To the Editor:
Leber congenital amaurosis (LCA) (MIM 204000/204100) is a clinically and genetically heterogeneous retinal disorder that occurs in infancy and is accompanied by profound visual loss, nystagmus, poor pupillary reflexes, and either a normal retina or varying degrees of atrophy and pigmentary changes (Leber 1869, 1871; François 1968). The electroretinogram (ERG) is extinguished or severely reduced (Franceschetti 1954). LCA is largely a recessive disease, although autosomal dominant pedigrees have been identified (Sorsby et al. 1960; Heckenlively 1988). To date, three genes for LCA have been identified and sequenced: retinal guanylate cyclase (GUCY2D) on chromosome 17p13; retinal pigment epithelium protein (RPE65) on chromosome 1p31; and cone-rod homeobox (CRX) on chromosome 19q13.3. One additional locus has been identified on chromosome 14q24 (Stockton et al. 1998). We show evidence for linkage to chromosome 6q11-16 in a multigenerational kindred of Old Order River Brethren. The disease gene maps to a 23-cM interval flanked by DNA polymorphic markers D6S1551 and D6S1694, with a maximum two-point LOD score of 3.38 (recombination fraction [θ] zero) at D6S391. Two candidate genes on chromosome 6 were screened for mutations: gamma aminobutyric acid rho1 and rho2 (GABRR1 and GABRR2) at 6q14-21 (Cutting et al. 1992), and interphotoreceptor matrix proteoglycan (IMPG1) at 6q13-15 (Gehrig et al. 1998).
The incidence of LCA is 3 in 100,000 persons and accounts for ⩾5% of all inherited retinal dystrophies (Perrault et al. 1996). Clinical and genetic heterogeneity have been demonstrated (Wardenburg 1961; Camuzat et al. 1996). The phenotype has been associated with familial juvenile nephronophthisis and cone-shaped epiphyses (Saldino-Mainzer syndrome) and with kidney disease (Senior-Loken syndrome), osteoporosis, metabolic diseases, and neurological abnormalities (Loken et al. 1961; Senior et al. 1961; Dekaban 1969; Mainzer et al. 1970; Ellis et al. 1984).
The first locus for LCA was mapped to 17p13 with the use of homozygosity mapping in consanguineous families of North African descent (Camuzat et al. 1996). Mutations in the retina-specific guanylate cyclase gene (RETGC 1), on chromosome 17p13, involved in phototransduction, were subsequently identified (Perrault et al. 1996). Mutations in RPE65 on chromosome 1p31, specific to the retinal pigment epithelium involved in retinoid metabolism, were reported in patients with LCA, thus establishing a second gene (LCA2) for this heterogeneous disease (Marlhens et al. 1997). The photoreceptor-specific homeobox gene CRX, on chromosome 19q13.3, has been implicated as the third gene, since mutations were demonstrated (Freund et al. 1998). A novel locus on chromosome 14q24 (LCA3) was identified in consanguineous Saudi Arabian families (Stockton et al. 1998).
We studied a consanguineous family belonging to the Old Order River Brethren, a religious isolate originating in eastern Pennsylvania. The Old Order River Brethren descended from the Swiss, who emigrated to America in the 1750s in pursuit of religious freedom (Breckvill 1972). The kindred includes three affected individuals in two related sibships (fig. 1) who were initially evaluated at the Johns Hopkins Center for Hereditary Eye Diseases (JHCHED) and who are being followed annually. The patients presented with visual acuities in the order of 20/100–20/400, nystagmus, high hypermetropia, poor pupillary reflexes, and normal fundi. Progressive hypermetropia and increasing peripheral retinal mottling, of varying degree, were noted. The ERG was abolished. Review of other systems was unremarkable. We report a novel locus for LCA (LCA5) in this pedigree, on chromosome 6q11-16, by linkage analysis and homozygosity mapping.
