Abstract
Low oxygen pressures exist in many solid tissues, including primary and secondary lymphoid organs. One key element in cellular adaptation to hypoxia is induced expression of hypoxia inducible factor (Hif) 1α. Here, we have examined the effect of Hif-1α, isolated from the myriad other effects of hypoxia, on T cell receptor (TCR) signaling in thymocytes. Because pVHL (von Hippel–Lindau protein) directs the proteolysis of Hif-1α under “normoxic” conditions, we achieved constitutive stabilization of Hif-1α through thymic deletion of Vhlh and reversed Hif-1α stabilization with double deletion of Vhlh and Hif-1α. We found that constitutive activity of Hif-1α resulted in diminished Ca2+ response upon TCR crosslinking despite equivalent activation of phospholipase Cγ1, normal intracellular Ca2+ stores, and normal entry of Ca2+ across the plasma membrane. Altered Ca2+ response was instead due to accelerated removal of Ca2+ from the cytoplasm into intracellular compartments, which occurred in association with Hif-1α-dependent overexpression of the calcium pump SERCA2 (sarcoplasmic/endoplasmic reticulum calcium ATPase 2). These data suggest a unique mechanism for control of TCR signaling through Hif-1α, which may be operative at the physiologic oxygen tensions seen in solid lymphoid organs.
Keywords: calcium, lymphocytes
The availability of oxygen varies widely among tissues (1, 2), and mammalian cells use oxygen-sensing pathways for adaptation. Many cellular responses to hypoxia are mediated through hypoxia inducible factors (HIFs), members of the PAS family of basic helix–loop–helix transcription factors (3). HIFs function as heterodimeric DNA-binding proteins composed of HIF-α and HIF-β [or aryl hydrocarbon receptor nuclear translocator (ARNT)] subunits. Both HIF-α and ARNT are constitutively expressed, but, under normoxic conditions, HIF-α subunits are hydroxylated on proline residues. As a result, pVHL (von Hippel–Lindau protein), a component of an E3 ubiquitin ligase complex, polyubiquitinates HIF-α and targets it for rapid proteasomal degradation (4, 5). Under hypoxia, limited availability of oxygen prevents the hydroxylation of HIF-α, thus stabilizing its expression. HIF-α dimerizes with ARNT in the nucleus and up-regulates the transcription of various hypoxia response element-containing genes such as VEGF.
Previous studies using microelectrodes demonstrated that normal oxygen tensions in lymphoid tissues (<5%) are much lower than those found in peripheral arterial blood (13%) (6–8). Pathological conditions that damage local microvasculature may also result in hypoxia. Moreover, a recent study has also shown that infiltrating T cells in synovia of patients with rheumatoid arthritis contain high levels of HIF-1α (9), consistent with an important role for hypoxia in the inflamed rheumatoid joint (10).
In vitro studies have shown that low oxygen tension influences activation, function, and survival of T cells (9, 11, 12). For instance, hypoxia delays the development of cytotoxic T lymphocyte activity in CD8+ T cells, although it ultimately increases lytic activity on a per-cell basis (7). However, hypoxia has multiple effects on cellular growth, metabolism, transcription, and translation (13–15), which are caused by energy depletion as well as HIF-mediated transcriptional activation. Thus, mechanistic investigations to define the specific effect of HIF-1α stabilization on T cells require an experimental system that tests the role of HIF in isolation from other consequences of exposure to hypoxia.
To study the effects of HIF on T cell signaling, we examined T cells lacking the gene encoding von Hippel–Lindau protein, Vhlh. Because germ-line deficiency in Vhlh results in embryonic lethality (16), we used mice containing a conditional Vhlh 2-lox allele alone or in combination with a Hif-1α 2-lox allele (12, 17, 18) as well as the Cre recombinase transgene under control of the proximal lck promoter (19). Deletion of Vhlh alone forces oxygen-independent stabilization of Hif-α at an early point in T cell ontogeny and induces transcription of HIF-responsive genes (12). In the present report, we use this model to show that Hif-1α negatively regulates Ca2+ signaling downstream of T cell receptor (TCR) ligation.
Materials and Methods
Generation and Genotyping of Mice. Mice with Vhlh and/or Hif-1α 2-lox alleles and Lck-Cre transgenic mice have been described in refs. 12 and 17–19. Mice with targeted deletion of Vhlh and Hif-1α were on mixed genetic backgrounds (BALB/c, 129Sv/J, and C57BL/6). Sex-matched, Cre-negative littermates were used as controls in all experiments. Protocols were approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
Calcium Imaging in Thymocytes. Total thymocytes were isolated in normal bath solution (145 mM NaCl/5 mM KCl/2 mM CaCl2/1 mM MgCl2/10 mM Hepes/10 mM glucose/5% FBS, pH 7.4) and loaded with 3 μM Fura-2 acetoxymethyl ester (Molecular Probes). Intracellular calcium was measured in single cells as described in ref. 20. Thymocytes labeled with biotinylated anti-CD3ε (145-2C11) and anti-CD4 (RM4-5, BD Pharmingen) were stimulated with 1 μg/ml streptavidin, 100 nM thapsigargin, or 1 μM ionomycin. LaCl3 (10 nM) was used to inhibit plasma membrane calcium ATPase (PMCA), and oligomycin (1 μM) and antimycin A (2 μM) were used to inhibit mitochondrial Ca2+ uptake. Data are presented as the mean [Ca2+]i (intracellular Ca2+ concentration) over time obtained from a field containing ≥100 cells.
