Perhaps our undying fascination with any topic related to sex arises from our desire to understand all features shared between man and woman and, more important, to discover those features which distinguish us from one another. During the past decade, scientists have begun to unlock differences between the sexes through molecular and genetic dissection of mammalian sexual differentiation. This area of research exemplifies a successful amalgamation of human, mouse, and molecular studies that are beginning to create a general understanding of this developmental event. Here, we have chosen not to cover the detailed embryology of gonadal and sexual differentiation (recently reviewed by Swain and Lovell-Badge 1999); we focus instead on concepts emerging from the numerous in vitro, in vivo, and genetic analyses. In vitro analyses have now outlined the molecular basis of testis development, although the function SRY/Sry, which apparently initiates this process, is still a complete mystery. Genetic analyses now suggest that, whereas gene dosage appears to be important in human sexual development, this effect is far less crucial in laboratory-mouse strains. On the other hand, reverse genetic experiments in these same mouse-model systems have led both to discovery and to elimination of candidate genes proposed to direct this developmental process. Finally, recent findings have served also to underscore the differences and similarities shared between mammalian and invertebrate sexual differentiation.
Gonadal and Sexual Development
Regardless of genetic sex, embryos undergo an indifferent stage of sexual development, marked by the appearance of bipotential gonads and internal and external genitalia that are sexually indeterminate. Both male and female embryos initially develop with primitive male and female reproductive tracts. The Müllerian duct gives rise to oviducts, uterus, and upper vagina, and the Wolffian duct is the precursor of the epididymis, vas deferens, seminal vesicle, and ejaculatory duct. In males, sexual determination culminates in the secretion of testicular hormones, which cause male-specific development of the bipotential reproductive system; this process is referred to as “primary sexual differentiation.” In the absence of gonads, embryos develop female reproductive tracts and genitalia, so female differentiation is considered a default pathway. Sex reversal in males can result from the absence of testicular hormones or from the failure of those hormones to signal in target tissues. Conversely, if female embryos are exposed to testicular hormones, they can undergo aspects of male sexual differentiation, despite the absence of testes.
Our understanding of male sexual development is much more extensive than our knowledge female development, largely because three essential testicular hormones have been identified. Several decades ago, two of these male-differentiation hormones were discovered when Jost performed the now-classic experiment of transplantation of fetal rabbit testes into female hosts. Sertoli cells secrete a TGF-β superfamily hormone called “Müllerian inhibiting substance” (MIS, also known as “anti-Müllerian hormone” [AMH]), which causes the Müllerian duct to regress (reviewed in Behringer 1995). Leydig cells secrete testosterone, which maintains the Wolffian duct; also, testosterone is metabolized into other steroids, which masculinize the external genitalia and specific brain regions. More recently, a third hormone has been identified by reverse genetics in mice. The Leydig-cell hormone, insulin-like hormone 3 (InsL3), is required for testicular descent (Zimmermann et al. 1999). Consistent with their role in testis development, MIS, InsL3, or steroidogenic enzymes producing testosterone are not present in the fetal ovary. Moreover, now it appears that synthesis of testosterone is actively repressed in the embryonic stages of female development (Vainio et al. 1999). Consequently, female differentiation occurs because (i) without MIS, Müllerian ducts are maintained; (ii) without testosterone, Wolffian ducts degenerate and female external genitalia develop; and (iii) without InsL3, gonads remain in the abdomen.
Regulatory Molecules in Mammalian Sex Differentiation
Although the hormonal basis of sexual differentiation is well understood, the molecular events initiated by these testicular hormones remain unclear. Much more is known about how expression of these testicular hormones becomes restricted to males. Identification of sex-determining genes either by human genetics or through promoter analyses of genes encoding male hormones has taught us three important facts. First, factors used to establish the early indifferent gonad regulate these testicular hormones and act at multiple stages in the embryonic and adult gonad. Second, these factors are neither sex specific nor gonad specific. Thus, whereas the expression of target genes, including those required for the synthesis of male hormones, is restricted to the embryonic testis, the regulators of these hormones are not. Finally, a straightforward cascade of gene activation and/or repression, as found in invertebrate systems, appears not to be used in mammalian sexual development. Rather, it seems that an intricate web of factors activates downstream targets and that each factor is itself regulated by a subset of these same genes.
Expression profiles of known sex-determining genes have been described, and the general temporal and sex-specific patterns, relative to each other, are shown in figure 1. Currently, three genes have been implicated in gonadal development—the orphan nuclear receptor Steroidogenic factor-1 (SF-1, or Ftz-F1), the zinc-finger factor, Wilms tumor 1 (WT1), and the homeobox gene, Lim-1. The only known sex-specific gene to be involved directly in testis development is the Y-linked gene, SRY, whose expression follows that of SF-1, WT1, and Lim-1. The autosomal genes—Sox9, SF-1, and DMRT1—all exhibit a sexually dimorphic expression pattern after commencement of testis determination, although SF-1 expression resurfaces in the postnatal ovary. DMRT1 is related to both mab-3 of Caenorhabditis elegans and doublesex of Drosophila and, thus far, represents the only structurally related gene known to participate in sexual differentiation in both vertebrates and invertebrates (Raymond et al. 1999). Wnt4 is implicated in ovarian development because of its role in the repression of steroidogenesis. Other genes implicated in the mammalian sex-determination pathway are desert hedgehog (Dhh), GATA-4, and DAX-1 (also known as “AHC”). However, as discussed below, studies of human and mouse mutants have formally ruled out roles for either DAX-1 or Dhh in gonadal development (table 1).
