10 CFU ml−1 and stored at 4°C for experimental use.
Endospore irradiation.
Spores were serially diluted (10-fold) into sterile DDW and aseptically deposited onto sterile 25-mm, 0.2-μm polycarbonate membrane filters (GE Osmonics Lab Store, Minnetonka, MN), to yield 1 to 8 log10 spores per filter. The spores were carefully distributed across the surface of the membrane in five 20-μl aliquots (100 μl on each filter), uniformly distributed to prevent spore clumping. The samples were allowed to air dry aseptically (12 to 18 h, 23°C) and then the membranes were placed spore side up within sterilized 5.5-cm by 8.9-cm paper envelopes (<0.1 mm thick). The envelopes were irradiated at various doses at Kent State University's NEO Beam facility (25) using a 5.0 MeV (Dynamitron) electron beam accelerator (Radiation Dynamics, Inc., Edgewood, NY). Envelopes were secured to a polystyrene platform and transported under the EB on a cart conveyor system (SI Handling Systems, Easton, PA). A thermocouple with an OM-3001 Portable Datalogger (Omega Technologies Co., Stamford, CT) was also secured to the polystyrene to record the temperature as the samples passed under the beam. Spores were irradiated from 0 to 40 kGy, in 5 kGy increments, to determine the approximate surface dose required to kill multiple concentrations of spores. Data were recorded for three separate irradiations, for a total of three samples (n = 3) per dose. The accelerator operated at a beam energy level of 5.0 MeV and a beam current of 10 mA (50 kW). Irradiated membranes containing spores were aseptically removed from their envelopes, transferred to 5-ml sterile nutrient broth tubes, and incubated at 36°C for 72 h. In separate experiments, irradiated membranes containing 8 log10 spores were mixed using a vortex in 10 ml sterile water for 3 min to dislodge spores into suspension. The suspended spores were serially diluted (10-fold) for standard plate counts (36°C for 48 h). The D10 value was determined over the EB dose range of 0 to 15 kGy (determined from preliminary experiments). Mean plate counts ± standard deviations (SD) were reported for each EB dose. Three irradiations were performed, each with triplicate samples (n = 9) per dose. Dosimetry was conducted with alanine pellets, using an e-scan spectrometer (Bruker BioSpin Corp., Billerica, MA).
Depth-dose-killing relationship.
Membrane filters with endospores were prepared as described above. A stack of envelopes was created, 5.5 cm tall, 114.6 g, with an average stack density of 0.418 g/cm3. Envelopes containing 8 log10 endospore-impregnated filters were positioned at the bottom of the stack. BioMax alanine film dosimeters (Eastman Kodak Co., Rochester, NY) were placed inside the top envelope and under the envelope containing spores at the bottom of the stack. The envelopes were secured to a cardboard platform and to the cart trays of the conveyor system. EB conditions were based upon the calculated results from an ITS Monte Carlo code (version 3.0; Oak Ridge National Laboratories, Oak Ridge, TN [www.ornl.gov/info/reports/1985/3445600037047.pdf]) used to predict the EB surface dose to achieve a target dose of 12.5 kGy at the stack bottom. Irradiated membrane filters were aseptically removed from the irradiated envelopes and mixed using a vortex in 10 ml sterile water for 3 min to dislodge spores into suspension. The suspended spores were serially diluted (1:10) for standard plate counts (36°C for 48 h). Dosimetry was conducted with an e-scan spectrometer (Bruker BioSpin Corp., Billerica, MA).
Statistical anyalysis.
A linear regression line from the plot of dose versus log10 CFU inactivation was used to predict the D10 value for B. atrophaeus when treated using a high-energy, high-beam current electron accelerator.
RESULTS
Examination of the inactivation of multiple concentrations of spores versus varying doses.
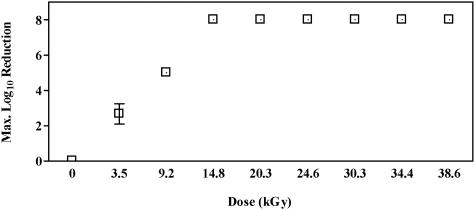
The results of spore germination after various electron beam dose treatments (0 to ∼40 kGy), as log10 reduction, are presented in Fig. 1. The data demonstrate that EB doses greater than or equal to 14.8 ± 0.3 kGy produced 8 log10 of spore kill. A dose of 9.2 ± 0.3 kGy was required to reduce spore population by 6 log10 (6 log10 and less), while as little as 3.5 ± 0.1 kGy appeared to cause a 3 log10 reduction.