Western Blots. Total thymocytes were isolated in cold Hanks' buffered salt solution (HBSS) (pH 7.4) and rested on ice for 15 min. For stimulations, 1 × 107 cells were treated with biotinylated anti-CD3ε and biotinylated anti-CD4 (10 μg/ml each) for 10 min on ice and then washed, resuspended in HBSS, and stimulated for 1 min with 1 μg/ml streptavidin at 37°C. Finally, cells were quickly pelleted and lysed in ice-cold RIPA buffer (0.15 mM NaCl/0.05 mM Tris·HCl, pH 7.2/1% Triton X-100/1% sodium deoxycholate/0.1% SDS) with protease and phosphatase inhibitor cocktails (Calbiochem). Equivalent amounts of total protein were used in each lane. Primary antibodies used for immunoblotting were as follows: anti-IP3R-I, II, and III (Santa Cruz Biotechnology); anti-phosphotyrosine (4G10, Upstate Biotechnology, Lake Placid, NY); anti-total PLCγ1 and anti-phosphoPLCγ1 (BD Transduction Laboratories); anti-total PMCA (5F10, Upstate Biotechnology); antibodies to SERCA (sarcoplasmic/endoplasmic reticulum calcium ATPase) (anti-SERCA2 and anti-SERCA3, Affinity BioReagents, Golden, CO); and anti-actin (Sigma-Aldrich). Horseradish peroxidase-conjugated secondary antibodies were used as appropriate for immunoblot detection of the above primary antibodies with enhanced chemiluminescence: anti-mouse IgG (Cell Signaling Technology, Beverly, MA) and anti-rabbit IgG (Amersham Pharmacia Biosciences).
Flow Cytometry. Total thymocytes were stained for 30 min at 37°C with either MitoTracker Green FM (25 nM, Molecular Probes) or JC-1 (1 μg/ml, Molecular Probes) followed by addition of TOPRO-3 (1 μM, Molecular Probes) at room temperature 10 min before analysis. Cells were gated on forward and side scatter as well as TOPRO-3 exclusion to define a population of live cells for data analysis.
Measurement of PMCA-Mediated Ca2+ Efflux. Intracellular calcium was monitored in thymocytes loaded with Fura-2 in normal bath solution. Cells were treated with thapsigargin (100 nM) for 300 sec and then perfused with Ca2+-free bath solutions. Ca2+ efflux rates were calculated from Δ[Ca2+]i during the first 10 sec in Ca2+-free solution. The Ca2+-free bath was 145 mM NaCl, 5 mM KCl, 0.25 mM EGTA, 1 mM MgCl2, 10 mM Hepes, and 10 mM glucose (pH 7.4).
45Ca2+ Uptake Assay. Freshly isolated control or Vhlh-deficient thymocytes were suspended (5 × 106 per ml) in 0.5 M sucrose/20 mM Tris, pH 7.8/mM DTT (to protect thiols in the SERCA pump from oxidative damage). The final reaction mixture contained 120 mM KCl, 20 mM Tris Hepes, 0.3 mM MgCl2, 0.5 mM EGTA, 0.5 mM N-(2-hydroxyethyl)ethylene-diaminetriacetic acid, 2 mM DTT, 1 mM MgATP, 10 mM phosphocreatine, 10 units/ml creatine kinase, 2 μM ruthenium red, 5 mM potassium oxalate, 4 μg/ml saponin, and 3 μCi/ml 45Ca2+ (1 Ci = 37 GBq). Ca2+ uptake by cells was measured for 30 min, and thapsigargin or A23187 (400 nM) was used to assess uptake by SERCA pumps or into other organelles. Assays were run in triplicate, and data are presented as mean ± standard deviation.
Results
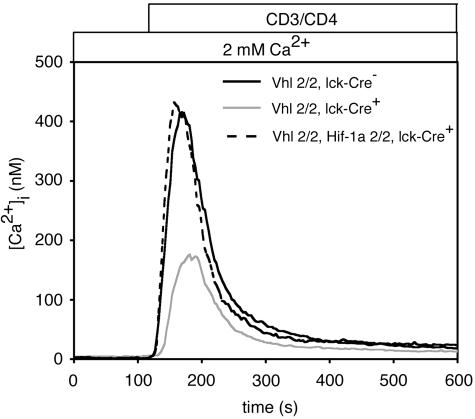
Hif-1α-Dependent Alterations in Ca2+ Signaling in Vhlh-/- Thymocytes. Ca2+ influx is a key event in TCR signaling, leading to activation of calcineurin and nuclear translocation of nuclear factor of activated T cells. Because hypoxia alters the functional effects of TCR stimulation in vitro, we tested the effects of Hif-1α stabilization on calcium mobilization in thymocytes after T cell activation. Enforced stabilization of Hif-α protein expression induced in Vhlh-/- thymocytes caused significantly diminished maximal [Ca2+]i together with retarded initial rates of [Ca2+]i elevation in response to CD3/CD4 coligation (Fig. 1). Because there are modest alterations in thymocyte subset percentages in Vhlh-/- mice [elevated percentages of double-positive cells and reduced numbers of single-positive cells (11)], we also analyzed the TCR-mediated calcium response in specific thymocyte subpopulations from Vhlh-/- mice and littermate controls (Fig. 6, which is published as supporting information on the PNAS web site). We confirmed that in all cases (double positive, CD4 single positive, and CD8 single positive), Vhlh-/- thymocytes had smaller responses than thymocytes from control mice. The lack of response in CD8 single-positive Vhlh-/- cells suggests that these cells are TCR-negative, immature, single-positive thymocytes, which are normally an immediate precursor of double-positive thymocytes in mice. Such cells are more common in thymii with increased numbers of cells making the double-negative to double-positive transition, which is consistent with the elevated percentages of double-negative cells in Vhlh-/- mice (12).