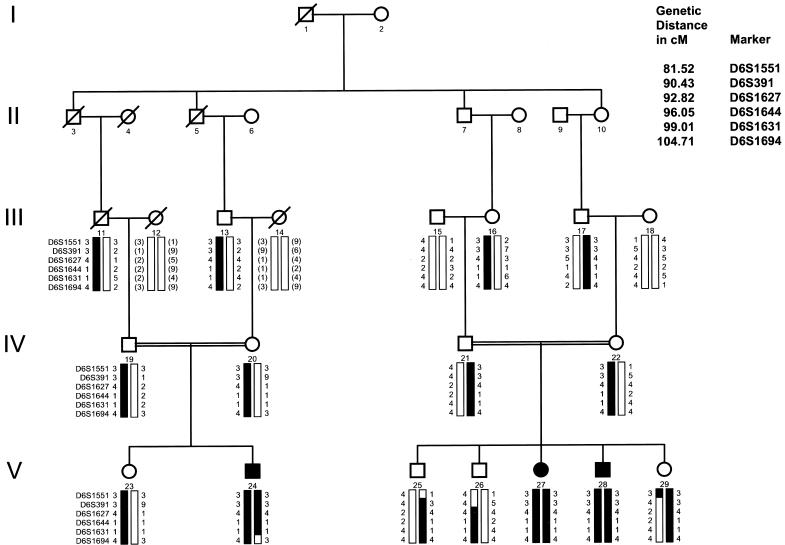
Figure 1.
Pedigree of family with LCA. Blackened symbols represent affected individuals. Blackened bars indicate disease-linked haplotype. Genotype (in parentheses) is inferred. Markers are listed in order, from centromere to telomere. Critical recombination events are noted in individuals 29 and 24 at markers D6S1551 and D6S1694, respectively.
Venous blood samples were obtained from 27 family members of the Old Order River Brethren community and a cheek brush sample was obtained from an infant (individual 29). Consents were obtained in accordance with regulations of the Johns Hopkins Medical Institutions' Joint Committee on Clinical Investigation. DNA was isolated from whole blood by means of the QIAamp Blood Kit (Qiagen), according to the manufacturer's instructions. The alkali method was used to obtain DNA from the single cheek sample.
Initially, the affected members and their first-degree relatives were screened to exclude linkage to the regions of the previously described genes involved in LCA on chromosomes 1, 17, and 19. The screen was then extended, by use of the whole-genome 8A multiplex version of markers spaced at 20 cM (Research Genetics). A region of homozygosity was identified on chromosome 6q. Further analysis, with additional markers in all potentially significant family members, was undertaken. Marker information was obtained from the Genome Database.
PCR-based genotyping, with fluorescent labeled markers, was performed by means of the Applied Biosystems 373 automated DNA sequencer. PCR reactions were performed in a 9600 Perkin Elmer thermocycler, and the PCR products were checked for amplification with a 3% agarose gel (Saiki et al. 1988).
The amplified PCR product was genotyped by means of the automated DNA sequencer. GENESCAN ANALYSIS 2.0.0 and GENOTYPER version 1.1 software were used, to size the PCR products and to analyze the data. Allele sizes were scored by two independent observers.
Two-point linkage analysis was performed by use of the MLINK option of the FASTLINK program, version 5.1 (Lathrop et al. 1984; Cottingham et al. 1993.) In this pedigree, LCA was analyzed as an autosomal recessive trait with complete penetrance, with an assumed allele frequency of .0032. A total of 40 microsatellite markers (Research Genetics) on chromosome 6 were analyzed, to determine the minimum region containing the new gene (Lander and Botstein 1987). Marker allele frequencies were estimated by means of the Genetic Analysis System, version 2.0 (Young), and GCONVERT (Duffy). The final LOD scores were computed by means of the allele frequencies generated by the GCONVERT program. Recombination frequencies for males and females were assumed to be equal. All inbreeding loops in the family were disconnected for computational reasons (Ott 1991) (fig. 1).
The GENEHUNTER program was used to perform multipoint linkage analysis against a fixed map of 17 informative markers, with an assumed equilibrium between marker and test loci (Kruglyak et al. 1996). These markers were selected from the original 40 microsatellite markers because they were highly polymorphic and their relative orders and map distances were well estimated in public databases. The comprehensive genetic map of the Center for Medical Genetics, Marshfield Medical Research Foundation, provided the sex-averaged genetic distances for the markers noted in figure 1. The same map provided the order for 37 markers. The remaining three markers were placed by means of the Genome Location Database. Subsequently, the genetic framework map from the Center for Medical Genetics, Marshfield Medical Research Foundation, reduced the 37 markers to 23, indicating that some markers occurred at identical positions. Thus, in the analyses in which multiple markers had identical positions, the markers with reduced information content were dropped in favor of those with better information content.