FIG. 1.
Maximum log10 reduction of B. atrophaeus spores (1 to 8 log10 CFU per filter) as a function of electron beam irradiation dose (n = 3).
Prediction of D10 value.
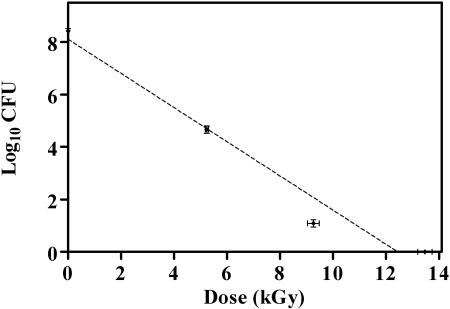
Killing of B. atrophaeus spores (8 log10 CFU ml−1) as a function of absorbed dose caused by EB irradiation is reported in Fig. 2. A D10 value of 1.53 ± 0.03 kGy was extrapolated from the regression line (r2 = 0.965). Error bars in both the x- and y-axis directions indicate the accuracy in the measurements of dose and colony counts, respectively.
FIG. 2.
Inactivation of B. atrophaeus spores as a function of electron beam irradiation dose (n = 9). Linear regression (r2 = 0.965) extrapolation produced a D10 value of 1.53 kGy.
Relationship between depth and dose.
The Monte Carlo code predicted an EB surface dose of 35 kGy so as to achieve an EB dose of 12.5 kGy at the stack bottom (data not shown). Experimentally, complete spore inactivation was achieved when a surface dose of 31.8 ± 0.5 kGy was used, resulting in a bottom dose of 14.6 ± 2.3 kGy. However, when the surface EB dose was 25.0 ± 0.3 kGy and the bottom EB dose was 12.4 ± 1.4 kGy, spore survival was variable (2.6 ± 2.8 log10 CFU ml−1).
Assessment of temperature increases with irradiations.
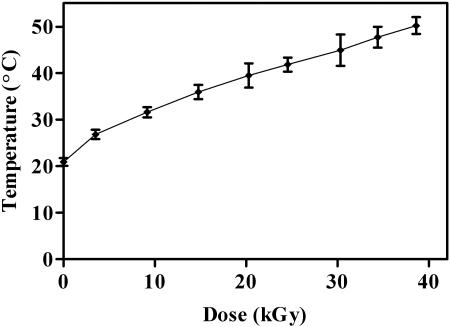
A temperature range of 20.9 ± 0.3°C to 49.2 ± 1.9°C was obtained over the dose range of 0 to 40 kGy, shown in Fig. 3. Error bars in both the x- and y-axis directions indicate the accuracy in the measurements of dose and temperature. Temperature data revealed that even at the slowest cart speed of 6 ft min−1, at a dose of 40 kGy, the maximum recorded temperature (49.2°C) lasted for only 1 to 2 s (data not shown).
FIG. 3.
Temperature recorded at various doses of electron beam irradiation, expressed as the mean ± SD (n = 3).
DISCUSSION
Before the anthrax attacks on the United States in the fall of 2001, there was little public information on the effects of EB ionizing radiation on species within the genus Bacillus. Few studies have been reported on the sensitivity of spores to EB radiation (3, 19). The one study that examined spore inactivation by EB suggested a D10 value of 3.35 kGy (19). Another study did not report a D10 value (3). Both previous studies present data where spore species were mixed with other materials during EB irradiation. Importantly, no reports on the EB dose required to kill 8 log10 or greater spores have been published. The present study evaluates the effect of EB on the inactivation of B. atrophaeus spores contained in paper envelopes. While this study was not meant to define specific practices for future EB irradiation of mail at postal facilities, it was designed to mirror reported USPS practices so as to study the impact of EB use on spores in paper envelopes.