Fig. 1.
Hif-1α stabilization reduces TCR-mediated Ca2+ signaling in thymocytes. Total thymocytes from control (Vhl2/2, Lck-Cre-) and Vhlh-deficient (Vhl2/2, Lck-Cre+) or Vhlh/Hif-1α double-deficient (Vhl2/2, Hif-1α2/2, Lck-Cre+) mice were stimulated in 2 mM extracellular Ca2+ bath. [Ca2+]i was measured in cells preloaded with the Ca2+ indicator Fura-2 (n ≥ 100). Biotinylated antibodies against CD3 and CD4 were crosslinked with streptavidin.
A normal Ca2+ signaling phenotype was completely restored in double knockout (Vhlh-/-, Hif-1α-/-) thymocytes, demonstrating that altered Ca2+ signaling in Vhlh-/- thymocytes is specifically attributable to Hif-1α stabilization. Although there are three known HIF-α gene products regulated by von Hippel–Lindau protein, the observation of rescued Ca2+ signaling in Vhlh-/-, Hif-1α-/- cells confirms our previous observation that Hif-2α is nonfunctional in thymocytes and that Hif-1α alone mediates the effects of Vhlh deficiency in this model (12).
The generation and resolution of intracellular Ca2+ elevations by TCR ligation involves several major elements (21), including (i) phospholipase Cγ1 (PLCγ1) activation and generation of inositol tris-phosphate (IP3), (ii) IP3-mediated release of intracellular Ca2+ from stores leading to Ca2+ release-activated Ca2+ (CRAC) channel activation, and (iii) clearance of cytoplasmic Ca2+ by means of PMCA, mitochondrial Ca2+ buffering, and reuptake into endoplasmic reticulum (ER) stores by means of SERCA pumps. To define the mechanism by which Hif-1α regulates TCR-mediated Ca2+ signaling in thymocytes, we examined each of these sequential steps in subsequent experiments.
Intact Proximal TCR Signaling in Vhlh-/- Thymocytes. We hypothesized that Hif-1α stabilization might blunt Ca2+ signaling in thymocytes by attenuating TCR proximal signaling events. To test this, we stimulated thymocytes in vitro and observed total and PLCγ1-specific tyrosine phosphorylation. Constitutive stabilization of Hif-1α in Vhlh-/- thymocytes did not prevent the rapid accumulation of tyrosine-phosphorylated proteins in activated thymocytes (Fig. 2A). Significantly, strong activation of PLCγ1 was observed in Vhlh-/- cells (Fig. 2B). These data suggest that constitutive stabilization of Hif-1α does not interfere with proximal TCR signaling.
Fig. 2.
Proximal TCR signaling is intact in Vhlh-deficient thymocytes. Total thymocytes from control or Vhlh-deficient mice were stimulated at 37°C for 1 min by crosslinking CD3 and CD4 as described in Materials and Methods. (A) Total phosphotyrosine signal was detected in resting and stimulated cells by using anti-phosphotyrosine mAb 4G10. Staining for β-actin is provided as a loading control. (B) Tyrosine-phosphorylated (pY783) and total PLCγ1 was detected in resting and stimulated cells.
Normal Store-Operated Ca2+ Entry in Vhlh-/- Thymocytes. We next examined the effects of HIF on Ca2+ release from intracellular stores. We first compared the size of intracellular Ca2+ stores present in control and Vhlh-/- cells, because their filling state controls CRAC channel activation (22, 23). If the amount of Ca2+ in the ER or other compartments is larger in Vhlh-/- cells, then the stores may be difficult to adequately deplete for CRAC channel activation. The drug thapsigargin blocks SERCA pump activity, unmasking a constant leak of Ca2+ from the ER into the cytoplasm. We found that thapsigargin-releasable stores were quantitatively equivalent in control and Vhlh-/- thymocytes (Fig. 7, which is published as supporting information on the PNAS web site). Moreover, the subsequent addition of ionomycin, which releases Ca2+ from all remaining (thapsigargin-insensitive) stores, produced similar elevations of [Ca2+]i in control and Vhlh-/- cells. Therefore, total and ER Ca2+ store quantity is not altered by Hif-1α stabilization.
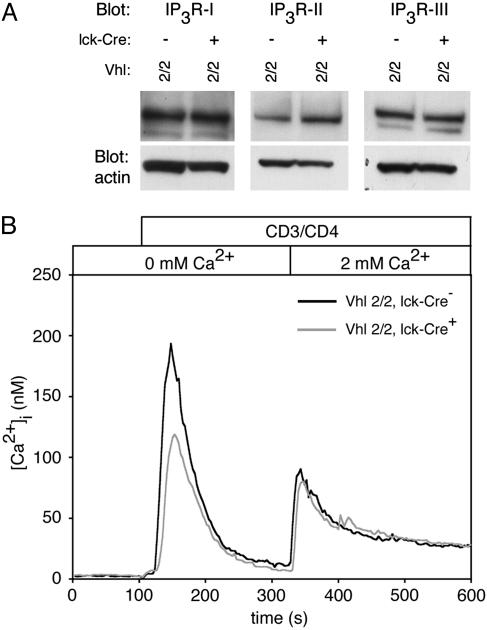
Significantly diminished expression of IP3 receptors (IP3Rs) might limit the depletion of intracellular stores subsequent to TCR activation. However, Western blots for IP3R-I, -II, and -III in resting total thymocytes revealed no significant alteration of IP3R protein expression in Vhlh-/- cells (Fig. 3A).