Multipoint LOD scores were computed with the same model described above for two-point analysis. Multipoint nonparametric linkage (NPL) scores were also computed with the use of only the affected individuals. Because of the inherent limitation on pedigree size in the GENEHUNTER program, the large pedigree was trimmed and broken into two separate units, accounting for some potential loss of power in the analysis. The data were examined for regions of allelic homozygosity in the affected individuals (Dib et al. 1996). Haplotype analysis was used to further define the interval containing the disease locus (Lathrop et al. 1985).
The following retina-specific genes on chromosome 6 were evaluated for the presence of disease-causing mutations: the GABRR1 and GABRR2 genes on chromosome 6q14.1-21 (Cutting et al. 1991, 1992) and the IMPG1 gene on chromosome 6q13-15 (Gehrig et al. 1998) (fig. 2).
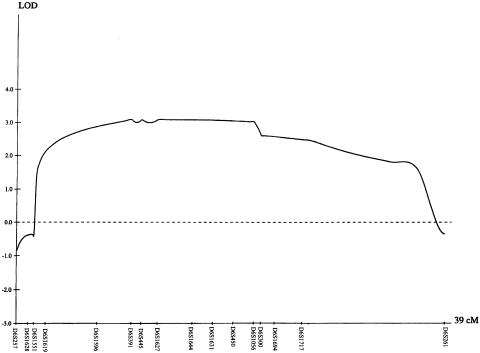
Figure 2.
Results of multipoint LOD score analysis using the GENEHUNTER program. The centromere is located toward the left of the graph. Maximum LOD scores were obtained between markers D6S391 and D6S450.
GABRR1 and GABRR2 are assumed to have arisen by gene duplication and share a 50% homology with each other. GABA is a neuroinhibitory transmitter mediating fast synaptic inhibition by activating chloride channels. GABRR1 is expressed largely in the retina; GABRR2 is expressed primarily in the brain. GABRR1 expression in the developing retina suggests its possible role as a candidate gene. In exons 1 and 4, polymorphic changes were identified. This did not change in the amino acid. These polymorphic changes were also identified in the normal population. No sequence changes were noted in GABRR2.
IMPG1, a novel gene encoding a major proteoglycan of the interphotoreceptor matrix, is expressed in the retina by both rods and cones and maps to 6q13-15; it was considered a further candidate gene for LCA (Gehrig et al. 1998). No significant changes were noted after the 17 coding exons of this gene were sequenced.
Linkage of LCA in the Old Order River Brethren was found at chromosome 6q11-16, supported by statistically significant two-point LOD scores with maximum LOD score (Zmax) 3.38 (θ=0) at D6S391 (table 1). Haplotype analysis of recombination events localizes the disease locus to a region of 23 cM, flanked by D6S1551 and D6S1694, thus identifying a new locus for LCA. Critical recombinant events were observed at marker D6S1551 in individual 29, who is unaffected, and at marker D6S1694 in individual 24, who is affected, defining the centromeric and telomeric boundaries, respectively. A common haplotype covers the region in all the affected individuals (fig. 1). Homozygosity of other highly informative markers across the candidate region was noted.
Table 1.
Two-Point LOD Scores for Linkage between LCA and Chromosome 6 Markers
|
LOD Score at θ = |
|||||||||
| Order | .00 | .01 | .50 | .10 | .20 | .30 | .40 | θmax | Zmax |
| D6S257 | −1.33 | −.17 | .67 | .92 | .93 | .71 | .39 | .145 | .97 |
| D6S1628 | −1.24 | 1.00 | 1.49 | 1.52 | 1.29 | .93 | .49 | .084 | 1.53 |
| D6S1658 | −1.23 | −.63 | −.15 | .04 | .19 | .18 | .11 | .239 | .20 |
| D6S430 | −1.17 | 1.41 | 1.85 | 1.82 | 1.46 | 1.00 | .51 | .066 | 1.86 |
| D6S1551 | −1.32 | .27 | .82 | .94 | .88 | .67 | .37 | .117 | .95 |
| D6S1619 | 1.20 | 1.17 | 1.03 | .87 | .59 | .36 | .17 | .001 | 1.20 |
| D6S1596 | 1.23 | 1.20 | 1.10 | .96 | .71 | .47 | .23 | .001 | 1.23 |
| D6S391 | 3.38 | 3.30 | 3.00 | 2.62 | 1.87 | 1.16 | .55 | .001 | 3.38 |