Data in Fig. 1 represent the irradiation of several concentrations of spores over a dose range of 0 to ∼40 kGy. As anticipated, spore survival was inversely related to EB dose. The lethal spore irradiation dose appears to be between 9.2 and 14.8 kGy. The lower EB dose of 3.5 kGy resulted in variable spore survival. This suggests that there is a certain degree of variability in the radiation sensitivity of the spore samples at that lower dose.
For filters containing 8 log10 spores enclosed in sterile envelopes and irradiated with a 5.0 MeV Dynamitron accelerator and a current of 10 mA, the D10 value was determined to be 1.53 ± 0.03 kGy (Fig. 2). Extrapolation of the D10 value from the linear regression line suggests that 12.24 kGy would be required to reduce a spore population by 8 log10. An 8 log10 reduction dose of approximately 12 kGy is also suggested by the data presented in Fig. 1.
The results of this study and two similar studies are summarized for comparison in Table 1. The three studies compared the effect of EB irradiation on spores from different Bacillus species. Furthermore, the three studies used different concentrations of spores and different packaging materials. The other studies reported EB effects on spores coated with dry milk (19) or kaolin (3). While no D10 value was reported, Auslender et al. indicated a 6 log10 reduction for a dose of 8.5 kGy using B. thuringiensis and 22 kGy for B. cereus (3). Niebuhr and Dickson (19) reported a D10 of 3.35 kGy for B. anthracis Sterne spores. We present a D10 of 1.53 kGy for B. atrophaeus. Clearly, the species, the number of spores present, the type of materials interacting with the spores (dried milk, kaolin, envelopes, etc.), and the depth of penetration of the beam all must be considered when designing experiments to evaluate EB effects on anthrax-contaminated mail.
TABLE 1.
Comparison of Bacillus spore inactivation by electron beam irradiation
| Study | Species | Sample size | Experimental conditions | EB accelerator and energy | D10 value |
|---|---|---|---|---|---|
| This report | B. atrophaeus | 8 log10 | Spores in water air dried on membrane filter, within one envelope | 5 MeV Dynamitron | 1.53 ± 0.03 kGy |
| 19 | B. anthracis, Sterne 34F2 | 5 log10 | Spores in ethanol inoculated in dry milk, within a plastic pouch and two envelopes | 10 MeV Circe III Linear Accelerator | 3.35 ± 0.02 kGy |
| 3 | B. cereus | 4 log10 | Frozen spores mixed in kaolin and lyophilized powder, within two envelopes | 2.4 MeV ILU-6 Impulse Linear Accelerator | Not reported |
| B. thuringiensis | 6 log10 | Spores in water dried in kaolin, within two envelopes |
Given the D10 of 1.53 kGy, a theoretical dose of 12.24 kGy would be needed to kill 8 log10 spores. The depth-dose experiments were designed to create a condition in which spores would receive a lethal dose, predicted by the D10, but were placed at the bottom of a stack of envelopes. A 5.5-cm envelope stack height was chosen based on the maximum penetration capabilities of the 5.0-MeV electron beam in paper (16) and using a value of 0.418 g/cm3 as the density of envelope materials. The ITS version 3.0 Monte Carlo code was used to predict the relationship between the EB dose required at the surface of the envelope stack to result in a lethal dose of at least 12.5 kGy (8 log10 reduction) at the bottom of the stack. The theoretical EB dose predicted by the Monte Carlo simulation to kill 100% of spores under a 5.5-cm envelope stack was 35 kGy at the surface (producing 12.5 kGy at the bottom). Experimental data suggest that an average surface EB dose of 25 kGy was insufficient to inactivate all spores, even though it produced an average bottom EB dose that should have inactivated 8 log10 of spores. Reasons for this discrepancy are not completely clear but may be due to subtle variations in EB penetration resulting in the observed variation (SD from the mean) in EB dose at the stack bottom. What is clear is that a surface dose of 31 kGy produced an EB dose of 14 kGy at the bottom of a 5.5-cm envelope stack, resulting in 100% spore inactivation.