Fig. 3.
Ca2+ store release and Ca2+ influx after stimulation. (A) Western blot analysis for expression of IP3R-I, -II, and -III using equivalent total protein from control and Vhlh-deficient total thymocyte lysates. (B) Total thymocytes from control and Vhlh-deficient mice were loaded with Fura-2 and stained with biotinylated antibodies against CD3 and CD4. Cells were stimulated by addition of streptavidin in Ca2+-free bath. After intracellular store release, 2 mM extracellular Ca2+ was restored as indicated (n ≥ 100).
Next, we directly tested the ability of Vhlh-/- thymocytes to release Ca2+ in response to TCR ligation. After CD3 and CD4 crosslinking, the rise in [Ca2+]i observed in Ca2+-free bath solution (representing release of store Ca2+) was somewhat smaller in Vhlh-/- cells (Fig. 3B). This phenotype could indicate diminished IP3-mediated store release or accelerated removal of TCR-mobilized Ca2+. Importantly, subsequent application of extracellular Ca2+ led to an equivalent increase in [Ca2+]i (representing plasma membrane permeabilization to Ca2+) in control and Vhlh-/- thymocytes, suggesting no differences in the extent of TCR channel-mediated Ca2+ influx between control and Vhlh-/- thymocytes. In particular, the initial rate of change in [Ca2+]i after addition of extracellular Ca2+, which is dictated primarily by the number of open CRAC channels, was equivalent in control and Vhlh-/- thymocytes (see the magnification of Fig. 3B, available as Fig. 8, which is published as supporting information on the PNAS web site). These data indicate that TCR-mediated store release in Vhlh-/- cells is sufficient to trigger a normal influx of Ca2+ across the plasma membrane. Similarly, when stores were depleted with thapsigargin in Ca2+-free bath solution followed by a switch to 2 mM extracellular Ca2+, Ca2+ influx was similar in control and Vhlh-/- thymocytes (data not shown). These data suggest that Hif-1α does not blunt Ca2+ responses by changing quantitative aspects of store release or Ca2+ influx.
Hif-1α Stabilization Accelerates Clearance of Cytoplasmic Ca2+. PMCA-mediated efflux. Because alterations in TCR proximal signaling, intracellular Ca2+ store content and release, and Ca2+ influx could not explain the effect of Hif-1α on Ca2+ signaling in thymocytes, we hypothesized that Hif-1α stabilization might increase the rate of removal of Ca2+ from the cytoplasm.
PMCA is the major efflux mechanism responsible for recovery of baseline cytoplasmic Ca2+ concentration (24). Total PMCA protein expression appeared similar in control and Vhlh-/- thymocytes (Fig. 4A). Moreover, experiments using La3+ (performed in a Ca2+-free bath to avoid effects from blockade of CRAC channels) revealed that inhibition of PMCA did not reverse the blunted elevation of [Ca2+]i in Vhlh-/- thymocytes (Fig. 4B). This finding was confirmed by measuring PMCA-mediated Ca2+ efflux rates. Thymocytes were Ca2+-loaded for 300 sec, using thapsigargin to block SERCA, deplete ER stores, and produce sustained CRAC-mediated elevation of [Ca2+]i, a protocol that fully activates PMCA pumps (24). PMCA-dependent Ca2+i efflux was then measured after a rapid switch to Ca2+-free bath. Under these conditions, SERCA-mediated reuptake into stores is blocked by thapsigargin, and mitochondrial Ca2+ uptake should be saturated after a prolonged enforced elevation of [Ca2+]i. As predicted, we observed similar efflux rates for control and Vhlh-/- thymocytes (Ca2+ efflux half-life: control, 16.5 ± 0.71 sec; Vhlh-/-, 16.8 ± 0.21 sec; see Fig. 9, which is published as supporting information on the PNAS web site). Together, these data suggest that alterations in PMCA activity do not produce the blunting of Ca2+ responses in Hif-1α-stabilized thymocytes.
Fig. 4.
PMCA and mitochondrial activity. (A) Western blot analysis of PMCA protein using mAb 5F10 in resting total thymocyte lysates from control and Vhlh-deficient mice. Staining for β-actin is provided as a loading control. (B) Fura-2-loaded thymocytes were stimulated as above in Ca2+-free bath containing 10 nM LaCl3 to inhibit PMCA activity (n ≥ 100). (C) Fura-2-loaded thymocytes from control or Vhlh-deficient mice were stimulated as above in bath solution containing 2 mM Ca2+ in the presence of oligomycin and antimycin A (O/A) to block mitochondrial Ca2+ uptake (n ≥ 100).
Mitochondrial-mediated clearance. We next considered that an increase in total mitochondrial mass might allow for more rapid removal of [Ca2+]i after influx due to increased mitochondrial uptake capacity (25, 26). However, flow cytometric analysis with MitoTracker Green FM, an indicator of total mitochondrial mass (27), demonstrated equivalent staining of control and Vhlh-/- thymocytes (Fig. 10, which is published as supporting information on the PNAS web site). Similarly, assessment of mitochondrial membrane potential (MMP) (28), the driving force for Ca2+ uptake, by staining with JC-1 (29) indicated no differences between control and Vhlh-/- cells (Fig. 9).