| D6S1707 | 3.15 | 3.07 | 2.76 | 2.37 | 1.63 | .98 | .47 | .001 | 3.15 |
| D6S251 | 3.22 | 3.14 | 2.83 | 2.45 | 1.71 | 1.06 | .50 | .001 | 3.22 |
| D6S445 | 2.97 | 2.89 | 2.59 | 2.23 | 1.56 | .98 | .48 | .001 | 2.97 |
| D6S1627 | 2.33 | 2.28 | 2.07 | 1.82 | 1.33 | .87 | .43 | .001 | 2.33 |
| D6S1644 | 2.20 | 2.15 | 1.93 | 1.66 | 1.17 | .74 | .35 | .001 | 2.20 |
| D6S1631 | 2.70 | 2.63 | 2.38 | 2.07 | 1.47 | .93 | .45 | .001 | 2.70 |
| D6S450 | 2.28 | 2.23 | 2.01 | 1.74 | 1.24 | .79 | .38 | .001 | 2.28 |
| D6S1056 | 1.47 | 1.42 | 1.22 | 1.02 | .70 | .46 | .23 | .001 | 1.47 |
| D6S300 | 1.24 | 1.33 | 1.43 | 1.36 | 1.07 | .71 | .35 | .050 | 1.43 |
| D6S1716 | .08 | .10 | .13 | .14 | .13 | .11 | .08 | .090 | .14 |
| D6S1694 | −.40 | −.31 | −.14 | −.07 | −.07 | −.07 | −.04 | .848 | .19 |
| D6S1717 | −.73 | −.74 | −.77 | −.77 | −.65 | −.43 | −.20 | .827 | .39 |
| D6S261 | −1.18 | −.60 | −.10 | .10 | .23 | .21 | .13 | .231 | .23 |
The maximum multipoint LOD score was 3.10 between D6S391 and D6S450, a 9.5-cM interval (fig. 2). The multipoint NPL score was significant, with P<.004 for a 23-cM region. Sequencing of candidate genes GABRR1 and GABRR2 and IMPG1 revealed polymorphic changes only.
LCA in the Old Order River Brethren, a highly inbred community, maps to a 23-cM interval on chromosome 6q11-16, as defined by linkage analysis and homozygosity mapping. Since this population is genetically isolated, and since LCA is quite rare, we presume that a single common ancestor was a carrier for this recessive trait (Lander and Botstein 1987). The large size of the region of homozygosity in the family and the history of migration indicate the recency of the mutation in the population (fig. 1).
LCA in this pedigree was not associated with multisystem abnormalities. Renal function remains normal. Neurological and hepatic function were within normal limits. The patients are of normal stature and intelligence. Neither photophobia nor photoattraction was reported in infancy, although pressing on the globes (the digito-ocular phenomenon of Franceschetti-Bamatter) played a prominent part in childhood behavior (Franceschetti 1954). Visual dysfunction, nystagmus, and the digito-ocular phenomenon were noticed in early infancy. A high hyperopic refractive correction was noted in all the patients (Wagner et al. 1985). Ophthalmoscopic examination in infancy revealed normal fundi, but in childhood, attenuated retinal vasculature with a varying degree of pigmentary changes was noticed. Electroretinography showed a markedly reduced response in the affected individuals. Vision has been stable in all affected members of the family who have been followed clinically at JHCHED.
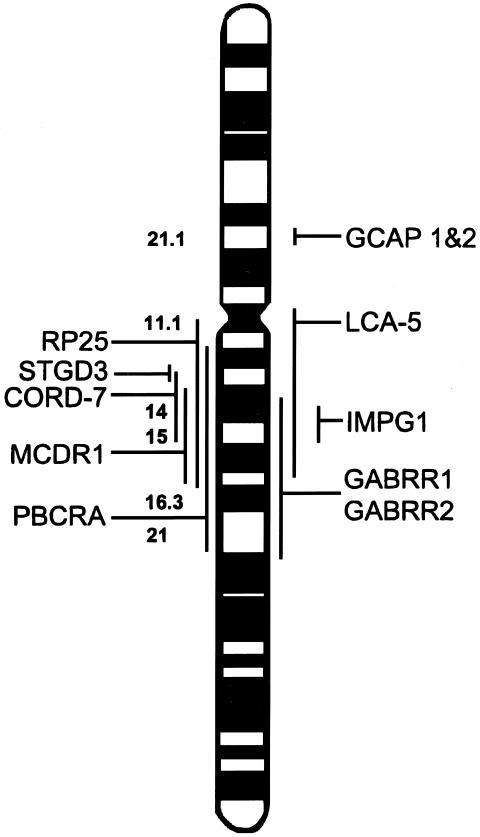
Genetic studies have identified a large region on chromosome 6q responsible for several retinal dystrophies (Small et al. 1992, 1993, 1997; Stone et al. 1994; Kelsell et al. 1995, 1998; Sauer et al. 1997; Griesinger et al. 1998; Rabb et al. 1998; Ruiz et al. 1998). LCA5 lies in the overlapping region of autosomal recessive RP at 6cen-q16 (Ruiz et al. 1998), progressive bifocal chorioretinal dystrophy (PBCRA) at 6q12-21 (Kelsell et al. 1995), North Carolina macular dystrophy at 6q14-16.2 (Small et al. 1993), Stargardt-like dominant macular degeneration (STGD3) at 6q13 (Stone et al. 1994; Griesinger et al. 1998), and dominant cone-rod dystrophy (CORD7) at 6q13-15 (Kelsell et al. 1998) (fig. 3). The occurrence of multiple loci so closely spaced in the genome could indicate the presence