The rise in temperature of the immediate spore environment was recorded (Fig. 3) as samples passed under the high-current, high-energy electron beam (5.0 MeV, 10 mA) to confirm that temperature does not play a role in spore death (26). The maximum temperature any of the samples received (at the highest dose of 38.6 kGy) was 49.2°C for up to 2 s. Previous studies demonstrated that spore death temperature (SDT, based on first-order inactivation kinetics) for this species was 99°C when samples were exposed for 10 min (26). The temperature over the dose range used to determine the D10 value in our studies was between 20 and 37°C, substantially lower than the SDT for this species and the maximum recorded temperature at the highest irradiation dose used in this study. Therefore, it is unlikely that the temperature increase generated by the EB over the dose range studied had an appreciable effect on the target material and spore viability.
In conclusion, this study demonstrates that a surface dose of 1.53 kGy is required to reduce an 8 log10 B. atrophaeus spore population (in paper envelopes) by 1 log10 when irradiated with a 5.0-MeV Dynamitron electron beam accelerator under the conditions described herein. Additionally, when inactivating spores not at a surface but under a 5.5-cm stack of envelopes, it appears that the surface EB dose must be sufficient to ensure a dose of at least 14 kGy at the bottom of the stack to produce 8 log10 spore reduction. Spore inactivation at these EB doses appears to be unrelated to EB-induced temperature increases. Finally, we conclude from our data that the use of 50 to 100 kGy of EB irradiation by the USPS appears to be appropriate, and within a significant margin of safety, if one seeks to render a highly concentrated spore sample (12 log10, for example) nonviable at the bottom of a 5.5-cm stack of paper.
Acknowledgments
We thank Anthony Berejka for helpful information and critical review of the manuscript.
REFERENCES
- 1.Ash, C., J. A. E. Farrow, S. Wallbanks, and M. D. Collins. 1991. Phylogenetic heterogeneity of the genus Bacillus revealed by comparative analysis of small-subunit-ribosomal RNA sequences. Lett. Appl. Microbiol. 13:202-206. [Google Scholar]
- 2.Aston, C. 2002. Neither snow, nor rain, nor anthrax. IEEE Spectrum 39:38-39. [Google Scholar]
- 3.Auslender, V., V. Vedernikov, M. Grachev, V. Drukker, A. Korchagin, E. Kruglyakov, A. Kudryavtsev, N. Kulikova, O. Netsvetaeva, O. Pavlova, V. Parfenova, E. Semenova, V. Serbin, I. Terkina, A. Tkov, and E. Chebykin. 2002. Sterilization of mail by means of an electron beam accelerator. Dokl. Biol. Sci. 385:306-309. [DOI] [PubMed] [Google Scholar]
- 4.Borick, P. M., and M. G. Fogarty. 1967. Effects of continuous and interrupted radiation on microorganisms. Appl. Microbiol. 15:785-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buttner, M. P., P. Cruz, L. D. Stetzenbach, A. K. Kilma-Comba, V. L. Stevens, and T. D. Cronin. 2004. Determination of the efficacy of two building decontamination strategies by surface sampling with culture and quantitative PCR analysis. Appl. Environ. Microbiol. 70:4740-4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christensen, E. A., and N. W. Holm. 1964. Inactivation of dried bacteria and bacterial spores by means of ionizing radiation. Acta Pathol. Microbiol. Scand. 60:253-264. [DOI] [PubMed] [Google Scholar]
- 7.Christopher, G. W., T. J. Cieslak, J. A. Pavlin, and E. M. Eitzen, Jr. 1997. Biological warfare. A historical perspective. JAMA 278:412-417. [PubMed] [Google Scholar]
- 8.Claus, D., and R. Berkeley. 1986. Endospore-forming Gram-positive rods and cocci. Genus Bacillus, p. 1104-1139. In P. H. A. Sneath (ed.), Bergey's manual of systematic bacteriology, 2nd ed., vol. 2. Williams and Wilkins, Baltimore, Md. [Google Scholar]
- 9.Dang, J. L., K. Heroux, J. Kearney, A. Arasteh, M. Gostomski, and P. A. Emanuel. 2001. Bacillus spore inactivation methods affect detection assays. Appl. Environ. Microbiol. 67:3665-3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fairand, B. P. 2002. Radiation sterilization for health care products: X-ray, gamma, and electron beam, p. 57-72. CRC Press, Boca Raton, Fla.