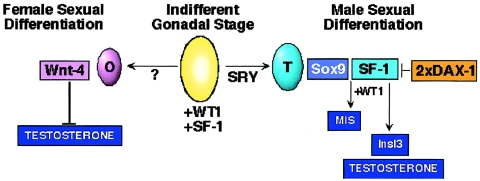
Figure 1.
Sex-specific and shared regulatory factors involved in male and female gonadal differentiation. Relative levels of expression in the testis and ovary are indicated, by color codes, for each factor. In mice, Sry expression commences at ∼10.5 dpc (Hacker et al. 1995; Jeske et al. 1995).
Table 1.
Dosage Effects and Phenotypes Observed in Human and Mouse Mutants of Sex-Linked and Autosomal Genes Involved in Mammalian Sex Determination
|
Effective Gene Dosage |
Other Phenotypes |
||||||||
| Karyotype and Genotype | SRY | SOX9 | WT1 | SF1 | DMRT | DAX1 | PhenotypicSex | Human | Mouse |
| 46,XX: | |||||||||
| Wild type | 0 | 2 | 2 | 2 | 2 | 2 | Female | ||
| 46,XY: | |||||||||
| Wild type | 1 | 2 | 2 | 2 | 2 | 1 | Male | ||
| SRY− | 0 | 2 | 2 | 2 | 2 | 1 | Female | Testicular dysgenesis | Not determined |
| SOX9−/+ | 1 | 1 | 2 | 2 | 2 | 1 | Female | Campomelic dysplasia in XX and XY heterozygote | Not determined |
| WT1−/+ | 1 | 2 | 1 | 2 | 2 | 1 | Female | Renal nephropathy, Wilms tumor in XX and XY heterozygote | Gonadal and kidney agenesis with defects in heart, lung in homozygote |
| SF1−/+ | 1 | 2 | 2 | 1 | 2 | 1 | Female | AdrenalInsufficiency in heterozygote | Gonadal, adrenal, gonadotropic, and ventromedial-hypothalamus agenesis in homozygote |
| DMRT−/+a | 1 | 2 | 2 | 2 | 1a | 1 | Female | Not determined | Not determined |
| Duplication of Xp21(DAX1) | 1 | 2 | 2 | 2 | 2 | 2 | Female | Not applicable | Not applicable |
| DAX1− | 1 | 2 | 2 | 2 | 2 | 0 | Male | Adrenal insufficiency, normal testes, sporadic hypogonadism/hypogonadotropism | Adrenal insufficiency, impaired spermatogenesis |
| 46,XX: | |||||||||
| DAX1−/− | 1 | 2 | 2 | 2 | 2 | 0 | Female | Adrenal insufficiency, normal ovaries | Adrenal insufficiency, normal ovaries |
Large deletion spanning both DMRT1 and DMRT2 (Raymond et al. 1999).
Dosage of Regulatory Factors
Gene dosage of regulatory factors appears to be critical in normal human sex determination, as indicated in table 1. This strategy for fine-tuning this developmental process has only been revealed by analyzing the genotype of human sex-reversed individuals, since dosage effects do not appear to operate in genetically engineered mouse-model systems. Heterozygous inbred Wt1 or SF-1 (ftz-f1) mouse mutants exhibit normal gonadal development, whereas human XY mutant heterozygotes may be fully sex reversed or may present with milder gonadal dysgenesis. Whether the phenotypic difference between heterozygote humans and mice represents a fine distinction in anatomy and physiology or whether it reflects the broad diversity of the human population versus an inbred strain bred with selective pressures for high fecundity is not clear. Given the conservation of factors shared between mice and men in sexual development, discussed below, the latter hypothesis seems more likely.
SRY
To date, the uniquely mammalian gene SRY, remains the only sex chromosome–specific gene that actively participates in gonadal development. Unsolved problems abound regarding the function of SRY. SRY/Sry was positionally cloned on the short arm of the Y chromosome (Koopman et al. 1990), and its candidacy as the testis-determining factor (TDF) was confirmed when a 14-kb mouse genomic-fragment transgene harboring Sry induced male sexual development in a proportion of XX mice (Koopman et al. 1991). In addition, data compiled from several labs revealed that ⩾20% of XY females with pure gonadal dysgenesis (complete female phenotype and streak gonads) carry missense or nonsense mutations clustered in the DNA-binding high-mobility group (HMG) domain of the intronless SRY gene. Oddly, as reviewed by Cameron and Sinclair (1997), 46,XY males with these HMG missense mutations may develop normally, indicating that the penetrance of these mutations is incomplete. Moreover, as shown in table 1, the presence of XY females who carry an intact SRY gene predicts that the developmental switch between male and female is under multiple levels of genetic control.
SRY has been known for its pivotal role as the sex-determining gene for almost 10 years, but how SRY initiates male gonadal differentiation at the molecular level is still not clear. Because it possesses a 79-amino-acid DNA-binding HMG domain, SRY is presumed to function as a DNA-binding protein to modify gene expression. As with other HMG-transcription factors, SRY binds preferentially to a conserved DNA sequence and causes DNA bending (Grosschedl et al. 1994). Despite extensive in vitro studies to define the mechanics and optimal DNA binding sites, bona fide downstream targets of SRY remain unidentified. By analogy to other HMG factors, it seems likely that SRY may function as a structural-bridging factor that nucleates the assembly of the transcriptional machinery. To date, potential SRY targets include those genes which are up-regulated during early testicular development, such as Sox9, SF-1, SF-1, DMRT1, GATA-4, Dhh, and the matrix metalloproteinase, Testatin (Töhönen et al. 1998). The other structural motifs in the SRY protein are not understood, and no functional interacting SRY protein partners have yet been reported.