of a number of retinal genes in continuum, since the phenotype of all these retinal dystrophies, their ophthalmologic appearance, age at onset, and the extent and pattern of visual loss are varied. On the other hand, like the ABCR gene mutations that cause autosomal recessive retinitis pigmentosa (Martinez-Mir et al. 1997, 1998), juvenile and late-onset fundus flavimaculatus (Allikmets et al. 1997a), cone-rod dystrophy (Cremers et al. 1998), age-related macular disease, and recessive Stargardt disease (Kaplan et al. 1993; Gerber et al. 1995, 1998; Allikmets et al. 1997b), leading to distinct phenotypes (Lewis et al. 1999), it is possible that a single large gene in the proximal centromeric portion of the long arm of chromosome 6 could cause a myriad of retinal dystrophies, LCA being the most severe.
Figure 3.
Chromosome 6 ideogram, showing the location of candidate genes screened and retinal disease loci in the region.
Perhaps LCA5 is allelic with STGD3, RP25, CORD7, MCDR, and PBCRA. It is conceivable that mutations in different sites cause different structural alterations in the predicted protein, predisposing to varying phenotypes (Rozet et al. 1998).
The three other genes causing LCA are known to cause other phenotypically varied retinal dystrophies as well, raising the possibility of a similar situation in the LCA5 gene. GUCY2D mutations (LCA1) have been identified in autosomal dominant cone-rod dystrophy (Kelsell et al. 1998), although RPE65 (LCA2) mutations cause autosomal recessive retinitis pigmentosa as well as LCA (Morimura et al. 1998). Mutations in the cone-rod homeobox gene are now known to cause autosomal dominant cone-rod dystrophy, LCA, and late-onset dominant retinitis pigmentosa (Sohocki et al. 1998). It is currently possible to identify mutations of the known LCA genes in less than one-third of the patients with LCA (Dharmaraj et al. 1999). The isolation of another locus for this retinal disorder, LCA5, on chromosome 6q, will account for an additional proportion of patients with an identifiable gene mutation. Recruitment of additional families with LCA to further narrow the critical region is under way, and candidate gene analysis continues.
Acknowledgments
We are grateful to the patients and their families for their cooperation and participation in the study. We would like to acknowledge the help of Garry Cutting, Marina Kniazeva, Joan Bailey-Wilson, Reza Vagefi, Naba Bora, Carrie Gruver, Kang Zhang, Suzanne M. Leal, Mary Anderson, Gregory Leppert, Karen A. Klima, and Pamela Maskell. This work was supported in part by grants from the Foundation for Retinal Research, The Grousbeck Foundation, and The Edel and Krieble Funds of The Johns Hopkins Center for Hereditary Eye Diseases.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- Center for Medical Genetics, Marshfield Medical Research Foundation, http://marshmed.org/genetics/
- Duffy LD (1995) Sib-pair program, http://www.qimr.edu.au/davidD/davidd.html
- Genome Database, http://www.gdb.org/ (for primer information)
- Genome Location Database, http://cedar.genetics.soton.ac.uk/pub/PROGRAMS/ldb (for placing markers)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim (for LCA [MIM 204000/204100])
- Research Genetics, http://www.resgen.com/
- Young A (1995) Genetic Analysis System, version 2.0, http://users.ox.ac.uk/~ayoung/gas.html
References
- Allikmets R, Shroyer NF, Singh N, Seddon JM, Lewis RA, Bernstein PS, Peiffer A, et al (1997a) Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science 277:1805–1807 [DOI] [PubMed]