Independent of mitochondrial mass and MMP, HIF-1α might modulate mitochondrial buffering capacity. Mitochondrial Ca2+ uptake can be diminished by treatment with electron transport uncoupling agents oligomycin and antimycin A (24, 30). We observed that such inhibition of mitochondrial buffering resulted in a minimal diminution of TCR-mediated peak [Ca2+]i in control cells (data not shown) but modestly rescued the blunted maximal [Ca2+]i elevation in Vhlh-/- cells (Fig. 4C), indicating that stabilization of Hif-1α increases mitochondrial removal of Ca2+ from the cytoplasm.
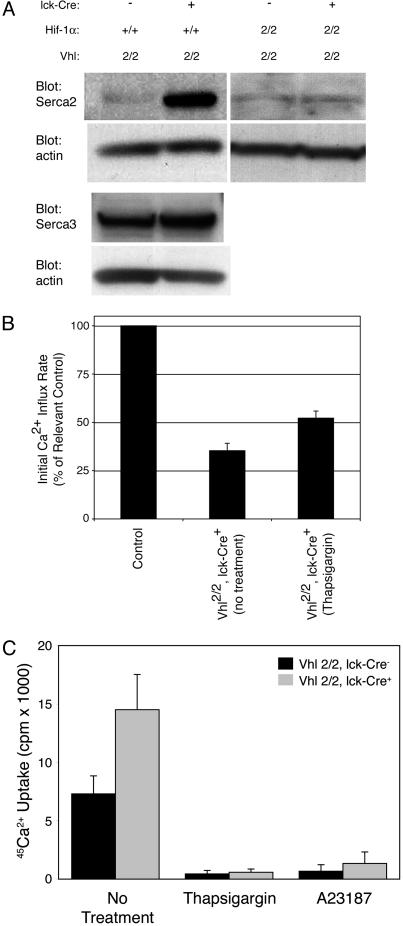
Calcium clearance by means of SERCA pumps. Finally, we reasoned that Hif-1α stabilization might increase the rate of cytoplasmic Ca2+ clearance by SERCA pump-mediated reuptake into the ER stores. Two of the three known SERCA pumps, Serca2 and Serca3, have been identified in murine T cells (31, 32). Western blot analysis of total protein lysates from resting thymocytes revealed no effect of Hif-1α on Serca3 protein expression levels but dramatic up-regulation of Serca2 in Vhlh-/- cells over littermate controls (Fig. 5A). Serca2 expression in double knockout (Vhlh-/-, Hif-1α-/-) thymocytes was similar to littermate controls, suggesting that Hif-1α directly or indirectly regulates Serca2 expression (Fig. 5A).
Fig. 5.
Expression and activity of SERCA pumps. (A) Western blot analysis for Serca2 and Serca3 in thymocyte lysates from resting Vhlh-deficient or Vhlh/Hif-1α double-deficient thymocytes. Staining for β-actin is provided as a loading control. (B) Fura-2-loaded thymocytes from control or Vhlh-deficient mice were stimulated in 2 mM Ca2+ bath solution by receptor crosslinking alone or in combination with thapsigargin (100 nM). The rate of rise in [Ca2+]i during the initial 10 sec was expressed for Vhlh-deficient thymocytes as a percentage ± SEM of the rate in identically treated control cells (n ≥ 100). (C) Ca2+ uptake rates were measured in permeabilized control or Vhlh-deficient thymocytes to assess SERCA activity. Thapsigargin was used to specifically block Ca2+ transport by SERCA pumps, and the Ca2+ ionophore A23187 was used to assess retention of Ca2+ within thapsigargin-sensitive and -insensitive intracellular compartments.
If Hif-1α activation results in accelerated uptake of cytoplasmic Ca2+ into intracellular stores due to increased SERCA pump expression, inhibition of these pumps should rescue blunted Ca2+ signaling in Vhlh-/- thymocytes. However, inhibition of SERCA pumps will also deplete these stores, trigger Ca2+ influx, and make the cells insensitive to TCR stimulation. To circumvent this, we added thapsigargin simultaneously with the CD3/CD4 crosslinker. Because this protocol may not permit full blockade of SERCA function before TCR-mediated store release, any observed reversal of blunted Ca2+ signaling in Vhlh-/- cells by thapsigargin would be an underestimate of SERCA activity. Despite this limitation, we observed partial reversal of the diminished initial rate of [Ca2+]i rise observed in Vhlh-/- thymocytes (Fig. 5B), demonstrating accelerated removal of cytoplasmic Ca2+ by means of SERCA pumps in Vhlh-/- cells.
The increased Serca2 protein levels we observe should produce an increased SERCA-mediated Ca2+ transport rate in Vhlh-/- thymocytes. We tested this theory by incubating measuring 45Ca2+ uptake into permeabilized thymocytes in the presence of ruthenium red, an inhibitor of mitochondrial Ca2+ uptake. We found that Vhlh-/- thymocytes exhibited significantly greater uptake of 45Ca2+ than controls during a 30-min incubation (Fig. 5C). Treatment with thapsigargin dramatically blocked uptake, demonstrating that Ca2+ sequestration in both cell types was highly dependent on SERCA activity. Similar results were found when the reaction mixture was pretreated with apyrase, leading to a broad inhibition of all ATPases (data not shown). Likewise, treatment with Ca2+ ionophore blocked retention of radiolabel, demonstrating that 45Ca2+ uptake was due to sequestration within intracellular compartments (i.e., the ER lumen). We conclude that Vhlh-/- thymocytes exhibit a faster rate of SERCA-dependent Ca2+ uptake compared with control thymocytes due to the observed increase in Serca2 expression.