The question of how SRY functions is further complicated by the striking sequence divergence observed among different mammalian SRY species (Gubbay et al. 1990; Sinclair et al. 1990). Both the overall topology and the protein sequence (outside the HMG motif) of SRY differ greatly among species. Why would a protein performing an important and conserved developmental program evolve so quickly? The largest sequence variations can be found at either the amino- or carboxyl-terminus of SRY, where the presence and/or number of glutamine (CAG) repeats differs significantly between mouse and rat and among different mouse species (Tucker and Lundrigan 1993; J. Shen and H. A. Ingrham, unpublished data). Further in vitro and in vivo analyses are needed to assess the potential roles of these non-HMG regions in the sex-differentiation pathways. In the absence of bona fide target genes, in vivo mapping or new innovative molecular strategies will be critical for a full dissection of the structure-function relationship of Sry.
The attempt to detect SRY transcripts and protein in mammals has met with limited success. To date, neither immunohistochemical nor western-blot analyses have confirmed the presence of Sry protein in embryonic gonadal tissue. Nonetheless, sensitive RNA-expression studies reveal Sry transcripts in the gonadal portion of the urogenital ridge, beginning at 10.5 days postcoitum (dpc), peaking at 11.0 dpc, and then declining (Hacker et al. 1995; Jeske et al. 1995; C. Nagamine, personal communication). The basis for this transient peak of Sry expression suggests that Sry triggers Sertoli-cell differentiation but that it is dispensable in the maintenance of testicular development. Promoter analyses of SRY/Sry in cultured cell lines have defined a proximal promoter region for expression in cultured cell lines (Desclozeaux et al. 1998). Indeed, several conserved binding sites—including those for SP-1, AP-1, and Oct-1—are found in multiple species, but none has been shown to be critical for Sry expression (Margarit et al. 1998).
Y chromosomes from Mus domesticus poschiavinus (Ypos) or Mus musculus domesticus (Ydom) have been introduced into the C57 Bl/6 genetic background, in order to study the function of Sry in mice. Both Ypos and Ydom chromosomes appear to carry a weakened allele of Sry, and, in this genetic context, they are associated with a variable rate of phenotypic female development in XY animals. Although this phenomenon remains unexplained at the molecular level, linkage analyses indicate that three autosomal loci (tda1, tda2, and tda3) modulate Sry action, to cause sex reversal (Eicher et al. 1996). Sex reversal can be variable in these Ydom mice, and the degree of sex reversal has shown to be associated with the duration of C57BL/6 Srydom expression (Lee and Taketo 1994), suggesting that timing and the expression levels of Srydom could account for this sex-reversal phenomenon. However, intrinsic differences among Srydom alleles and/or strain-specific protein interactions with different Srydom alleles are also possible. If precise timing and expression levels of Sry are critical for normal male sexual development, as suggested by these data, then finely tuned regulatory mechanisms governing Sry's temporal and spatial expression must exist. The ability to sex-convert an XX mouse with the Sry genomic fragment predicts that no other Y-specific genes are required in this transcriptional event.
The DAX-1 Paradox
DAX-1 (also known as “AHC”) was initially identified as being a factor underlying familial X-linked adrenal hypoplasia congenita, a condition that presents as early-onset adrenal insufficiency (Muscatelli et al. 1994; Zanaria et al. 1994). Excitement following the discovery of the DAX-1 gene arose in part because this X-linked gene was proposed to be the long-awaited ovary-determining factor, because it resided in the X-chromosome duplicated region associated with dosage-sensitive sex reversal (DSS). DSS occurs in XY individuals who carry a tandem duplication of a 160-kb region on the short arm of the X chromosome (table 1; also see and Bardoni et al. 1994). However, in Dax1−/− mice, ovarian development proceeds normally, with only a subtle defect observed in the adult ovary of sporadic fused follicles (table 1; also see Yu et al. 1998). A rare homozygous DAX1-mutant female patient has also been identified who exhibits small but normal ovaries (table 1; also see Merke et al. 1999). Collectively, these data exclude an overt role for DAX1 in ovarian development, as originally proposed elsewhere (Zanaria et al. 1994; Swain et al. 1996).
Sex reversal in 46,XY patients with a duplication of DAX1 led to the obvious hypothesis that heightened expression of the DAX1 protein, an unusual orphan nuclear receptor, antagonizes the male program. Thus far, there is good molecular support for this dosage hypothesis (discussed below), but it does not fully account for data obtained in mouse transgenic studies. Indeed, transgenic XY mice carrying multiple copies of Dax1 are not feminized, despite an excess of both Dax1 mRNA and protein in the testes (Swain et al. 1998). However, an antagonistic effect of Dax1 on male development was unmasked in the Ypos x C57/Bl6 mouse strain, which, as described above, carries a weakened Sry allele. Moreover, introduction of a Sry transgene into XX mice, which normally initiates testis development, fails to do so in strains that carry multiple copies of the Dax1 transgene, suggesting that Dax1 can antagonize this Sry-mediated sex conversion. Collectively, these data are provocative, but they leave one pondering the simple hypothesis that two doses of DAX1 lead to DSS. The fact that Dax1 overexpression fails to sex-reverse a normal XY mouse is intriguing and suggests that mechanisms of sex-reversal in DSS patients may be more complex that simple dosage effects. Specifically, the site of the transgene integration might not recapitulate a tandem duplication in DAX1, or surrounding chromosomal information in this 160-kb region may also contribute to the DSS phenotype. Finally, it is formally possible that another gene in this region is responsible for DSS.