- Allikmets R, Singh N, Sun H, Shroyer NF, Hutchinson A, Chidambaram A, Gerrad B, et al (1997b) A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet 15:236–246 [DOI] [PubMed]
- Breckvill LT (1972) History Old Order River Brethren. Breckvill and Stickler, Pennsylvania [Google Scholar]
- Camuzat A, Rozet JM, Dollfus H, Gerber S, Perrault I, Weissenbach J, Munnich A, et al (1996) Evidence of genetic heterogeneity of Leber's congenital amaurosis (LCA) and mapping of LCA1 to chromosome 17p13. Hum Genet 97:798–801 [DOI] [PubMed]
- Cottingham RW Jr, Idury RM, Schaffer AA (1993) Faster sequential genetic linkage computations. Am J Hum Genet 53:252–263 [PMC free article] [PubMed]
- Cremers FP, van de Pol DJ, Van Driel M, den Hollander AI, van Haren FJ, Knoers NV, Tijmes N, et al (1998) Autosomal recessive retinitis pigmentosa and cone-rod dystrophy caused by splice site mutations in the Stargardt's disease gene ABCR. Hum Mol Genet 7:355–362 [DOI] [PubMed]
- Cutting GR, Curristin S, Zoghbi H, O'Hara B, Seldin MF, Uhl GR (1992) Identification of a putative gamma-aminobutyric acid receptor subunit rho 2 cDNA and localization of the genes rho 2 (GABRR2) and rho 1 (GABRR1) to human chromosome 6q14-q21 and mouse chromosome 4. Genomics 12:801–806 [DOI] [PubMed]
- Cutting GR, Lu L, O'Hara BF, Kasch LM, Montrose-Rafizadeh C, Donovan DM, Shimada S, et al (1991) Cloning of the gamma-aminobutyric acid GABA RHO 1 cDNA: a receptor subunit highly expressed in the retina. Proc Natl Acad Sci USA 88:2673–2677 [DOI] [PMC free article] [PubMed]
- Dekaban AS (1969) Hereditary syndrome of congenital retinal blindness (Leber), polycystic kidneys and maldevelopment of the brain. Am J Ophthalmol 68:1029–1037 [DOI] [PubMed]
- Dharmaraj S, Silva E, Li YY, Loyer M, Koenekoop RK, Maumenee IH (1999) Mutational analysis in one hundred consecutive patients with Leber congenital amaurosis. Invest Ophthalmol Vis Sci 40:A2983 [Google Scholar]
- Dib C, Faune S, Fizames C, Samson D, Dvout N, Vignal A, Millasseau P, et al (1996) A comprehensive genetic map of the human genome based on 5262 microsatellites. Nature 380:152–154 [DOI] [PubMed]
- Ellis DS, Heckenlively JR, Martin CL, Lachman RS, Sakati NA, Rimoin DL (1984) Leber's congenital amaurosis associated with familial juvenile nephronophthisis and cone-shaped epiphyses of the hands (the Saldino-Mainzer syndrome). Am J Ophthalmol 97:233–239 [DOI] [PubMed]
- Franceschetti A, Dieterle P (1954) L'importance diagnostique de l'electrorétinogramme dans les dégénérescences tapéto-rétinennes avec rétrécissement du champ visuel et héméralopie. Conf Neurol 14:184–186 [PubMed] [Google Scholar]
- François J (1968) Leber's congenital tapeto-retinal degeneration. Int Ophthalmol Clin 8:929–947 [PubMed]
- Freund CL, Wang QL, Chen S, Muskat B, Wiles CD, Sheffield VC, Jacobson SG, et al (1998) De novo mutations in the CRX homeobox gene associated with Leber congenital amaurosis. Nat Genet 18:311–312 [DOI] [PubMed]
- Gehrig A, Felbor U, Kelsell R, Hunt DM, Maumenee IH, Weber BHF (1998) Assessment of the interphotoreceptor matrix proteoglycan-1 (IMPG1) localized to 6q13-q15 in autosomal dominant Stargardt-like disease (ADSTGD), progressive bifocal chorioretinal atrophy (PBCRA), and North Carolina macular dystrophy (MCDR1). J Med Genet 35:641–645 [DOI] [PMC free article] [PubMed]
- Gerber S, Rozet JM, Bonneau D, Souied E, Camuzat A, Dufier JL, Amalric P, et al (1995) A gene for late-onset fundus flavimaculatus with macular dystrophy maps to chromosome 1p13. Am J Hum Genet 56:396–399 [PMC free article] [PubMed]