- 11.Florig, H. K. 2002. Public health: is safe mail worth the price? Science 295:1467-1468. [DOI] [PubMed] [Google Scholar]
- 12.Fritze, D., and R. Pukall. 2001. Reclassification of bioindicator strains Bacillus subtilis DSM 675 and Bacillus subtilis DSM 2277 as Bacillus atrophaeus. Int. J. Syst. Evol. Microbiol. 51:35-37. [DOI] [PubMed] [Google Scholar]
- 13.Hansen, J. M., and H. L. Shaffer. 2001. Sterilization and preservation by radiation sterilization, p. 729-745. In S. S. Block (ed.), Disinfection, sterilization, and preservation, 5th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 14.Hanson, D. J. 2002. Zapping the mail. Chem. Eng. News 80:30-32. [Google Scholar]
- 15.Jernigan, D. B., P. L. Raghunathan, B. P. Bell, R. Brechner, E. A. Bresnitz, J. C. Butler, M. Cetron, M. Cohen, T. Doyle, M. Fischer, C. Greene, K. S. Griffith, J. Guarner, J. L. Hadler, J. A. Hayslett, R. Meyer, L. R. Petersen, M. Phillips, R. Pinner, T. Popovic, C. P. Quinn, J. Reefhuis, D. Reissman, N. Rosenstein, A. Schuchat, W.-J. Shieh, L. Siegal, D. L. Swerdlow, F. C. Tenover, M. Traeger, J. W. Ward, I. Weisfuse, S. Wiersma, K. Yeskey, S. Zaki, D. A. Ashford, B. A. Perkins, S. Ostroff, J. Hughes, D. Fleming, J. P. Koplan, and J. L. Gerberding. 2002. Investigation of bioterrorism-related anthrax, United States, 2001: epidemiologic findings. Emerg. Infect. Dis. 8:1019-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McLaughlin, W. L., A. Boyd, K. Chadwick, J. McDonald, and A. Miller. 1989. Dosimetry for radiation processing, p. 28-31. Taylor & Francis, London, United Kingdom.
- 17.Nakamura, L. K. 1989. Taxonomic relationship of black-pigmented Bacillus subtilis strains and a proposal for Bacillus atrophaeus sp. nov. Int. J. Syst. Bacteriol. 39:295-300. [Google Scholar]
- 18.Neergaard, L. 18 November 2001. Officials weigh risk to medical mailings from irradiation, p. A7. The Washington Post, Washington, D.C.
- 19.Niebuhr, S., and J. Dickson. 2003. Destruction of Bacillus anthracis strain Sterne 34F2 spores in postal envelopes by exposure to electron beam irradiation. Lett. Appl. Microbiol. 37:17-20. [DOI] [PubMed] [Google Scholar]
- 20.Ritter, S. 2001. Ousting anthrax. Chem. Eng. News 79:24-26. [Google Scholar]
- 21.Rotz, L. D., A. S. Kahn, S. R. Lillibridge, S. M. Ostroff, and J. M. Hughes. 2002. Public health assessment of potential biological terrorism agents. Emerg. Infect. Dis. 8:225-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruane, M. E. 23 October 2001. Irradiation explored as answer to anthrax, p. A11. The Washington Post, Washington, D.C.
- 23.Simon, J. D. 1997. Biological terrorism. Preparing to meet the threat. JAMA 278:428-430. [DOI] [PubMed] [Google Scholar]
- 24.Spotts Whitney, E. A., M. E. Beatty, T. H. Taylor, Jr., R. Weyant, J. Sobel, M. J. Arduino, and D. A. Ashford. 2003. Inactivation of Bacillus anthracis spores. Emerg. Infect. Dis. 9:623-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uribe, R., and C. Vargas-Aburto. 2001. Proceedings of the 16th International Conference on the Application of Accelerators in Research and Industry, Denton, TX, 2000. American Institute of Physics, New York, N.Y.
- 26.Warth, A. D. 1977. Relationship between the heat resistance of spores and the optimum and maximum growth temperatures of Bacillus species. J. Bacteriol. 134:699-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu, D., and J.-C. Cote. 2003. Phylogenetic relationships between Bacillus species and related genera inferred from comparison of 3′ end 16S rDNA and 5′ end 16S-23S ITS nucleotide sequences. Int. J. Syst. Evol. Microbiol. 53:695-704. [DOI] [PubMed] [Google Scholar]