Modulation of SF-1 by DAX1
Impaired spermatogenesis in Dax1−/− mice is consistent with the testicular-expression pattern of Dax1 (Ikeda et al. 1996; Tamai et al. 1996; Nachtigal et al. 1998; Yu et al. 1998) but is difficult to reconcile with the model suggesting that Dax1, as a single-copy gene, acts strictly by antagonizing Sry. Instead, these data support the notion that Dax1 contributes to embryonic and adult testis function in both Sertoli and Leydig cells. Moreover, the shared phenotypes observed in SF-1 and DAX1/Dax1 loss-of-function mutants would predict that DAX1 either activates SF-1 target genes or interacts directly with SF-1 as a positive cofactor. Contrary to this hypothesis, however, assays in transfected cells show that Dax1 represses SF-1–mediated transcription (Ito et al. 1997; Lalli et al. 1997; Crawford et al. 1998; Nachtigal et al. 1998).
In vivo data generated from yeast and mammalian two-hybrid assays, as well as from biochemical experiments, show that both the N-terminus and the ligand-binding domain (LBD) of DAX1 physically interact with SF-1 (Crawford et al. 1998; Nachtigal et al. 1998; M. Nachtigal and H. A. Ingraham, unpublished data). Human and mouse DAX1 share a series of conserved LXXLL motifs at their N-termini, in addition to one such motif in the LBD region, motifs that are known to nucleate protein-protein interactions by nuclear receptor–superfamily members. The outcome of a Dax1:SF-1 protein partnership might depend on which LXXLL motif interacts with a similar motif in the LBD of SF-1. Additionally, because classic nuclear receptors require ligand to relieve repression by corepressors such as SMRT and N-CoR, the binding of a still unknown DAX1 ligand may unlock the activation functions of DAX1. Thus far, all DAX1 missense mutations that are associated with DAX1 dysfunction occur in the putative LBD region; thus the function of the N-terminal repeats remains elusive (reviewed in Zhang et al. 1998).
Natural or Targeted Mutations in Regulators of Sexual Development
Perhaps surprisingly, no mouse strain with a null allele of Sry has been described, despite the striking sex-reversal and gonadal-dysgenesis phenotypes in people with SRY mutations. For other autosomal genes that are implicated in the sex-determination cascade, human and mouse mutants exist, but they do not always phenocopy one another; this is especially true in the heterozygous state (table 1). For instance, the heterozygote 46,XY human mutants of SOX9, WT1, and SF-1 are all sex-reversed, whereas heterozygote mouse mutants of these same genes appear to be normal. It will be of great interest to see whether this trend continues for other genes, such as DMRT1, that are implicated in the sex-determination pathway (Raymond et al. 1999). Presently, neither sex-reversed 46,XY humans carrying just one allele of DMRT1 nor mice carrying a targeted disruption of the Dmrt1 gene have been reported. Despite apparent differences, in gene-dosage effects, between mice and humans, mutants of both species illustrate that these sex-determining factors are essential for other developmental processes, which are unrelated to gonadal differentiation; these are outlined briefly in table 1.
Within the past decade, genetic studies have identified many of the key players in the mammalian sex-determination pathway. It is uncertain how many more genes will be found in this developmental pathway. One of the great challenges will be to determine the molecular and biochemical nature of this pathway, in which genetic variation leads to such complex and variable phenotypes. We now turn to some the recent progress in this area.
MIS: A Molecular Model of Sex-Specific Gene Expression
MIS is one of the few male-specific genes known, and it has served as a model of sex-specific gene expression. Consequently, MIS transcription is the best-understood aspect of sexual differentiation at a molecular level, and it is regulated by many of the autosomal factors described above. Promoter studies of the MIS gene reveal a 180-bp region that is both necessary for reporter-gene expression in Sertoli cells (Shen et al. 1994) and sufficient to recapitulate the normal expression pattern of MIS in transgenic mice (Giuili et al. 1997).
Regulation of MIS by SF-1/Sox9/WT1/GATA-4
A binding site for SF-1 within the 180-bp proximal promoter is required for MIS promoter activity both in vitro and in vivo, and SF-1 activates transcription from the MIS promoter when transfected into heterologous cell lines (Shen et al. 1994; Nachtigal et al. 1998). These findings provide strong evidence that, in addition to its function in gonadal formation, SF-1 is a key regulator of MIS in vivo. The recent identification of a human SF-1 heterozygote with a missense mutation that abolishes sequence-specific DNA binding corroborates roles for SF-1 in testis determination and in MIS regulation, since this XY individual possesses streak gonads and retains fully developed Müllerian structures (Achermann et al. 1999).