- Gerber S, Rozet JM, van de Pol TJ, Hoyng CB, Munnich A, Blankenagel A, Kaplan J, et al (1998) Complete exon-intron structure of the retina-specific ATP binding transporter gene (ABCR) allows the identification of novel mutations underlying Stargardt disease. Genomics 48:139–142 [DOI] [PubMed]
- Griesinger IB, Sieving PA, Chandrasekharappa SC, Ayyagari R (1998) Macular degeneration with highly variable phenotype localized to chromosome 6q. Am J Hum Genet 63:A30 [Google Scholar]
- Heckenlively JR (1988) Retinitis pigmentosa. Philadelphia, Lippincott, pp 125–149 [Google Scholar]
- Kaplan J, Gerber S, Larget-Piet D, Rozet JM, Dollfus H, Dufier JL, Odent S, et al (1993) A gene for Stargardt's disease (fundus flavimaculatus) maps to the short arm of chromosome 1. Nat Genet 5:308–311 [DOI] [PubMed]
- Kelsell RE, Godley BF, Evans K, et al (1995) Localization of the gene for progressive bifocal chorioretinal atrophy (PBCRA) to chromosome 6q. Hum Mol Genet 4:1653–1656 [DOI] [PubMed]
- Kelsell RE, Gregory-Evans K, Gregory-Evans CY, Holder GE, Jay MR, Weber BH, Moore AT, et al (1998) Localization of a gene (CORD7) for a dominant cone-rod dystrophy to chromosome 6q. Am J Hum Genet 63:274–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruglyak L, Daly MJ, Reeve-Daly MP, Lander ES (1996) Parametric and nonparametric linkage analysis: a unified multipoint approach. Am J Hum Genet 58:1347–1363 [PMC free article] [PubMed]
- Lander ES, Botstein D (1987) Homozygosity mapping: a way to map human recessive traits with the DNA of inbred children. Science 236:1567–1570 [DOI] [PubMed]
- Lathrop GM, Lalouel JM, Julier C, Ott J (1984) Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci USA 81:3443–3446 [DOI] [PMC free article] [PubMed]
- ——— (1985) Multipoint linkage analysis in humans: detection of linkage and estimation of recombination. Am J Hum Genet 37:482–498 [PMC free article] [PubMed]
- Leber T (1869) Über retinitis pigmentosa und angeborene amaurose. Albrecht von Graefe's Arch Klin Exp Ophthalmol 15:1–25 [Google Scholar]
- ——— (1871) Über anormale formen der retinitis pigmentosa. Arch für Ophthalmol 17:314–341 [Google Scholar]
- Lewis RA, Shroyer NF, Singh N, Allikmets R, Hutchinson A, Li Y, Lupski JR, et al (1999) Genotype/phenotype analysis of a photoreceptor-specific ATP-binding cassette transporter gene ABCR, in Stargardt disease. Am J Hum Genet 64:422–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loken AC, Hanssen O, Halvolsen S, Jolster NB (1961) Hereditary renal dysplasia and blindness. Acta Paediatr 50:177-194 [DOI] [PubMed] [Google Scholar]
- Mainzer F, Saldino RM, Ozonoff MB, Minagi H (1970) Familial nephropathy associated with retinitis pigmentosa, cerebellar ataxia and skeletal abnormalities. Am J Med 49:556–562 [DOI] [PubMed]
- Marlhens F, Bareil C, Griffoin JM, Zrenner E, Amalric P, Eliaou C, Liu SY, et al (1997) Mutations in RPE65 cause Leber's congenital amaurosis. Nat Genet 17:139–141 [DOI] [PubMed]
- Martinez-Mir A, Bayes M, Vilageliu L, Grinberg D, Ayuso C, Del Rio T, Garcia-Sandoval B, et al (1997) A new locus for autosomal recessive retinitis pigmentosa (RP19) maps to 1p13-1p21. Genomics 40:142–146 [DOI] [PubMed]
- Martinez-Mir A, Palona E, Allikmets R, Ayuso C, Del Rio T, Dean M, Vilageliu L, et al (1998) Retinitis pigmentosa caused by a homozygous mutation in the Stargardt disease gene ABCR. Nat Genet 18:11–12 [DOI] [PubMed]