Discussion
In our murine primary cell model, we observed Hif-1α-dependent diminished Ca2+ mobilization after CD3/CD4 stimulation of thymocytes. However, tyrosine phosphorylation and PLCγ1 activation were intact, intracellular Ca2+ stores were normal, and TCR crosslinking led to successful store depletion and equivalent Ca2+ influx across the plasma membrane in control and Vhlh-/- thymocytes. Accelerated removal of cytoplasmic Ca2+ contributed to diminished Ca2+ signaling. Although faster efflux by means of PMCA was excluded, we observed moderately increased mitochondrial buffering of Ca2+ and reuptake into ER stores in Vhlh-/- thymocytes. Most dramatically, Hif-1α stabilization caused markedly increased protein expression of a Ca2+ pump that is responsible for refilling intracellular stores, Serca2, and also increased overall Serca-mediated Ca2+ transport. These results suggest that both hypoxic and nonhypoxic mediated Hif-1α stabilization may minimize the strength and duration of Ca2+ signaling by means of acceleration of cytoplasmic Ca2+ clearance.
Our results showing that constitutive stabilization of Hif-1α leads to overexpression of the Serca2 pump and a phenotype of diminished Ca2+ signaling in thymocytes are consistent with previously reported findings demonstrating that targeted cardiomyocyte deficiency in Hif-1α causes diminished expression of hypoxia response genes, prolonged Ca2+ signaling, and impaired cardiac contractility (33). Most importantly, expression of Serca2 was diminished in Hif-1α-/- cardiomyocytes, suggesting that Serca2 is regulated directly or indirectly by Hif-1α. Although putative Hif-1α binding regions exist in the Serca2 promoter, little HIF-dependent transcriptional activation was reported using in vitro Serca2 promoter analyses. The control of Serca2 by Hif-1α may require other parallel regulatory pathways, or transcription of Serca2 might be accomplished through an unknown intermediary transcription factor.
These results are relevant for T cell activation because Ca2+ signaling is central to TCR signaling (34). In fact, divergent patterns of Ca2+ signaling (e.g., steady-state elevation versus oscillation of [Ca2+]i) can selectively control activation of nuclear factor of activated T cells and NF-κB (35) and influence thymocyte motility in situ (36). In this way, Ca2+ oscillations could regulate TCR signaling in T lymphocytes by controlling the stability of interactions with antigen-presenting cells. Because Ca2+ oscillations result from the interaction among CRAC channels, ER Ca2+ stores, and mitochondria (26, 37, 38), the ability of Hif-1α to alter Serca2 expression and mitochondrial buffering may have consequences for dictating patterns of Ca2+ signaling and transcription factor activation.
Because Hif-1α modulates Ca2+ signaling during TCR stimulation in thymocytes, we hypothesize that a similar regulation may exist in activated mature T cells, an important consideration given that HIF-1α may be induced upon TCR stimulation (39). In addition to hypoxia, two important events in T cell activation, signaling by phosphoinositide 3-kinases and cytoplasmic acidification, are known to induce HIF-1α protein expression (40–42). It should also be noted that tissue oxygen tensions are likely relatively “hypoxic” compared with atmospheric oxygen tensions used for in vitro tissue culture. Moreover, mature T cells were recently shown to exhibit reduced proliferative responses when stimulated under the low oxygen concentrations (compared with atmospheric oxygen) that normally are found in lymphoid tissues (43). Our findings present a potential mechanistic explanation for this observation. Unfortunately, as our previous work has found that many of the mature T cells present in Vhlh-/- mice are derived from those thymocytes that have failed to delete the Vhlh gene (12), the role of Hif-1α in mature T cell calcium signaling cannot be examined at present.
There are a number of biological scenarios wherein modulation of lymphocyte activation by hypoxia might prove relevant, including ischemia due to vessel occlusion or damage to microvasculature after sterile tissue injury (11). In both cases, the resulting tissue hypoxia may result in local necrotic cell death with uncontrolled self-antigen release. Necrosis promotes inflammatory responses by myeloid cells, and these responses are Hif-dependent (44). We hypothesize that injury-associated hypoxia might engage a parallel protective mechanism that directly prevents the undesirable consequences of T cell activation by means of stabilization of Hif-1α and suppression of TCR-mediated Ca2+ signaling. However, it should be noted that mice lacking HIF-1α in both T and B cells (made by RAG-2 deficient blastocyst complementation) displayed autoimmunity that was associated with B cell abnormalities and autoantibodies (45). In addition, HIF-1α may be able to oppose proinflammatory cytokine production by T cells (11, 46), suggesting a complex role for this transcription factor in regulating adaptive immune responses in vivo.
Hif also promotes the bactericidal capability of phagocytic cells (47). However, because the adaptive immune system has little role in the immediate response to bacterial pathogens, Hif-mediated down-regulation of T cell responses would not be predicted to have an adverse effect on the response to these pathogens in vivo. Finally, because we have previously demonstrated that Hif-1α stabilization in thymocytes causes increased apoptosis (12), it is tempting to speculate that the effects of Hif-1α on mitochondrial Ca2+ uptake may contribute to shaping the mature T cell repertoire.