Factors besides SF-1 are required to ensure tissue- and sex-specific expression of MIS. Like other genes in sexual development, SF-1 functions in many tissues in addition to the gonad, such as the adrenal gland and hypothalamus, whereas MIS expression is restricted to the fetal testis and postnatal gonads. Furthermore, MIS transcripts are detected just before SF-1 levels rise dramatically in males, suggesting that other factors initiate MIS expression. Three additional transcription factors—Sox9, WT-1, and GATA-4—also regulate MIS-gene expression, and, interestingly, these factors, like SF-1, appear to act at early stages in the sex-determination cascade or in the development of nongonadal tissues. The first of these factors to be expressed in a testis-specific fashion is Sox9, which binds to a more distal site within the 180-bp proximal MIS promoter. This HMG factor also interacts with SF-1 and cooperates in the activation of a MIS reporter gene (De Santa Barbara et al. 1998).
A second factor proposed to participate in the expression of MIS is the product of WT1. WT-1 is coexpressed with SF-1 during testis development and, like SF-1, is required for gonad formation. Although WT-1 does not bind the MIS promoter sequence or activate transcription by itself, it physically interacts with SF-1 and dramatically augments MIS expression in vitro. Synergy between SF-1 and WT-1 is abolished by mutations in WT-1 that are found in patients with Wilms tumor who have genital abnormalities (Nachtigal et al. 1998). Increased dosage of DAX1, which causes XY sex-reversal in humans (Muscatelli et al. 1994; Zanaria et al. 1994), also abrogates WT-1 synergy with SF-1 on the MIS promoter in vitro (Nachtigal et al. 1998). Finally, the third gene implicated in MIS regulation encodes the zinc-finger transcription factor GATA-4. GATA-4 protein is critical for development of heart and erythroid cells and is also expressed in gonadal somatic cells. During development, GATA-4 is down-regulated in the embryonic ovary, but it persists in the testis. Although it activates the 180-bp MIS promoter in vitro (Viger et al. 1998), GATA-4 acquires its sexually dimorphic expression only late in gonadal development, at a stage, in mice, when Müllerian-duct regression is complete. Hence it is unlikely to contribute to sex-specific expression of MIS, but it may participate in late embryonic expression of MIS.
Accounting for the Tissue and Sex-Specific Expression of MIS
Although embryonic expression of MIS is restricted to the testis, none of the factors that control sexually dimorphic expression of MIS are testis specific, in that they are expressed in nongonadal tissues and also in the fetal ovary. MIS expression may require the combined activities of several factors—such as SF-1, Sox-9, and WT-1—and thus may occur only in the gonad, where all of these factors overlap, but this model cannot explain why MIS is silent in the fetal ovary, despite the presence of low levels of SF-1, Sox9, and WT-1. Unknown testis-specific factors may be required for MIS expression in the embryonic testis, or, alternatively, unidentified ovarian factors may repress expression of MIS or may degrade MIS transcripts in the ovary. Precedence for this suggestion comes from a recent study showing that WNT4 inhibits androgen biosynthesis in the embryonic ovary (see below and Vainio et al. 1999).
SF-1: A Master Regulator of Male Sexual Differentation
Activation and Suppression of Steroidogenesis in Fetal Gonads
During differentiation of the internal genitalia, embryonic Leydig cells express enzymes that are required for the synthesis of sex steroids, enzymes such as 17α-hydroxylase (P45017α/CYP17) and 3β-hydroxysteroid dehydrogenase. By contrast, the fetal ovary does not express these enzymes, illustrating the sexually dimorphic nature of steroid biosynthesis in the embryo (Ikeda et al. 1994; Vainio et al. 1999; also see Davey et al. 1999 [in this issue]). As postulated for the adult adrenal and gonads, the coordinate regulation of steroidogenesis in fetal Leydig cells is most likely mediated by SF-1 (Parker and Schimmer 1997). Although direct proof that SF-1 regulates hydroxylase expression in fetal Leydig cells is hindered by the fact that Sf-1–null mice lack gonads, a human patient with an inactivating SF-1 mutation possesses abnormal gonads and is phenotypically female, suggesting that SF-1 controls fetal androgen biosynthesis (Achermann et al. 1999).
Despite low levels of SF-1 in the fetal ovary, steroidogenic enzymes are not expressed. Recent genetic studies in mice show that steroidogenesis is actively suppressed in the fetal ovary by a member of the Wnt gene family, Wnt-4. In contrast to Sox9, Sox9, Gata-4, and Dmrt, Wnt-4 appears to be down-regulated in the embryonic testis. Female Wnt-4−/− mice display masculinized internal genitalia; that is, they possess Wolffian-duct structures, owing to inappropriate testosterone biosynthesis (Vainio et al. 1999). However, Wnt-4–null ovaries do not express the Sertoli cell–specific genes MIS or DHH, indicating that Wnt-4 is not a general repressor of testis determination. Sertoli cell–gene expression may be repressed in ovary by as-yet-unknown factors, or factors required for the activation of these genes may simply be absent in the ovary. Wnt-4–null mice provide the first example of paracrine regulation of testicular hormone expression. Future studies aimed at delineating both how Wnt signaling suppresses steroidogenesis in the ovary and whether other male-specific genes are repressed by similar mechanisms will be of great interest.