- Morimura H, Fishman GA, Grover SA, Fulton AB, Berson EI, Dryja TP (1998) Mutations in the RPE65 gene in patients with autosomal recessive retinitis pigmentosa or Leber congenital amaurosis. Proc Natl Acad Sci USA 95:3088–3093 [DOI] [PMC free article] [PubMed]
- Ott J (ed) (1991) Analysis of human genetic linkage. 2d ed. Johns Hopkins University Press, Baltimore [Google Scholar]
- Perrault I, Rozet JM, Calvas P, Gerber S, Camuzat A, Dollfus H, Chatelin S, et al (1996) Retinal-specific guanylate cyclase gene mutations in Leber's congenital amaurosis. Nat Genet 14:461–466 [DOI] [PubMed]
- Rabb MF, Mullen L, Yelchits S, Udar N, Small KW (1998) A North Carolina macular dystrophy phenotype in a Belizean family maps to the MCDR1 locus. Am J Ophthalmol 125:502–508 [DOI] [PubMed]
- Rozet JM, Gerber S, Souied E, Perrault I, Chatelin S, Ghazi I, Leowski C, et al (1998) Spectrum of ABCR gene mutations in autosomal recessive macular dystrophies. Eur J Hum Genet 6:291–295 [DOI] [PubMed]
- Ruiz A, Borrego S, Marcos I, Antinolo G (1998) A major locus for autosomal recessive retinitis pigmentosa on 6q, determined by homozygosity mapping of chromosomal regions that contain gamma-aminobutyric acid-receptor clusters. Am J Hum Genet 62:1452–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki RK, Gelfand DH, Stoffel S, Sharf SJ, Higuchi R, Horn GT, Mullis KB, et al (1988) Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 239:487–491 [DOI] [PubMed]
- Sauer CG, Schworm HD, Ulbig M, Blankenagel A, Rohrshneider K, Pauleikhoff D, Grimm T, et al (1997) An ancestral core haplotype defines the critical region harbouring the North Carolina macular dystrophy gene (MCDR1). J Med Genet 34:961–966 [DOI] [PMC free article] [PubMed]
- Senior B, Friedman AI, Brando JL (1961) Juvenile familial nephropathy with tapetoretinal degeneration. Am J Ophthalmol 52:625–633 [DOI] [PubMed] [Google Scholar]
- Small KW, Puech B, Mullen L, Yelchits S (1997) North Carolina macular dystrophy phenotype in France maps to the MCDR1 locus. Mol Vis 3:1–6 [PubMed]
- Small KW, Weber J, Roses A, Pericak-Vance P (1993) North Carolina macular dystrophy MCDR1: a review and refined mapping to 6q14-q16.2. Ophthalmic Paediatr Genet 14:143–150 [DOI] [PubMed]
- Small KW, Weber JL, Roses A, Lennon F, Vance JM, Pericak-Vance MA (1992) North Carolina macular dystrophy is assigned to chromosome 6. Genomics 13:681–685 [DOI] [PubMed]
- Sohocki MM, Sullivan LS, Mintz-Hittner HA, Birch D, Heckenlively JR, Freund CL, McInnes RR, et al (1998) A range of clinical phenotypes associated with CRX, a photoreceptor transcription-factor gene. Am J Hum Genet 63:1307–1315 [DOI] [PMC free article] [PubMed]
- Sorsby A, Williams CE (1960) Retinal aplasia as a clinical entity. Br Med J 1:293–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockton DW, Lewis RA, Abboud EB, Rejhi-Manzer AA, Anderson KH, Lupski JR (1998) A novel locus for Leber congenital amaurosis on chromosome 14q24. Hum Genet 103:328–333 [DOI] [PubMed]
- Stone EM, Nichols BE, Kimura AE, Weingeist TA, Drack A, Sheffield VC (1994) Clinical features of a Stargardt-like dominant progressive macular dystrophy with genetic linkage to chromosome 6q. Arch Ophthalmol 112:765–772 [DOI] [PubMed]
- Waardenburg PJ (1961) Congenital and early infantile retinal dysfunction (high-graded) amblyopia and amaurosis Leber. In: Waardenburg PJ, Franceschetti A, Klein D (eds) Genetics and ophthalmology. 1st ed. Blackwell Scientific, Oxford, pp 1567–1581 [Google Scholar]
- Wagner RS, Caputo AR, Nelson LB, Zanoni D (1985) High hyperopia in Leber's congenital amaurosis. Arch Ophthalmol 103:1507–1509 [DOI] [PubMed]