Supplementary Material
Acknowledgments
We thank Gary Koretzky, Devon Taylor, and Patrick Walsh for helpful discussions and comments regarding the manuscript. This work was supported by National Institutes of Health Grant AI-43626 (to L.A.T.) and American Heart Association Grant 0365342U (to V.H.H.).
Author contributions: A.K.N., J.Y., M.P.B., S.K.J., V.H.H., B.D.F., and L.A.T. designed research; A.K.N., J.Y., M.P.B., S.K.J., and B.D.F. performed research; R.S.J. contributed new reagents/analytic tools; A.K.N., J.Y., M.P.B., S.K.J., V.H.H., B.D.F., and L.A.T. analyzed data; and A.K.N., V.H.H., B.D.F., and L.A.T. wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Hif, hypoxia inducible factor; TCR, T cell receptor; SERCA, sarcoplasmic/endoplasmic reticulum calcium ATPase; [Ca2+]i, intracellular Ca2+ concentration; PMCA, plasma membrane calcium ATPase; PLCγ1, phospholipase Cγ1; IP3, inositol tris-phosphate; IP3R, IP3 receptor; CRAC, Ca2+ release-activated Ca2+; ER, endoplasmic reticulum.
References
- 1.Semenza, G. L. (2003) Annu. Rev. Med. 54, 17-28. [DOI] [PubMed] [Google Scholar]
- 2.Michiels, C. (2004) Am. J. Pathol. 164, 1875-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaelin, W. G., Jr. (2002) Genes Dev. 16, 1441-1445. [DOI] [PubMed] [Google Scholar]
- 4.Huang, L. E., Gu, J., Schau, M. & Bunn, H. F. (1998) Proc. Natl. Acad. Sci. USA 95, 7987-7992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maxwell, P. H., Wiesener, M. S., Chang, G. W., Clifford, S. C., Vaux, E. C., Cockman, M. E., Wykoff, C. C., Pugh, C. W., Maher, E. R. & Ratcliffe, P. J. (1999) Nature 399, 271-275. [DOI] [PubMed] [Google Scholar]
- 6.Hale, L. P., Braun, R. D., Gwinn, W. M., Greer, P. K. & Dewhirst, M. W. (2002) Am. J. Physiol. 282, H1467-H1477. [DOI] [PubMed] [Google Scholar]
- 7.Caldwell, C. C., Kojima, H., Lukashev, D., Armstrong, J., Farber, M., Apasov, S. G. & Sitkovsky, M. V. (2001) J. Immunol. 167, 6140-6149. [DOI] [PubMed] [Google Scholar]
- 8.Braun, R. D., Lanzen, J. L., Snyder, S. A. & Dewhirst, M. W. (2001) Am. J. Physiol. 280, H2533-H2544. [DOI] [PubMed] [Google Scholar]
- 9.Makino, Y., Nakamura, H., Ikeda, E., Ohnuma, K., Yamauchi, K., Yabe, Y., Poellinger, L., Okada, Y., Morimoto, C. & Tanaka, H. (2003) J. Immunol. 171, 6534-6540. [DOI] [PubMed] [Google Scholar]
- 10.Distler, J. H., Wenger, R. H., Gassmann, M., Kurowska, M., Hirth, A., Gay, S. & Distler, O. (2004) Arthritis Rheum. 50, 10-23. [DOI] [PubMed] [Google Scholar]
- 11.Sitkovsky, M. V., Lukashev, D., Apasov, S., Kojima, H., Koshiba, M., Caldwell, C., Ohta, A. & Thiel, M. (2004) Annu. Rev. Immunol. 22, 657-682. [DOI] [PubMed] [Google Scholar]
- 12.Biju, M. P., Neumann, A. K., Bensinger, S. J., Johnson, R. S., Turka, L. A. & Haase, V. H. (2004) Mol. Cell. Biol. 24, 9038-9047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schofield, C. J. & Ratcliffe, P. J. (2004) Nat. Rev. Mol. Cell. Biol. 5, 343-354. [DOI] [PubMed] [Google Scholar]
- 14.Semenza, G. L. (2001) Curr. Opin. Cell Biol. 13, 167-171. [DOI] [PubMed] [Google Scholar]
- 15.Wenger, R. H. (2002) FASEB J. 16, 1151-1162. [DOI] [PubMed] [Google Scholar]
- 16.Gnarra, J. R., Ward, J. M., Porter, F. D., Wagner, J. R., Devor, D. E., Grinberg, A., Emmert-Buck, M. R., Westphal, H., Klausner, R. D. & Linehan, W. M. (1997) Proc. Natl. Acad. Sci. USA 94, 9102-9107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haase, V. H., Glickman, J. N., Socolovsky, M. & Jaenisch, R. (2001) Proc. Natl. Acad. Sci. USA 98, 1583-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryan, H. E., Poloni, M., McNulty, W., Elson, D., Gassmann, M., Arbeit, J. M. & Johnson, R. S. (2000) Cancer Res. 60, 4010-4015. [PubMed] [Google Scholar]
- 19.Lee, P. P., Fitzpatrick, D. R., Beard, C., Jessup, H. K., Lehar, S., Makar, K. W., Perez-Melgosa, M., Sweetser, M. T., Schlissel, M. S., Nguyen, S., et al. (2001) Immunity 15, 763-774. [DOI] [PubMed] [Google Scholar]