InsL3, Testicular Descent, and SF-1
In addition to Müllerian-duct regression and the onset of steroidogenesis, a critical and late event in male sexual development is the descent of the testes, through the inguinal canals to the scrotum. Proper positioning of the testes is determined by two genital ligaments, the cranial suspensory ligament and the gubernaculum, or caudal genital ligament. In males the gubernaculum grows, allowing the testis to descend; by contrast, in females this ligament fails to develop. Recently, reverse genetics has identified Insl3 as a factor required for gubernaculum development. In Insl3−/− male mice, this ligament fails to develop, resulting in bilateral cryptorchidism and sterility (Zimmermann et al. 1999). InsL3 shows a male-specific pattern of expression in the fetal testis, beginning at E13.5 in mouse, and is not detected in the fetal ovary. As with other hormones involved in male sexual differentiation, there is good in vitro evidence that SF-1 regulates InsL3 (Zimmermann et al. 1998). Because the timing and cell-specific expression of InsL3 differ from those of MIS (Leydig cells vs. Sertoli cells), different SF-1 cofactors and/or factors must interact with SF-1 on the InsL3 and MIS promoters, to create distinct expression patterns.
Collectively, in vitro and in vivo data generated from multiple investigations allow for tentative placement of several autosomal genes in the mammalian sex-differentiation pathway. Our current interpretation of existing findings, which we have outlined in the text above, is shown in figure 2. Clearly, a molecular understanding of the latter stages of male sexual differentiation is much more complete than that for initial events, which specify testicular and ovarian development. Thus, efforts to complete our understanding of this pathway will surely be focused on determining how SRY directs testis development and how or whether SRY links directly with autosomal factors, such as SOX-9 and SF-1. Additionally, the field anxiously awaits additional factors that, by either repressing the male program (as shown for Wnt-4) or actively promoting ovarian determination, contribute to ovarian development (fig. 2).
Figure 2.
Mammalian sex determination: a molecular view. Factors implicated in formation of the bipotential gonad and differentiation of the ovary and testis are shown. Testis determination is initiated by SRY; however, molecular downstream targets of SRY and direct connections of SRY with other regulatory factors are undetermined. During testis differentiation, Sox9, SF-1 and WT-1 regulate MIS. SF-1 also directs expression of steroidogenic enzymes to produce testosterone and the Insl-3 factor, which is required for proper testis descent. Mutation of SRY, SOX9, SF-1, or WT1 leads to 46,XY sex-reversed females. An additional dose of the DAX1 gene (or increased DAX1 protein) is postulated to result in 46,XY sex reversal by direct antagonism of SF-1. By contrast, Wnt-4 blocks production of testosterone and therefore may block segments of the male program in the ovary. The placement of DMRT1 in this pathway remains uncertain. Cross-regulation of SRY, SOX9, SF-1, WT-1, and DAX1 may occur at multiple levels and is not depicted here.
Sex Determination, Gender, and Sexual Behavior
As our current knowledge of the mammalian sex-differentiation pathway expands, a connection of this process to the potential genetic regulation of sexual behavior and sex-specific development of the brain is natural. At least in invertebrate systems, a tangible genetic link has been forged between the sex-development pathway and normal courtship behavior. Two genes, fruitless and dissatisfaction, are now known to contribute to normal male courtship behavior, by specifying the identity of neurons required for execution of the reproductive behaviors. Additionally, these genes begin to define new branches of the Drosophila sex-determination pathway that are downstream of the transformer gene (tra) and apparently independent of one another (Finley et al. 1998, and references therein). These findings offer an interesting new perspective for those contemplating the potential mechanisms governing sex differences in the mammalian brain.
Previous studies have shown that steroids affect sex-specific brain development during critical periods before and after birth, with consequences for adult behavior (for review, see Breedlove 1992). For instance, perinatal exposure of female animals to sex steroids can masculinize adult behaviors, and androgens can alter the morphology of brain nuclei, such as in the case of the sexually dimorphic nucleus of the preoptic area of the hypothalamus. Defining the precise contributions of individual nuclei to adult sexual behavior has been difficult. Moreover, the effects of sex steroids on brain development are complex, because these steroids are locally metabolized, as in the case of androgens. One major metabolite of the aromatization of testosterone is estrogen, which is generally assumed to be required in male-specific brain development. The persistence of a masculine preoptic area in androgen-insensitive rats suggests that some developmental effects of testosterone occur after aromatization to estrogen (Breedlove 1992). Interestingly, deficiency for the estrogen-receptor subtype ERα in mice disrupts male-specific aggressive and mating behaviors but does not affect male sexual motivation (Ogawa et al. 1997). Moreover, male mice lacking aromatase are fully capable of breeding (Fisher et al. 1998). These recent studies underscore how little is understood regarding the molecular basis of sex steroids and their cognate nuclear receptors, in brain development and sex differences in the brain.
Continued molecular and genetic investigations will be required to identify which steroids and what nuclei or brain regions are involved in sexually dimorphic brain development. These studies promise to be challenging, given the complexity of (a) steroid action on early brain development and modulation of adult behavior and (b) the indirect effects of steroid action on peripheral tissues. It will be of great interest to know whether mammals use a steroid-independent arm of the sex-determination cascade to dictate sex differences in the brain, as Drosophila does. During the next decade, the convergence of vertebrate genetics, behavior analyses, and genomics should elucidate the remaining mysteries of gonadal development and may begin to provide a genetic rationale for neurological and behavioral differences between men and women.
Footnotes
*This article represents the opinion of the authors and has not been peer reviewed.
References
- Achermann J, Ito M, Ito M, Hindmarsh P, Jameson J (1999) A mutation in the gene encoding steroidogenic factor-1 causes XY sex reversal and adrenal failure in humans. Nat Genet 22:125–126 [DOI] [PubMed]
- Bardoni B, Zanaria E, Guioli S, Floridia G, Worley KC, Tonini G, Ferrante E, et al (1994) A dosage sensitive locus at chromosome Xp21 is involved in male to female sex reversal. Nat Genet 7:497–501 [DOI] [PubMed]
- Behringer RR (1995) The mullerian inhibitor and mammalian sexual development. Philos Trans R Soc Lond B Biol Sci 350:285–288 (discussion, 289) [DOI] [PubMed]
- Breedlove SM (1992) Sexual dimorphism in the vertebrate nervous system. J Neurosci 12:4133–4142 [DOI] [PMC free article] [PubMed]
- Cameron FJ, Sinclair AH (1997) Mutations in SRY and SOX9: testis-determining genes. Hum Mutat 9:388–395 [DOI] [PubMed]
- Crawford PA, Dorn C, Sadovsky Y, Milbrandt J (1998) Nuclear receptor DAX-1 recruits nuclear receptor corepressor N-CoR to steroidogenic factor 1. Mol Cell Biol 18:2949–2956 [DOI] [PMC free article] [PubMed]
- Davey HW, Wilkins RJ, Waxman DJ (1999) Human sexual development stat5 signaling in sexually dimorphic gene expression and growth patterns. Am J Hum Genet 65:959–965 (in this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santa Barbara P, Bonneaud N, Boizet B, Desclozeaux M, Moniot B, Sudbeck P, Scherer G, et al (1998) Direct interaction of SRY-related protein SOX9 and steroidogenic factor 1 regulates transcription of the human anti-Müllerian hormone gene. Mol Cell Biol 18:6653–6665 [DOI] [PMC free article] [PubMed]
- Desclozeaux M, Poulat F, de Santa Barbara P, Soullier S, Jay P, Berta P, Boizet-Bonhoure B (1998) Characterization of two Sp1 binding sites of the human sex determining SRY promoter. Biochim Biophys Acta 1397:247–252 [DOI] [PubMed]
- Eicher EM, Washburn LL, Schork NJ, Lee BK, Shown EP, Xu X, Dredge RD, et al (1996) Sex-determining genes on mouse autosomes identified by linkage analysis of C57BL/6J-YPOS sex reversal. Nat Genet 14:206–209 [DOI] [PubMed]
- Finley KD, Edeen PT, Foss M, Gross E, Ghbeish N, Palmer RH, Taylor BJ, et al (1998) Dissatisfaction encodes a tailless-like nuclear receptor expressed in a subset of CNS neurons controlling Drosophila sexual behavior. Neuron 21:1363–1374 [DOI] [PubMed]
- Fisher CR, Graves KH, Parlow AF, Simpson ER (1998) Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc Natl Acad Sci USA 95:6965–6970 [DOI] [PMC free article] [PubMed]
- Giuili G, Shen WH, Ingraham HA (1997) The nuclear receptor SF-1 mediates sexually dimorphic expression of Mullerian inhibiting substance, in vivo. Development 124:1799–1807 [DOI] [PubMed]
- Grosschedl R, Giese K, Pagel J (1994) HMG domain proteins: architectural elements in the assembly of nucleoprotein structures. Trends Genet 10:94–100 [DOI] [PubMed]
- Gubbay J, Collignon J, Koopman P, Capel B, Economou A, Münsterberg A, Vivian N, et al (1990) A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature 346:245–250 [DOI] [PubMed]
- Hacker A, Capel B, Goodfellow P, Lovell-Badge R (1995) Expression of Sry, the mouse sex determining gene. Development 121:1603–1614 [DOI] [PubMed]
- Ikeda Y, Shen WH, Ingraham HA, Parker KL (1994) Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol Endocrinol 8:654–662 [DOI] [PubMed]
- Ikeda Y, Swain A, Weber TJ, Hentges KE, Zanaria E, Lalli E, Tamai KT, et al (1996) Steroidogenic factor 1 and Dax-1 colocalize in multiple cell lineages: potential links in endocrine development. Mol Endocrinol 10:1261–1272 [DOI] [PubMed]
- Ito M, Yu R, Jameson JL (1997) DAX-1 inhibits SF-1-mediated transactivation via a carboxy-terminal domain that is deleted in adrenal hypoplasia congenita. Mol Cell Biol 17:1476–1483 [DOI] [PMC free article] [PubMed]
- Jeske YW, Bowles J, Greenfield A, Koopman P (1995) Expression of a linear Sry transcript in the mouse genital ridge. Nat Genet 10:480–482 [DOI] [PubMed]
- Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R (1991) Male development of chromosomally female mice transgenic for Sry. Nature 351:117–121 [DOI] [PubMed]
- Koopman P, Münsterberg A, Capel B, Vivian N, Lovell-Badge R (1990) Expression of a candidate sex-determining gene during mouse testis differentiation. Nature 348:450–452 [DOI] [PubMed]
- Lalli E, Bardoni B, Zazopoulos E, Wurtz JM, Strom TM, Moras D, Sassone-Corsi P (1997) A transcriptional silencing domain in DAX-1 whose mutation causes adrenal hypoplasia congenita. Mol Endocrinol 11:1950–1960 [DOI] [PubMed]