- 20.Freedman, B. D., Liu, Q. H., Somersan, S., Kotlikoff, M. I. & Punt, J. A. (1999) J. Exp. Med. 190, 943-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis, R. S. (2001) Annu. Rev. Immunol. 19, 497-521. [DOI] [PubMed] [Google Scholar]
- 22.Zweifach, A. & Lewis, R. S. (1993) Proc. Natl. Acad. Sci. USA 90, 6295-6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Putney, J. W., Jr., Broad, L. M., Braun, F. J., Lievremont, J. P. & Bird, G. S. (2001) J. Cell Sci. 114, 2223-2229. [DOI] [PubMed] [Google Scholar]
- 24.Bautista, D. M., Hoth, M. & Lewis, R. S. (2002) J. Physiol. 541, 877-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunter, T. E., Yule, D. I., Gunter, K. K., Eliseev, R. A. & Salter, J. D. (2004) FEBS Lett. 567, 96-102. [DOI] [PubMed] [Google Scholar]
- 26.Hoth, M., Fanger, C. M. & Lewis, R. S. (1997) J. Cell Biol. 137, 633-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oubrahim, H., Stadtman, E. R. & Chock, P. B. (2001) Proc. Natl. Acad. Sci. USA 98, 9505-9510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friel, D. D. & Tsien, R. W. (1994) J. Neurosci. 14, 4007-4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smiley, S. T., Reers, M., Mottola-Hartshorn, C., Lin, M., Chen, A., Smith, T. W., Steele, G. D., Jr., & Chen, L. B. (1991) Proc. Natl. Acad. Sci. USA 88, 3671-3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bautista, D. M. & Lewis, R. S. (2004) J. Physiol. 556, 805-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Launay, S., Bobe, R., Lacabaratz-Porret, C., Bredoux, R., Kovacs, T., Enouf, J. & Papp, B. (1997) J. Biol. Chem. 272, 10746-10750. [DOI] [PubMed] [Google Scholar]
- 32.Poch, E., Leach, S., Snape, S., Cacic, T., MacLennan, D. H. & Lytton, J. (1998) Am. J. Physiol. 275, C1449-C1458. [DOI] [PubMed] [Google Scholar]
- 33.Huang, Y., Hickey, R. P., Yeh, J. L., Liu, D., Dadak, A., Young, L. H., Johnson, R. S. & Giordano, F. J. (2004) FASEB J. 18, 1138-1140. [DOI] [PubMed] [Google Scholar]
- 34.Winslow, M. M., Neilson, J. R. & Crabtree, G. R. (2003) Curr. Opin. Immunol. 15, 299-307. [DOI] [PubMed] [Google Scholar]
- 35.Dolmetsch, R. E., Xu, K. & Lewis, R. S. (1998) Nature 392, 933-936. [DOI] [PubMed] [Google Scholar]
- 36.Bhakta, N. R., Oh, D. Y. & Lewis, R. S. (2005) Nat. Immunol. 6, 143-151. [DOI] [PubMed] [Google Scholar]
- 37.Dolmetsch, R. E. & Lewis, R. S. (1994) J. Gen. Physiol. 103, 365-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rizzuto, R., Pinton, P., Carrington, W., Fay, F. S., Fogarty, K. E., Lifshitz, L. M., Tuft, R. A. & Pozzan, T. (1998) Science 280, 1763-1766. [DOI] [PubMed] [Google Scholar]
- 39.Lukashev, D., Caldwell, C., Ohta, A., Chen, P. & Sitkovsky, M. (2001) J. Biol. Chem. 276, 48754-48763. [DOI] [PubMed] [Google Scholar]
- 40.Laughner, E., Taghavi, P., Chiles, K., Mahon, P. C. & Semenza, G. L. (2001) Mol. Cell. Biol. 21, 3995-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang, B. H., Jiang, G., Zheng, J. Z., Lu, Z., Hunter, T. & Vogt, P. K. (2001) Cell Growth Differ. 12, 363-369. [PubMed] [Google Scholar]
- 42.Mekhail, K., Gunaratnam, L., Bonicalzi, M. E. & Lee, S. (2004) Nat. Cell. Biol. 6, 642-647. [DOI] [PubMed] [Google Scholar]
- 43.Atkuri, K. R., Herzenberg, L. A. & Herzenberg, L. A. (2005) Proc. Natl. Acad. Sci. USA 102, 3756-3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cramer, T., Yamanishi, Y., Clausen, B. E., Forster, I., Pawlinski, R., Mackman, N., Haase, V. H., Jaenisch, R., Corr, M., Nizet, V., et al. (2003) Cell 112, 645-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kojima, H., Gu, H., Nomura, S., Caldwell, C. C., Kobata, T., Carmeliet, P., Semenza, G. L. & Sitkovsky, M. V. (2002) Proc. Natl. Acad. Sci. USA 99, 2170-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sitkovsky, M. & Lukashev, D. (2005) Nat. Rev. Immunol. 5, 712-721. [DOI] [PubMed] [Google Scholar]
- 47.Peyssonnaux, C., Datta, V., Cramer, T., Doedens, A., Theodorakis, E. A., Gallo, R. L., Hurtado-Ziola, N., Nizet, V. & Johnson, R. S. (2005) J. Clin. Invest. 115, 1806-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.