- Lee CH, Taketo T (1994) Normal onset, but prolonged expression, of Sry gene in the B6.Ydom sex-reversed mouse gonad. Dev Biol 165:442–452 [DOI] [PubMed]
- Margarit E, Guillén A, Rebordosa C, Vidal-Taboada J, Sánchez M, Ballesta F, Oliva R (1998) Identification of conserved potentially regulatory sequences of the SRY gene from 10 different species of mammals. Biochem Biophys Res Commun 245:370–377 [DOI] [PubMed]
- Merke DP, Tajima T, Baron J, Cutler GB Jr (1999) Hypogonadotropic hypogonadism in a female caused by an X-linked recessive mutation in the DAX1 gene. N Engl J Med 340:1248–1252 [DOI] [PubMed]
- Muscatelli F, Strom TM, Walker AP, Zanaria E, Récan D, Meindl A, Bardoni B, et al (1994) Mutations in the DAX-1 gene give rise to both X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism. Nature 372:672–676 [DOI] [PubMed]
- Nachtigal MW, Hirokawa Y, Enyeart-VanHouten DL, Flanagan JN, Hammer GD, Ingraham HA (1998) Wilms' tumor 1 and Dax-1 modulate the orphan nuclear receptor SF-1 in sex-specific gene expression. Cell 93:445–454 [DOI] [PubMed]
- Ogawa S, Lubahn DB, Korach KS, Pfaff DW (1997) Behavioral effects of estrogen receptor gene disruption in male mice. Proc Natl Acad Sci USA 94:1476–1481 [DOI] [PMC free article] [PubMed]
- Parker KL, Schimmer BP (1997) Steroidogenic factor 1: a key determinant of endocrine development and function. Endocr Rev 18:361–377 [DOI] [PubMed]
- Raymond CS, Parker ED, Kettlewell JR, Brown LG, Page DC, Kusz K, Jaruzelka J, et al (1999) A region of human chromosome 9p required for testis development contains two genes related to known sexual regulators. Hum Mol Genet 8:989–996 [DOI] [PubMed]
- Shen WH, Moore CC, Ikeda Y, Parker KL, Ingraham HA (1994) Nuclear receptor steroidogenic factor 1 regulates the müllerian inhibiting substance gene: a link to the sex determination cascade. Cell 77:651–661 [DOI] [PubMed]
- Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, Foster JW, et al (1990) A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 346:240–244 [DOI] [PubMed]
- Swain A, Lovell-Badge R (1999) Mammalian sex determination: a molecular drama. Genes Dev 13:755–767 [DOI] [PubMed]
- Swain A, Narvaez V, Burgoyne P, Camerino G, Lovell-Badge R (1998) Dax1 antagonizes Sry action in mammalian sex determination. Nature 391:761–767 [DOI] [PubMed]
- Swain A, Zanaria E, Hacker A, Lovell-Badge R, Camerino G (1996) Mouse Dax1 expression is consistent with a role in sex determination as well as in adrenal and hypothalamus function. Nat Genet 12:404–409 [DOI] [PubMed]
- Tamai KT, Monaco L, Alastalo TP, Lalli E, Parvinen M, Sassone-Corsi P (1996) Hormonal and developmental regulation of DAX-1 expression in Sertoli cells. Mol Endocrinol 10:1561–1569 [DOI] [PubMed]
- Töhönen V, Osterlund C, Nordqvist K (1998) Testatin: a cystatin-related gene expressed during early testis development. Proc Natl Acad Sci USA 95:14208–14213 [DOI] [PMC free article] [PubMed]
- Tucker PK, Lundrigan BL (1993) Rapid evolution of the sex determining locus in Old World mice and rats. Nature 364:715–717 [DOI] [PubMed]
- Vainio S, Heikkilä M, Kispert A, Chin N, McMahon AP (1999) Female development in mammals is regulated by Wnt-4 signalling. Nature 397:405–409 [DOI] [PubMed]
- Viger RS, Mertineit C, Trasler JM, Nemer M (1998) Transcription factor GATA-4 is expressed in a sexually dimorphic pattern during mouse gonadal development and is a potent activator of the Müllerian inhibiting substance promoter. Development 125:2665–2675 [DOI] [PubMed]
- Yu RN, Ito M, Saunders TL, Camper SA, Jameson JL (1998) Role of Ahch in gonadal development and gametogenesis. Nat Genet 20:353-357 [DOI] [PubMed]
- Zanaria E, Muscatelli F, Bardoni B, Strom TM, Guioli S, Guo W, Lalli E, et al (1994) An unusual member of the nuclear hormone receptor superfamily responsible for X-linked adrenal hypoplasia congenita. Nature 372:635–641 [DOI] [PubMed]
- Zhang Y-H, Guo W, Wagner RL, Huang B-L, McCabe L, Vilain E, Burris TP, et al (1998) DAX1 mutations map to putative structural domains in a deduced three-dimensional model. Am J Hum Genet 62:855–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann S, Schwärzler A, Buth S, Engel W, Adham IM (1998) Transcription of the Leydig insulin-like gene is mediated by steroidogenic factor-1. Mol Endocrinol 12:706–713 [DOI] [PubMed]
- Zimmermann S, Steding G, Emmen JMA, Brinkmann AO, Nayernia K, Engel W, Adham IM (1999) Targeted disruption of the Insl3 gene causes bilateral cryptorchidism. Mol Endocrinol 13:681–691 [DOI] [PubMed]